Abstract
B cells are recognized as effector cells in allograft rejection that are dependent upon T cell help to produce alloantibodies causing graft injury. It is not known if B cells can also help T cells differentiate into memory cells in the alloimmune response. We found that in B cell-deficient hosts, differentiation of alloreactive T cells into effectors was intact whereas their development into memory T cells was impaired. To test if B cell help for T cells was required for their continued differentiation into memory T cells, activated T cells were sorted from alloimmunized mice and transferred either with or without B cells into naïve adoptive hosts. Activated T cells co-transferred with B cells gave rise to more memory T cells than those transferred without B cells and upon recall, mediated accelerated rejection of skin allografts. Co-transfer of B cells led to increased memory T cells by enhancing activated CD4 T cell proliferation and activated CD8 T cell survival. These results indicate that B cells help alloreactive T cell differentiation, proliferation and survival to generate optimal numbers of functional memory T cells.
Keywords: B cells, T cells, memory and transplantation
Materials and Methods
Mice
C57BL/6 (CD45.2, H-2b; hereafter wt), B6.SJL-Ptprca Pepcb/BoyJ (CD45.1, H-2b; hereafter CD45.1), B6.129S2-Igh-6tm1Cgn/J (CD45.2, H-2b; hereafter µMT) [1] and BALB/c (H-2d) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All animals were maintained under SPF conditions and procedures were performed as per IACUC guidelines.
Skin transplantation, donor specific transfusion and co-stimulation blockade
Partial thickness skin transplantation was performed using BALB/c donor abdominal skin [2, 3]. Skin grafts were monitored daily and rejection was defined as >95% graft necrosis. In some experiments, allograft recipients were treated with donor specific transfusion (DST) (BALB/c splenocytes, 2 × 107, day 0) and anti-CD40L (MR1, 1mg, days 0, 7, 14) (Bioexpress Inc, MA). Logrank test (Graphpad prism 4.0, Graphpad Software, Inc) was used to assess differences in allograft survival and p value of < 0.05 was considered significant.
Flow cytometry and intracellular cytokine staining
Fluorochrome-tagged antibodies CD8a (53-6.7), CD4 (RM4–5), CD44 (IM7), CD62L (MEL-14), CD45.1 (A20), CD69 (H1.2 F3), CD25 (PC61), CD29 (eBioHMb1-1), CD127 (A7R34), Bcl-2 (3F11) and IFNγ (XMG1.2) for flow cytometry were purchased from BD Pharmingen (San Diego, CA) and eBioscience (San Diego, CA). Intracellular IFNγ in lymphocytes was measured after re-stimualtion ex-vivo with BALB/c splenocytes (H-2d) (1:1) for 6-hrs. Flow cytometry acquisition was performed on LSRII analyzers (BD Biosciences, San Diego, CA), and data analyzed using Flowjo software (Treestar, Ashland, OR). BALB/c-reactive IFNγ+ T cells present within the responder CD4 and CD8 T cell populations were quantitated after gating on the H-2d negative population.
Cytotoxicity assays
Recipients of BALB/c skin allografts were used as memory mice at 8-weeks after allograft rejection. To assess in-vivo cytotoxicity, naïve and memory mice were treated with 200µg of anti-NK1.1 (PK136) (days -2 and -1) to deplete NK cells and injected with equal numbers of CFSE labeled H-2b (2 × 107, 0.2µM B6, syngeneic) and H-2d (2 × 107, 2µM, BALB/c, allogeneic) splenocytes (day 0). 24-hrs later, in-vivo killing of allogeneic cells was measured as loss of H-2d target cells compared to loss of H-2b syngeneic cells in mice vs. naïve control mice using the following formula: 100 - [(%H-2d cells in memory mice/ %H-2b cells in memory mice ÷ %H-2d cells in naïve mice / %H-2b cells in naïve mice) × 100] [4]. To assess in-vitro cytoxicity, spleen (SP) and lymph node (LN) cells from memory mice were purified for T cells by MACS depletion of B220+, NK1.1+, CD11b+ and CD11c+ cells and incubated with calcein labeled BALB/c splenocytes (0.3mM, 100:1) at 37°C for 4hrs. BALB/c cell killing was calculated using the formula: (% dead target cells – spontaneous dead target cells/100 – spontaneous dead targets) × 100 [5, 6].
Sorting of B cells and activated T cells for adoptive transfer
CD45.1 mice were immunized (3 × 107 BALB/c splenocytes, i.p.) and 8-days later, SP and LN cells were harvested to isolate activated T cells. Harvested cells were labeled with antibodies against CD4, CD8, CD44, and B220, and sorted for CD8+ CD44high, CD4+ CD44high and B220+ (CD4− and CD8−) populations (purity > 95%) on BD FACS Aria. 1 × 106 CD4+ CD44high or CD8+ CD44high T cells were transferred with or without 1.5 × 107 B220+ B cells into µMT and wt hosts. In some experiments, B220+ B cells were sorted from unimmunized naïve mice. In experiments testing allograft rejection, 2 × 106 CD8+ CD44high and CD4+ CD44high T cells were transferred into adoptive hosts with or without 1.5 × 107 B220+ B cells. CD8+ CD44high and CD4+ CD44high T cells were labeled with CFSE (2µM, Molecular Probes) prior to adoptive transfer in specified experiments [7].
Cell harvest and enumeration after adoptive transfer
Cells were harvested from spleen, LN, and bone marrow (BM) of adoptive hosts at indicated times points (1, 2, 3 and 8–12 weeks) after transfer of CD4+ CD44high or CD8+ CD44high T cells with or without B220+ B cells. Bone marrow cells were obtained from femurs and tibia, multiplied by a factor of 5.4 to estimate total body bone-marrow cells [3, 8]. Harvested live cells from tissues were counted using trypan blue exclusion on a hemacytometer, stained with FACS antibodies and analyzed by flow cytometry after gating on CD4+ or CD8+, CD45.1+ population. Apoptosis was determined after staining cells for Annexin V and 7-AAD (BD Pharmingen, San Diego CA). Statistical analyses were performed using unpaired t test (Graphpad Software, Inc) and differences with p < 0.05 were considered significant.
Introduction
B cells and memory T cells contribute to treatment resistant acute and chronic allograft rejection [9–13]. Traditionally, B cells are viewed as antibody producing effector cells that are dependent upon T cell help for their differentiation [14, 15]. Preformed and de-novo alloantibodies in patients often precede not only humoral but also cellular, acute and chronic rejection episodes [16–22]. Also, allograft biopsies from patients with acute and chronic rejection show evidence of B and memory T cell infiltrates [10, 11, 23–25]. These observations suggest a link between B cell and T cell activation in the alloimmune response.
Long-lived memory T cells have quantitative and qualitative advantages over naïve T cells that are essential for protective immune responses, and jeopardize allograft survival [12, 26–28] [29]. Studies on the requirement for B cells in the development of memory responses and protective immunity have yielded conflicting results. B cells were required for CD4 effector and memory T cell responses in Pneumocystis carinii, acute LCMV and Chlamydia trachomatis lung infections, and protein antigens such as KLH (keyhole lympet hemocyanin), PCC (pigeon cytochrome c) and conalbumin [30–34]. In the absence of B cells, CD8 T cell memory was impaired in chronic LCMV infection [35]. Similarly, B cells were required for T cell activation in rheumatoid arthritis and lupus nephritis [36, 37]. In contrast to the above studies, B cells were dispensable for CD4 T cell memory in influenza virus lung and Chlamydia trachomatis genital infections [38, 39]. Also, CD8 T cell memory to HY antigen, acute LCMV and Listeria monocytogenes infections was intact in B cell-deficient mice [40–42]. Differences in the type of pathogen studied, route of antigen entry and site of immune response, antigen load, extent of inflammation and requirement for neutralizing antibodies for pathogen clearance could have contributed to the contradictory results observed in these studies. The contribution of B cells to alloimmunity has been largely attributed to alloantibody production. A transplanted organ represents a unique milieu of foreign antigen and it is not known if development of T cell memory in this setting requires B cells. Understanding the role of B cells in T cell alloimmunity would assist in the design of therapies to target B cells that inhibit not only antibody production but also T cell immunity for lasting allograft survival.
Several functions of B cells such as antigen presentation, co-stimulation and cytokine production can modulate T cell differentiation and cellular immunity [43–46]. It has been shown that in the absence of antigen presentation by B cells, CD4 T cell activation and alloantibody production were impaired resulting in delayed heart but not skin allograft rejection [47]. However, it was not clear whether B cells had any impact on alloreactive T cell memory. We studied the development of CD4 and CD8 memory T cells in B cell-deficient and B cell-competent hosts after allogeneic skin transplantation. We report that in the absence of B cells, CD4 and CD8 T cells differentiate into alloreactive effectors but their development into memory T cells is impaired resulting in decreased numbers of alloreactive memory T cells. We find that B cells promote proliferation of activated CD4 T cells and survival of CD8 T cells leading to increased numbers of memory T cells, and thus, contribute to the quantitative advantage of T cell memory.
Results
Alloreactive T cell memory is impaired in B cell-deficient hosts
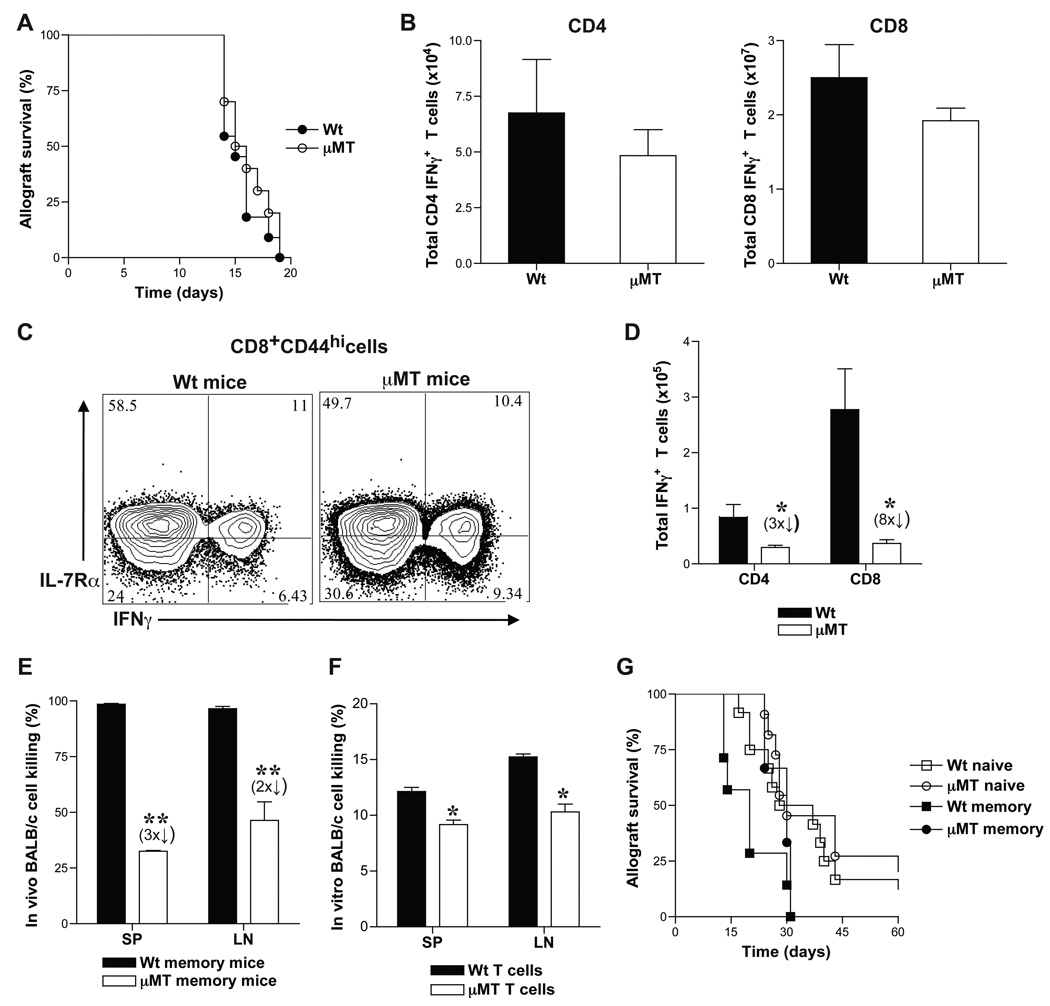
We investigated the role of B cells in alloreactive T cell responses by using B cell-deficient, µMT (B6, H-2b) mice. Allogeneic BALB/c (H-2d) skin grafts were transplanted onto µMT mice and allograft rejection was compared to that in B cell-competent wt mice. µMT and wt mice rejected skin allografts comparably (Fig. 1A) consistent with previous reports [47–49]. Spleen (SP), lymph node (LN) and bone marrow (BM) cells were harvested from µMT and wt recipients of BALB/c skin grafts at 14-days after transplantation (effector phase) and at 8 weeks after allograft rejection (memory phase) to measure alloreactive effector and memory T cells, respectively. Alloreactive T cells were identified as IFNγ+ cells present within the responding CD4+ and CD8+ T cell populations after 6-hr ex-vivo rechallenge with BALB/c splenocytes. Since CD4 and CD8 IFNγ+ cells have been reported to mark effector T cells that give rise to memory T cells [50], we utilized ex-vivo alloreactive IFNγ production to identify antigen specific cells. CD4+ and CD8+ BALB/c-reactive IFNγ+ T cell numbers were comparable between wt and µMT mice during the effector phase (Fig. 1B). However, the number of CD8 memory T cell precursors identified as BALB/c-reactive IFNγ+ IL-7Rα+ effector T cells within the CD8+ CD44hi population were decreased in µMT chimeras compared to wt chimeras (45 ± 8% vs. 58 ± 5%, respectively) (Fig. 1C). CD4 and CD8 BALB/c-reactive IFNγ+ T cells in the memory phase were significantly fewer in µMT recipients than in wt mice (Fig. 1D). Thus, despite comparable skin allograft rejection and differentiation into alloreactive effector T cells in µMT and wt mice, resulting memory T cells were significantly reduced in number in the absence of B cells. Since µMT mice have altered splenic architecture and contain fewer splenic T cells than wt mice [49, 51], alloreactive T cell responses were also tested in chimeras generated in irradiated µMT mice by transplanting syngeneic bone marrow cells from either µMT or wt donors. BALB/c-reactive IFNγ+ CD4 and CD8 memory T cells in µMT chimeras were 4- and 6-fold fewer, respectively, than in wt chimeras (data not shown). To test whether the difference in memory T cell numbers was significant to affect recall responses, cytotoxic function and allograft rejection were tested in µMT and wt mice at 8-weeks after BALB/c skin allograft rejection. µMT recipients mediated significantly less BALB/c cell lysis than wt mice in the memory phase (Fig. 1E–F). To assess allograft rejection mediated by memory T cells, µMT and wt memory mice were re-challenged with BALB/c skin allografts and treated with DST and anti-CD40L to inhibit endogenous naïve T cell activation [27, 52, 53]. Wt memory recipients rejected BALB/c skin allografts in a significantly accelerated manner compared to µMT memory mice (MST = 20 vs. 30 days, respectively, n = 4/grp; p = 0.03) (Fig. 1G). Allograft rejection in µMT memory mice was comparable to that of naïve mice (MST = 30 days in both groups, n = 4 – 9/grp) (Fig. 1G). Although alloantibodies could contribute to in-vivo allogeneic cell lysis in wt memory mice, skin allograft rejection occurs independently of alloantibody mediated graft injury in mice [54–56]. Therefore, these data suggest that impaired recall function in µMT memory mice is due to decreased numbers of alloreactive memory T cells in these mice compared to wt memory mice. Thus, in the absence of B cells, although alloreactive effector T cell differentiation occurs, their subsequent development into memory T cells is significantly impaired resulting in decreased numbers of functional alloreactive memory T cells.
Figure 1. Development of alloreactive memory T cells is impaired in B-cell deficient mice.
A. Rejection of BALB/c (H-2d) skin allografts transplanted to µMT (H-2b) and wt (H-2b) mice (MST = 16 and 15 days, respectively; p > 0.05; n = 10 mice/grp). Quantitiation of alloreactive effector (B) and memory (D) T cells in µMT and wt mice. Spleen (SP), lymph node (LN) and bone marrow (BM) cells were harvested from BALB/c skin grafted µMT and wt recipients at 14-days (effector phase) and at 8-weeks after rejection (memory phase) for quantitation of alloreactive T cells. Harvested cells were re-stimulated ex-vivo for 6-hrs with BALB/c splenocytes and IFNγ producing CD4 and CD8 T cells were assessed by flow cytometry and enumerated (Mean ± SD; *, p < 0.05; n = 4 mice/grp). C. CD8 memory precursors within alloreactive effector T cells in µMT and wt mice. Representative FACS plots of IL-7Rα expression on BALB/c-reactive IFNγ+ population within CD8+ CD44hi splenic T cells harvested at 14-days after BALB/c skin transplantation are shown. E–F. Cytotoxic function of µMT and wt memory T cells was assessed by in-vivo (E) and in-vitro (F) allogeneic cell lysis at 8-weeks after BALB/c skin graft rejection (memory phase). µMT and wt mice were depleted of NK cells and equal numbers of CFSE labeled H-2b (2 × 107, 2µM B6 wt, syngeneic) and H-2d (2 × 107, 0.2µM, BALB/c, allogeneic) splenocytes were injected (i.v.). 24-hrs later, in-vivo killing of BALB/c cells in comparison to B6 cells was measured by flow cytometry (Mean ± SD; **, p < 0.005; n = 3 – 4 mice/grp). Purified T cells from SP and LN cells of µMT and wt memory mice were incubated with calcein labeled BALB/c splenocytes (0.3mM, 100:1) and 4-hrs later, in-vitro killing of BALB/c cells was measured by flow cytometry (Mean ± SD; *, p < 0.05; n = 3 –4 mice/grp). G. Allograft rejection in memory µMT and wt mice. At 8-weeks after BALB/c allograft rejection (memory phase), µMT and wt recipients were re-challenged with BALB/c skin grafts and treated with DST (2 × 107) and anti-CD40L (MR1, 1mg on days 0, 7 and 14 after transplantation). Allograft rejection was assessed in µMT and wt memory mice, and compared to naïve mice (MST = 30, 20 and 30 days, respectively; p < 0.05; n = 4 – 9 mice/grp).
B cells promote differentiation of activated T cells into memory T cells in adoptive hosts
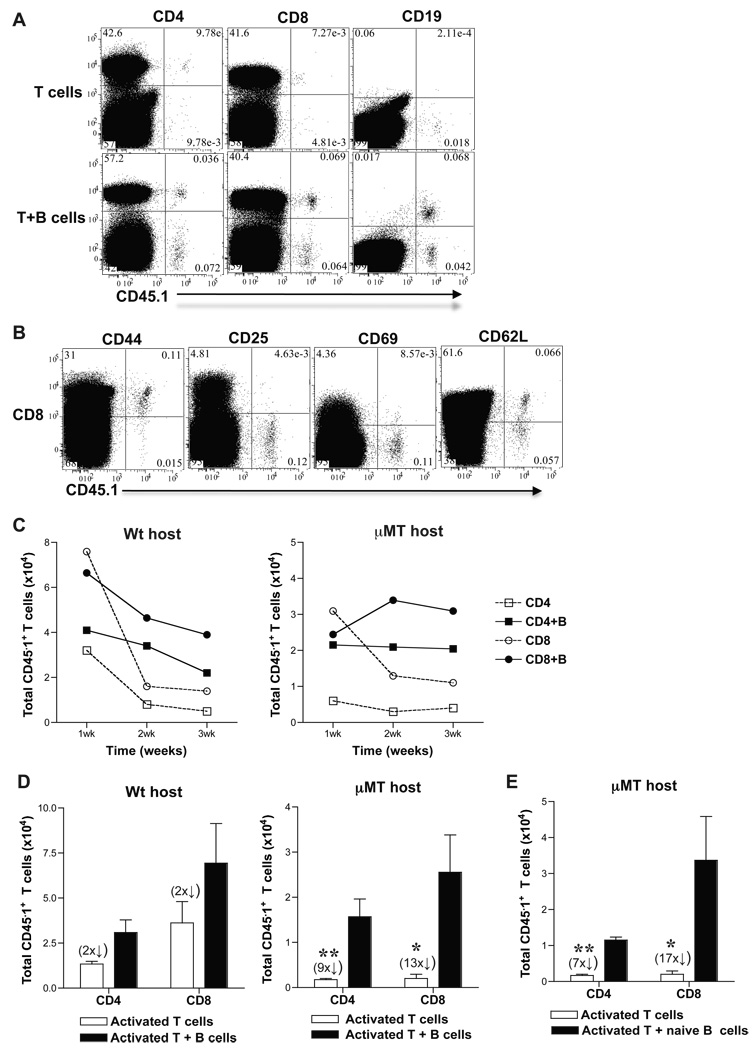
Results in µMT and wt hosts showed that in the absence of B cells, alloreactive T cell differentiation into effectors was preserved but their development into memory T cells was impaired. These findings raised the question whether B cells are required for the continued differentiation of alloreactive effector T cells into memory T cells. To answer this question, we adoptively transferred activated T cells sorted from alloimmunized mice either with or without B cells into naïve µMT and wt mice and tested their differentiation into functional memory T cells. CD4+ and CD8+ CD44hi activated T cells and B220+ B cells were sorted from SP and LN cells of CD45.1 (B6, H-2b) mice at 8-days after immunization with BALB/c splenocytes (3 × 107, i.p.). Sorted CD4+ CD44hi or CD8+ CD44hi activated T cells (>95% purity) (1 × 106) were transferred either alone or together with B220+ B cells (>97% purity) (1.5 × 107) into congenic naïve µMT (CD45.2) or wt (CD45.2) mice. Sorted T cells were CD44hi, 1B11hi and contained a population of CD69hi, CD25hi and CD62Llo cells consistent with phenotype of activated T cells (data not shown) [3]. SP, LN and BM cells were harvested from adoptive hosts, phenotyped and enumerated after gating on CD45.1+ T cells at 1, 2, 3 and 8–12-weeks after transfer. Activated CD45.1+ CD4 and CD8 T cells co-transferred with B cells were found to persist along with CD45.1+ B cells in adoptive hosts better than activated T cells that were transferred alone at 10-weeks after transfer (Fig. 2A). Gating on CD8 T cells within the harvested cells, a CD45.1+ population that is CD44hi, CD25lo, CD69lo consistent with memory phenotype and containing CD62Lhi central and CD62Llo effector memory T cells was observed (Fig. 2B). CD45.1+ CD4 T cells harvested from µMT hosts were also of similar phenotype and comparable to those harvested from wt adoptive hosts (data not shown). Thus, activated T cells transferred into naïve adoptive hosts had differentiated into memory T cells. Activated T cells that were transferred alone declined more than those co-transferred with B cells over time in adoptive hosts (Fig. 2C). The decay of activated T cells in adoptive hosts was most pronounced at 2-weeks with the exception of activated CD4 T cells in µMT hosts that appeared to have declined already at 1-week after transfer (Fig. 2C). Although wt hosts had endogenous B cells, co-transfer of B cells from alloimmunized mice was required to attenuate the contraction of activated T cells after transfer suggesting a possible role for cognate interaction between B and T cells (Fig. 2C). Upon quantitation of CD45.1+ memory T cells at 8–12-weeks after transfer into µMT recipients, 9-fold more CD4 and 13-fold more CD8, CD45.1+ memory T cells were obtained from activated T cells co-transferred with B cells than from those transferred alone (Fig. 2D). In wt recipients, activated T cells co-transferred with B cells yielded 2-fold more CD4 and CD8 CD45.1+ memory T cells than those transferred alone (Fig. 2D). Activated T cells co-transferred with B cells from naïve mice also led to formation of 7-fold more CD4 and 17-fold more CD8, CD45.1+ memory T cells in µMT recipients (Fig. 2E). These results were comparable to memory T cell numbers obtained from activated T cells transferred alone into wt recipients containing endogenous B cells (Fig. 2D) suggesting a possible non-cognate interaction between B and T cells. Thus, in the absence of B cells, activated T cells decayed over time yielding fewer memory T cells. These findings suggest that B cell help for activated T cells promotes their differentiation into memory T cells.
Figure 2. B cells promote differentiation of activated T cells to memory T cells in adoptive hosts.
CD45.1 mice were immunized with BALB/c splenocytes (3 × 107) and 8-days later, SP and LN cells were harvested and sorted for CD4+ and CD8+ CD44hi T cells and B220+ B cells. Sorted CD4+ or CD8+ CD44hi activated T cells (1 × 106) were transferred (i.v.) with or without B220+ B cells (1.5 × 107) into µMT and wt mice. SP, LN and BM cells from µMT and wt hosts were harvested at 1, 2, 3 and 8–12 weeks (C and D, respectively) after adoptive transfer and were enumerated after gating on CD4+ or CD8+ CD45.1+ T cells. A. CD45.1+ T and B cells in µMT adoptive hosts. Representative FACS plots from spleen cells harvested at 10-weeks after transfer from µMT recipients of activated T cells transferred with or without B cells are shown. y-axis legend is depicted on top of the FACS plots. B. Memory phenotype of CD45.1+ T cells harvested from adoptive hosts at 10-weeks after transfer. Representative FACS plots are shown from spleen cells of µMT adoptive recipient of CD8+ CD44hi activated T cells co-transferred with B220+ B cells. y-axis legend is depicted on top of the FACS plots. C. Activated T cells transferred without B cells decline over time in adoptive hosts (Mean ± SD, n = 2 – 3 mice/grp). D–E. Activated T cells co-transferred with B cells develop into more memory T cells in adoptive hosts. B220+ B cells were sorted from either CD45.1 mice immunized with BALB/c splenocytes at 8-days (D) or from unimmunized naïve CD45.1 mice (E) (Mean ± SD; *, p < 0.05, **, p < 0.005; n = 4 – 6 mice/grp).
B cells enhance proliferation and survival of activated T cells to generate more memory T cells
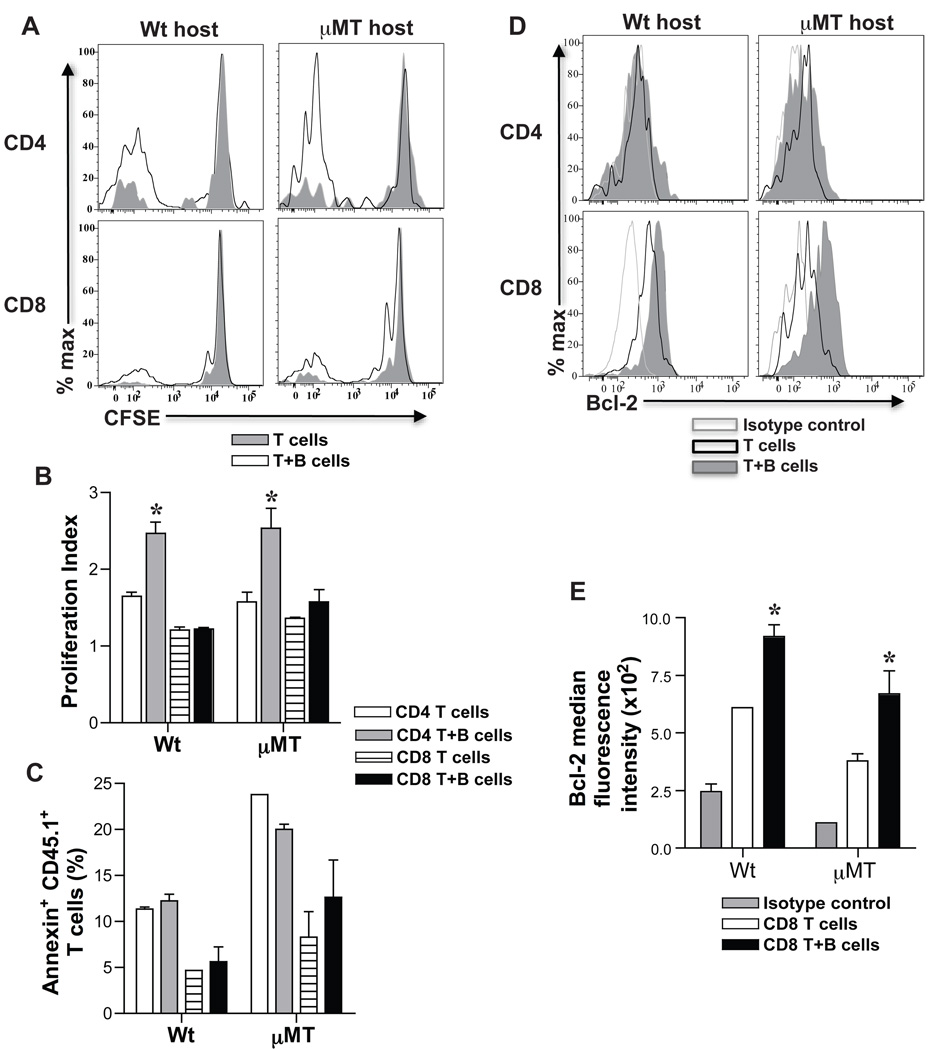
To test how B cells led to development of more memory T cells from activated T cells, proliferation, apoptosis and survival of activated T cells following transfer into adoptive hosts was assessed. CFSE labeled CD45.1+ activated CD4 and CD8 T cells were transferred with or without CD45.1+ B cells into naïve wt and µMT mice. Two weeks later, SP and LN cells from adoptive hosts were harvested, cell proliferation was evaluated by CFSE dilution and cell death was assessed by Annexin V expression on CD4+ and CD8+ CD45.1+ T cells. Activated CD4 and CD8 T cells co-transferred with B cells diluted CFSE more than those transferred alone (Fig. 3A). Proliferation index of activated CD4 T cells co-transferred with B cells was 1.5-fold higher than activated CD4 T cells transferred alone whereas activated CD8 T cells transferred with or without B cells showed comparable proliferation index (Fig. 3B). There was no difference in Annexin V+ cells detected within the harvested CD45.1+ T cells from adoptive recipients of activated T cells with or without B cells (Fig. 3C). Bcl-2 was significantly upregulated in harvested CD8+ CD45.1+ T cells that were co-transferred with B cells compared to those transferred alone and this was not observed in harvested CD4+ CD45.1+ T cells (Fig. 3D–E). Despite differences in Bcl-2 expression, apoptosis of transferred CD8 T cells with or without B cells was comparable (Fig. 3C) possibly due to a difference that was too small or gradual to be measured, or due to rapid clearance of apoptotic cells in-vivo. Taken together, these results suggest that B cells enhance proliferation of activated CD4 T cells and survival of activated CD8 T cells by upregulation of Bcl-2, thus, significantly increasing the number of memory T cells formed.
Figure 3. Proliferation and survival of activated T cells in adoptive hosts.
Activated CD4+ or CD8+ CD44hi T cells and B220+ B cells were sorted from SP and LN cells of immunized CD45.1 mice (BALB/c splenocytes, 3 × 107) at 8-days. Sorted CD45.1 CD4+ and CD8+ CD44hi T cells (1 × 106) were CFSE labeled and transferred with or without B220+ B cells (1.5 × 107) into µMT and wt adoptive hosts. At 2-weeks after transfer, CFSE dilution was analyzed on harvested SP and LN cells after gating on CD4+ or CD8+ CD45.1+ T cells. Representative histogram overlays of CFSE dilution in CD4+ or CD8+ CD45.1+ T cells from harvested spleens of µMT and wt adoptive hosts are shown (A). B. B cells help proliferation of activated CD4 T cells in adoptive hosts. Proliferation index is calculated using Flowjo software after gating on CD4+ or CD8+ CD45.1+ T cells (Mean ± SD; *, p < 0.05; n = 3 – 4 mice/grp). C. Apoptosis of activated T cells in adoptive hosts. Harvested SP and LN cells from µMT and wt adoptive hosts were examined for Annexin V+ staining after gating on CD4+ or CD8+ CD45.1+ T cells (Mean ± SD; n = 3 – 4 mice/grp). D. Bcl-2 expression in CD4+ and CD8+ CD45.1+ T cells in adoptive hosts. Representative histogram overlays of Bcl-2 expression are shown after intracellular staining and gating on harvested CD4+ or CD8+ CD45.1+ T cells from adoptive hosts. E. Bcl-2 expression is upregulated in developing CD8 memory T cells in adoptive hosts harboring co-transferred B cells. Median fluorescence intensity of Bcl-2 expression on CD8+ CD45.1+ T cells harvested from adoptive hosts is shown in comparison to isotype control (Mean ± SD; *, p < 0.05, n = 3 – 4 mice/grp).
Memory T cells that develop with B cell help mediate accelerated allograft rejection
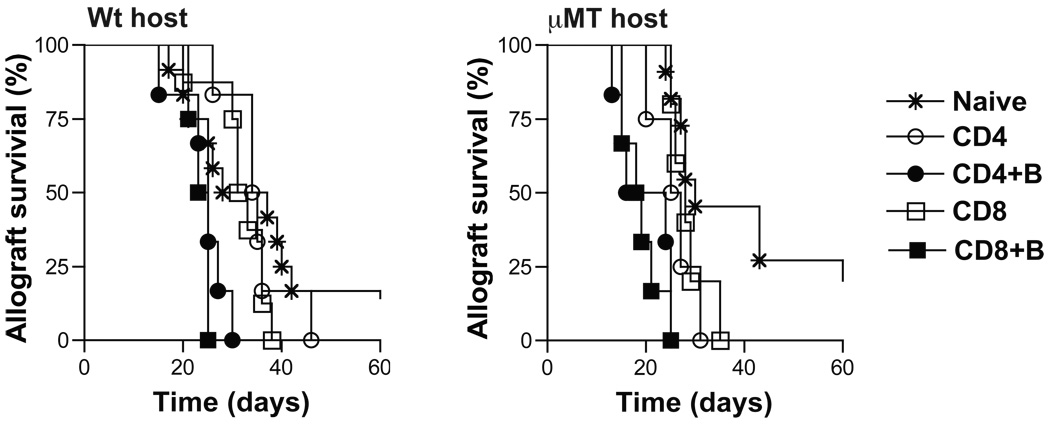
We tested the function of memory T cells that developed from transferred activated T cells in adoptive hosts by re-challenging with BALB/c skin transplants. Activated CD4+ or CD8+ CD45.1+ T cells were transferred into µMT and wt adoptive hosts with or without B cells. 12-weeks later, to assess function of memory T cells that developed, mice were treated with DST and anti-CD40L to inhibit endogenous naïve T cell activation and transplanted with BALB/c skin grafts. BALB/c skin allograft rejection in µMT and wt adoptive hosts of activated CD4 or CD8 T cells co-transferred with B cells was significantly accelerated compared to recipients of activated CD4 or CD8 T cells alone (µMT MST = 20 and 19 days vs. 26 and 28 days, and wt MST = 35 and 32 days vs. 25 and 24 days, respectively, n = 4–6/grp; p = 0.003) (Fig. 4). BALB/c skin allograft survival in adoptive hosts of activated CD4 or CD8 T cells was comparable to that in naïve recipients (Fig. 4). These findings suggest that activated T cells co-transferred with B cells develop into functional memory T cells in adoptive hosts. It’s possible that the number of memory T cells in adoptive hosts of activated CD4 or CD8 T cells alone were too few to effect accelerated rejection and may not reflect impaired function of memory T cells on a per cell basis. Since significantly more memory T cells develop from activated T cells with B cell help (Fig. 2D–E), taken together, these data suggest that B cells promote the quantitative advantage of memory T cells essential for recall function.
Figure 4. Allograft rejection in adoptive hosts harboring memory T cells.
Memory T cells that developed from activated CD4+ or CD8+ CD45.1+ T cells co-transferred with or without B220+ B cells into adoptive hosts were re-challenged with BALB/c skin grafts at 12-weeks after adoptive transfer (memory phase). Skin allograft recipients were treated with DST (2 × 107) and anti-CD40L (MR1, 1mg on days 0, 7 and 14 after transplantation). Allograft rejection in µMT and wt adoptive hosts harboring memory T cells was compared to naïve mice. MST in wt naïve and adoptive hosts of activated CD4 vs. CD4 + B, CD8 vs. CD8 + B cells were 33, 35 vs. 25 and 32 vs. 24 days, respectively (**, p < 0.005; n = 4 – 9 mice/grp). MST in µMT naïve and adoptive hosts of activated CD4 vs. CD4 + B, CD8 vs. CD8 + B cells were 30, 26 vs. 20 and 28 vs. 19 days, respectively (**, p < 0.005; n = 4 – 9 mice/grp).
Discussion
We investigated in this study whether B cells were required for the development of effector and memory alloreactive T cell responses. We found that skin allograft rejection and effector T cell responses were comparable between B-cell deficient and B-cell replete hosts. However, development of alloreactive memory T cells was defective in the absence of B cells resulting in fewer memory T cells and impaired recall function. These findings suggested that although B cells were not required for activation of alloreactive T cells, their subsequent development into memory T cells was dependent upon B cells. Activated T cells were transferred with or without B cells into adoptive hosts to test if B cells were required for their differentiation into memory T cells. Activated T cells co-transferred with B cells gave rise to more memory T cells than activated T cells transferred alone and upon recall, caused accelerated rejection of skin allografts. These results show that B cells help alloreactive T cells differentiate into memory T cells during the alloimmune response yielding increased numbers of memory T cells and contribute to the quantitative advantage of memory responses. It remains to be determined if B cell help also impacts the ‘quality’ of memory T cells generated in the alloimmune response.
Decreased alloreactive T cell memory in µMT mice despite intact differentiation into effectors (Fig. 1B–D) and accelerated decline of activated T cells in µMT adoptive hosts (Fig. 2C) is consistent with increased contraction of CD4 and CD8 T cells reported in LCMV and Listeria monocytogenes infections, respectively in the absence of B cells [32, 42]. Enhanced IL-12 production by DCs in µMT mice due to a relative IL-10 deficient milieu could be contributing to the increased contraction of activated T cells observed in these mice [57, 58]. Impaired expansion of CD4 T cells in adoptive hosts contributed to decreased CD4 memory in the absence of B cells (Fig. 3B). Attenuated IL-7Rα expression (Fig. 1C) and diminished Bcl-2 (Fig. 3E) in activated CD8 T cells differentiating without B cell help suggests that reduced CD8 T cell survival contributed to decreased CD8 memory [59]. It remains to be determined whether impaired CD8 T cell memory in the absence of B cells is due to lack of B cell help for CD8 T cells or due to suboptimal CD4 T cell differentiation resulting in deficient CD4 T cell help for CD8 T cells.
Activated CD4 and CD8 T cells transferred without B cells declined rapidly in adoptive hosts by 2-weeks suggesting an exaggerated contraction phase in the absence of B cells (Fig. 2C) [32, 42]. It’s possible that B cells also play a role in the maintenance of memory T cells since memory CD4 and CD8 T cells derived from activated T cells transferred alone were many fold fewer in µMT than in wt adoptive hosts (Fig. 2D) suggesting a continued decline over time in the absence of endogenous B cells. This decline in µMT adoptive hosts is attenuated upon co-transfer of naïve B cells resulting in memory T cell numbers comparable to wt mice (Fig. 2D–E) suggesting a possible non-cognate role for B cells in promoting survival of memory T cells. Maintenance of CD8 memory T cells is dependent upon IL-15 while IL-7 plays a role in maintenance of both CD4 and CD8 memory T cells [60–63]. It remains to be tested whether endogenous B cells in wt hosts are promoting maintenance of memory T cells directly or indirectly by affecting lymphoid architecture and survival of stromal cells that secrete homeostatic cytokines, IL-7 and IL-15 [64]. Despite a modest difference in memory T cell numbers, wt recipients of activated T cells co-transferred with B cells mediate accelerated graft rejection compared to wt recipients of activated T cells alone (Fig. 4) suggesting that cognate B cell help for T cells promoted their differentiation into functional memory T cells. Further elucidation of cognate vs. non-cognate roles of B cells in the development of alloreactive T cell memory is required and needs models in which antigen-specific B and T cells can be identified and tracked.
Early co-stimulatory interactions via CD28/B7 and CD40/CD40L and IL-2 regulate clonal expansion of CD4 and CD8 T cells following antigen exposure [65]. The role of B cells in this phase could be an indirect one in maintaining lymphoid architecture, shuttling antigen and formation of immune complexes that amplifies the interactions between DCs and T cells [64, 66–68]. CD27/CD70, 4-1BB/4-1BBL, OX40/OX40L and ICOS/ICOSL co-stimulatory interactions that occur in the later phase of activation determine the number and quality of CD4 and CD8 memory T cells that are generated [69–72]. Activated B cells can entrap antigen and function as potent APCs driving CD4 T cell expansion and differentiation [31, 34, 43, 73]. B cells upregulate CD40, ICOSL, CD70, OX40L co-stimulatory ligands and secrete cytokines upon activation that can modulate T cell differentiation [44–46]. B cells can thus provide help for T cells via multiple mechanisms promoting T cell proliferation, differentiation and survival to generate memory T cells (Fig. 5). Therefore, B cell depletion/inhibition early in the alloimmune response could possibly prevent not only alloantibody formation but also generation of long-lived memory T cells improving graft survival.
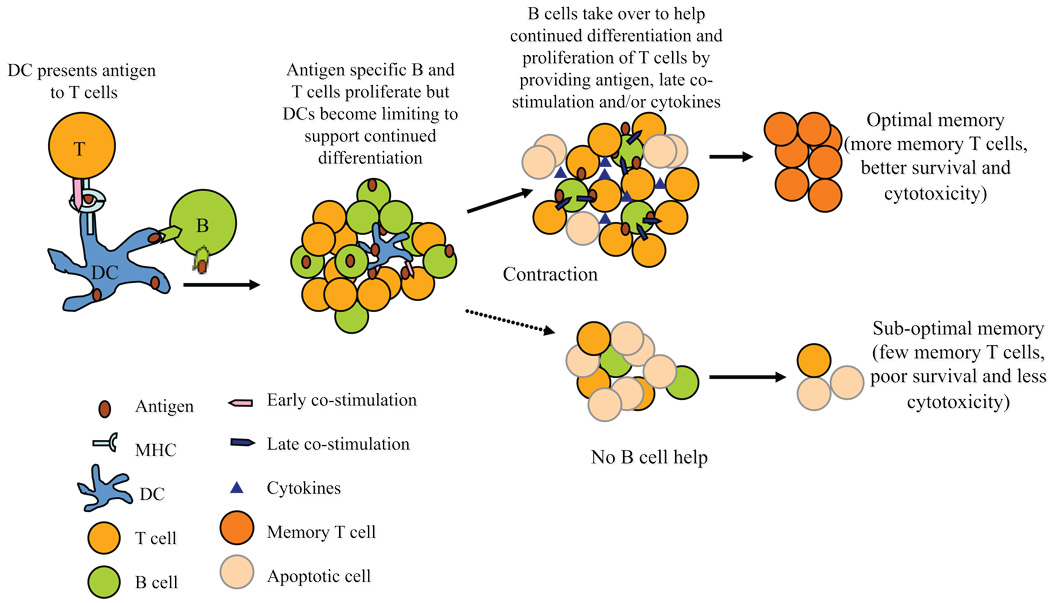
Figure 5. Model of B cell help for T cell differentiation to memory.
Early in the immune response professional APCs such as dendritic cells (DCs) present antigen to T cells and provide early co-stimulatory signals. As antigen specific B and T cells proliferate, the non-dividing DCs become limiting to support further differentiation of T cells. B cells then take over as ‘helper’ cells to promote continued differentiation and survival of T cells. B cells present entrapped antigen, provide cytokines and/or co-stimulation to T cells to support their differentiation into long-lived memory T cells.
Acknowledgements
We thank Ms. Autumn Marlowe for technical assistance; Dr. Hongmei Shen for FACS sorting; and Dr. Fadi Lakkis for critical reading of the manuscript.
Abbreviations used in this paper
- DC
dendritic cell
- APC
antigen presenting cell
- LN
lymph node
- BM
bone marrow
- SP
spleen
- DST
donor specific transfusion
Footnotes
This work is supported by NIH grants AI059137, AI073578 and AI079177 (GC) and John Merrill Transplant Research Scholar Award (GC) from the American Society of Nephrology and the American Society of Transplantation. Y-HN is a recipient of American Heart Association Physician Scientist Fellowship Award. MHO is a recipient of the American Society of Transplantation International Fellowship Award and the Thomas E. Starzl Fellowship in Transplantation Biology.
References
- 1.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg AS. Skin Allograft Rejection. Hoboken, NJ: John Wiley & Sons, Inc.; 1991. [Google Scholar]
- 3.Obhrai J, Oberbarnscheidt M, Hand T, Diggs L, Chalasani G, Lakkis F. Effector T cell differentiation and memory T cell maintenance outside secondary lymphoid organs. J Immunol. 2006;176:4051–4058. doi: 10.4049/jimmunol.176.7.4051. [DOI] [PubMed] [Google Scholar]
- 4.Barber D, Wherry E, Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J Immunol. 2003;171:27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
- 5.Papadopoulos N, Dedoussis G, Spanakos G, Gritzapis A, Baxevanis C, Papamichail M. An improved fluorescence assay for he determination of lymphocyte-mediated cytotoxicity using flow cytometry. J Immunol Methods. 1994;177:101–111. doi: 10.1016/0022-1759(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 6.Mattis A, Bernhardt G, Lipp M, Forster R. Analyzing cytotoxic lymphocyte activity: a simple and reliable flow cytometry-based assay. J Immunol Methods. 1997;204:135–142. doi: 10.1016/s0022-1759(97)00047-1. [DOI] [PubMed] [Google Scholar]
- 7.Oberbarnscheidt MH, Ng Y, Chalasani G. The roles of CD8 central and effector memory T-cell subsets in allograft rejection. Am J Transplant. 2008;8:1809–1818. doi: 10.1111/j.1600-6143.2008.02335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chervenick PA, Boggs DR, Marsh JC, Cartwright GE, Wintrobe MM. Quantitative studies of blood and bone marrow neutrophils in normal mice. Am J Physiol. 1968;215:353–360. doi: 10.1152/ajplegacy.1968.215.2.353. [DOI] [PubMed] [Google Scholar]
- 9.Sarwal M, Chua M, Kambham N, Hsieh S, Satterwhite T, Masek M, et al. Molecular Heterogeneity in Acute Renal Allograft Rejection Identified by DNA Microarray Profiling. N Engl J med. 2003;349:125–138. doi: 10.1056/NEJMoa035588. [DOI] [PubMed] [Google Scholar]
- 10.Ibrahim S, Dawson D, Sanfilippo F. Predominant infiltration of rejecting human renal allografts with T cells expressing CD8 and CD45RO. Transplantation. 1995;59:724–728. doi: 10.1097/00007890-199503150-00015. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim S, Dawson D, Van Tright P, Sanfilippo F. Differential infiltration by CD45RO and CD45RA subsets of T cells associated with human heart allograft rejection. Am J Pathol. 1993;142:1794–1803. [PMC free article] [PubMed] [Google Scholar]
- 12.Heeger PS, Greenspan NS, Kuhlenschmidt S, Dejelo C, Hricik DE, Schulak JA, et al. Pretransplant frequency of donor-specific, IFN-γ-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999;163:2267–2275. [PubMed] [Google Scholar]
- 13.Najafian N, Salama A, Fedoseyeva E, Benichou G, Sayegh M. Enzyme-linked immunosorbent spot assay analysis of peripheral blood lymphocyte reactivity to donor HLA-DR peptides: potential novel assay for prediction of outcomes for renal transplant recipients. J Am Soc Nephrol. 2002;13:252–259. doi: 10.1681/ASN.V131252. [DOI] [PubMed] [Google Scholar]
- 14.Steele D, Laufer T, Smiley S, Ando Y, Grusby M, Glimcher L, et al. Two levels of help for B cell alloantibody production. J Exp Med. 1996;183:699–703. doi: 10.1084/jem.183.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernard A, Coitot S, Bremont A, Bernard G. T and B cell cooperation: A dance of life and death. Transplantation. 2005;79:S8–S11. doi: 10.1097/01.tp.0000153290.75695.31. [DOI] [PubMed] [Google Scholar]
- 16.Itescu S, Tung T, Burke E, Weinberg A, Moazami N, JH A, et al. Preformed IgG antibodies against major histocompatibility complex class II antigens are major risk factors for high-grade cellular rejection in recipients of heart transplantation. Circulation. 1998;98:786–793. doi: 10.1161/01.cir.98.8.786. [DOI] [PubMed] [Google Scholar]
- 17.Pelletier R, Hennessy P, Adams P, VanBuskirk A, RM F, Orosz C. Clinical significance of MHC-reactive alloantibodies that develop after kidney or kidney-pancreas transplantation. Am J Transplant. 2002;2:134–141. doi: 10.1034/j.1600-6143.2002.020204.x. [DOI] [PubMed] [Google Scholar]
- 18.Girnita AL, McCurry KR, Iacono AT, Duquesnoy R, Corcoran TE, Awad M, et al. HLA-specific antibodies are associated with high-grade and persistent-recurrent lung allograft acute rejection. J Heart Lung Transplant. 2004 Oct;23(10):1135–1141. doi: 10.1016/j.healun.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 19.Di Filippo S, Girnita A, Webber S, Tsao S, Boyle G, Miller S, et al. Impact of ELISA-detected anti-HLA antibodies on pediatric cardiac allograft outcome. Hum Immunol. 2005;66:513–518. doi: 10.1016/j.humimm.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Girnita A, Duquesnoy R, Yousem S, Iacono A, Corcoran T, Buzoianu M, et al. HLA-specific antibodies are risk factors for lymphocytic bronchiolitis and chronic lung allograft dysfunction. Am J Transplant. 2005;5:131–138. doi: 10.1111/j.1600-6143.2004.00650.x. [DOI] [PubMed] [Google Scholar]
- 21.Piazza A, Poggi E, Ozzella G, Borrelli L, Scornejenghi A, Laria G. Post-transplant donor-specific antibody production and graft outcome in kidney transplantation: Results of sixteen year monitoring by flow cytometry. In: Terasaki PI, et al., editors. Clinical Transplants. Los Angeles: Terasaki Foundation Laboratory; 2006. pp. 323–336. [PubMed] [Google Scholar]
- 22.Lachman N, Terasaki PI, Schonemann C. Donor-specific HLA antibodies in chronic renal allograft rejection: a prospective trial with a four-year follow-up. In: Terasaki PI, editor. Clinical Transplants. Los Angeles: Terasaki Foundation Laboratory; 2006. pp. 171–200. [PubMed] [Google Scholar]
- 23.Steinmetz O, Panzer U, Kneissler U, Harendza S, Lipp M, Helmchen U, et al. BCA-1/CXCL13 expression is associated with CXCR5-positive B-cell cluster formation in acute renal transplant rejection. Kidney Int. 2005;67:1616–1621. doi: 10.1111/j.1523-1755.2005.00244.x. [DOI] [PubMed] [Google Scholar]
- 24.Reinke P, Fietze E, Docke W, Kern F, Ewert R, Volk H. Late acute rejection in long-term renal allograft recipients. Diagnostic and predictive value of circulating activated T cells. Transplantation. 1994;58:35–41. [PubMed] [Google Scholar]
- 25.Zarkhin V, Kambham N, Li L, Kwok S, Hsieh S, Salvatierra O, et al. Characterization of intra-graft B cells during renal allograft rejection. Kidney Int. 2008;74:664–673. doi: 10.1038/ki.2008.249. [DOI] [PubMed] [Google Scholar]
- 26.Chalasani G, Dai Z, Konieczny BT, Baddoura FK, Lakkis FG. Recall and propagation of allospecific memory T cells independent of secondary lymphoid organs. Proc Natl Acad Sci U S A. 2002;99:6175–6180. doi: 10.1073/pnas.092596999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valujskikh A, Pantenburg B, Heeger P. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2:501–509. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 28.Valujskikh A, Lakkis FG. In remembrance of things past: memory T cells and transplant rejection. Immunol Rev. 2003;196:65–74. doi: 10.1046/j.1600-065x.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 29.Macedo C, Orkis E, Popescu I, Elinoff B, Zeevi A, Shapiro R, et al. Contribution of naïve and memory T-cell populations to the human alloimmune response. Am J Transplant. 2009;9:2057–2066. doi: 10.1111/j.1600-6143.2009.02742.x. [DOI] [PubMed] [Google Scholar]
- 30.Yang X, Brunham R. Gene knockout B cell-deficient mice demonstrate that B cells play an important role in the initiation of T cell responses to Chlamydia trachomatis (mouse pneumonitis) lung infection. J Immunol. 1998;161:1439–1446. [PubMed] [Google Scholar]
- 31.Lund F, Hollifield M, Schuer K, Lines J, Randall T, Garvy B. B cells are required for generation of protective effector and memory CD4 cells in response to Pneumocystis lung infection. J Immunol. 2006;176:6147–6154. doi: 10.4049/jimmunol.176.10.6147. [DOI] [PubMed] [Google Scholar]
- 32.Whitmire J, Asano M, Kaech S, Sarkar S, Hannum L, Shlomchik M, et al. Requirement of B cells for generating CD4+ T cell memory. J Immunol. 2009;182(4):1868–1876. doi: 10.4049/jimmunol.0802501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Constant S, Schweitzer N, West J, Ranney P, Bottomly K. B lymphocyte can be competent antigen-presenting cells for priming CD4+ T cells to protein antigen in vivo. J Immunol. 1995;155:3734–3741. [PubMed] [Google Scholar]
- 34.Linton P, Harbertson J, Bradley L. A critical role for B Cells in the development of memory CD4 cells. J Immunol. 2000;165:5558–5565. doi: 10.4049/jimmunol.165.10.5558. [DOI] [PubMed] [Google Scholar]
- 35.Christensen J, Kauffman S, Thomsen A. Deficient CD4+ T cell priming and regression of CD8+ T cell functionality in virus-infected mice lacking a normal B cell compartment. J Immunol. 2003;171:4733–4741. doi: 10.4049/jimmunol.171.9.4733. [DOI] [PubMed] [Google Scholar]
- 36.Takemura S, Klimiuk P, Braun A, Goronzy J, Weyand C. T cell activation in rheumatoid synovium is B cell dependent. J Immunol. 2001;167:4710–4718. doi: 10.4049/jimmunol.167.8.4710. [DOI] [PubMed] [Google Scholar]
- 37.Chan O, Madaio M, Shlomchik M. B cells are required for lupus nephritis in the polygenic, Fas-intact MRL model of systemic autoimmunity. J Immunol. 1999;163:3592–3596. [PubMed] [Google Scholar]
- 38.Topham D, Tripp R, Hamilton-Easton A, Sarawar S, Doherty P. Quantitative analysis of the influenza virus-specific CD4+ T cell memory in the absence of B cells and Ig. J Immunol. 1996;157:2947–2952. [PubMed] [Google Scholar]
- 39.Johansson M, Lycke N. Immunological memory in B-cell-deficient mice conveys long-lasting protection against genital tract infection with Chlamydia trachomatis by rapid recruitment of T cells. Immunology. 2001;102:199–208. doi: 10.1046/j.1365-2567.2001.01167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Rosa F, Matzinger P. Long-lasting CD8 T cell memory in the absence of CD4 T cells or B cells. J Exp Med. 1996;183:2153–2163. doi: 10.1084/jem.183.5.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asano M, Ahmed R. CD8 T cell memory in B cell-deficient mice. J Exp Med. 1996;183:2165–2174. doi: 10.1084/jem.183.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen H, Whitmire J, Fan X, Shedlock D, Kaech S, Ahmed R. A specific role for B cells in the geneartion of CD8 T cell memory by recombinant Listeria monocytogenes. J Immunol. 2003;170:1443–1451. doi: 10.4049/jimmunol.170.3.1443. [DOI] [PubMed] [Google Scholar]
- 43.Crawford A, MacLeod M, Schumacher T, Corlett L, Gray D. Primary T cell expansion and differentiation in vivo requires antigen presentation by B cells. J Immunol. 2006;176:3498–3506. doi: 10.4049/jimmunol.176.6.3498. [DOI] [PubMed] [Google Scholar]
- 44.Akiba H, Oshima H, Takeda K, Atsuta M, Nakano H, Nakajima A, et al. CD28-independent co-stimulation of T cells by OX40 ligand and CD70 on activated B cells. J Immunol. 1999;162:7058–7065. [PubMed] [Google Scholar]
- 45.Linton P, Bautista B, Biederman E, Bradley E, harberton J, Kondrack R, et al. Costimulation via OX40L expressed by B cells is sufficient to determine the extent of primary CD4 cell expansion and Th2 cytokine secretion in vivo. J Exp Med. 2003;197:875–883. doi: 10.1084/jem.20021290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harris D, Haynes L, Sayles P, Duso D, Eaton S, Lepak N, et al. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol. 2000;1:475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 47.Noorchasm H, Reed G, Rostami S, Mozaffari R, Zekavat G, Koeberlein B, et al. B Cell-Mediated Antigen Presentation Is Required for the Pathogenesis of Acute Cardiac Allograft Rejection. J Immunol. 2006;177:7715–7722. doi: 10.4049/jimmunol.177.11.7715. [DOI] [PubMed] [Google Scholar]
- 48.Epstein M, Di Rosa F, Jankovic D, Sher A, Matzinger P. Successful T cell priming in B cell-deficient mice. J Exp Med. 1995;182:915–922. doi: 10.1084/jem.182.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nozaki T, Rosenblum J, Ishii D, Tanabe K, Fairchild R. CD4 T cell-mediated rejection of cardiac allografts in B cell-deficient mice. J Immunol. 2008;15:5257–5263. doi: 10.4049/jimmunol.181.8.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harrington L, Janowski K, Oliver J, Zajac A, Weaver C. Memory CD4 T cells emerge from effector T-cell progenitors. Nature. 2008;452:356–360. doi: 10.1038/nature06672. [DOI] [PubMed] [Google Scholar]
- 51.Homann D, Tishon A, Berger D, Weigle W, Von Herrath M, Oldstone M. Evidence for an underlying CD4 helper and CD8 T-cell defect in B-cell-deficient mice: Failure to clear persistent virus infection after adoptive immunotherapy with virus-specific memory cells from µMT/µMT mice. J Virol. 1998;72:9208–9216. doi: 10.1128/jvi.72.11.9208-9216.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Larsen C, Elwood E, Alexander D, Ritchie S, Hendrix R, Tucker-Burden C, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 53.Zhai Y, Meng L, Gao F, Busuttil R, Kupiec-Weglinski J. Allograft rejection by primed/memory CD8+ T cells is CD154 blockade resistant: therapeutic implications for sensitized transplant recipients. J Immunol. 2002;169:4667–4673. doi: 10.4049/jimmunol.169.8.4667. [DOI] [PubMed] [Google Scholar]
- 54.Steinmuller D. Passive transfer of immunity to skin homografts in rats. Ann N Y Acad Sci. 1962;99:629–644. doi: 10.1111/j.1749-6632.1962.tb45346.x. [DOI] [PubMed] [Google Scholar]
- 55.Tyan M, Cole L. Differential effects of the specific antisera on the rejection of allogeneic and xenogeneic skin grafts sublethally X-irradiated mice. J Immunol. 1963;91:621–624. [PubMed] [Google Scholar]
- 56.Yoshimura R, Chargui J, Aitouche A, Veyron P, Touraine J. Induction of hyperacute rejection of skin allografts by CD8+ lymphocytes. Transplantation. 2000;69:1452–1457. doi: 10.1097/00007890-200004150-00041. [DOI] [PubMed] [Google Scholar]
- 57.Moulin V, Andris F, Thielemans K, Maliszewski C, Urbain J, Moser M. B lymphocytes regulate dendritic cell (DC) function in vivo: increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation. J Exp Med. 2000;192:475–482. doi: 10.1084/jem.192.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pearce E, Shen H. Generation of CD8 T cell memory is regulated by IL-12. J Immunol. 2007;179:2074–2081. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- 59.Klonowski K, Williams K, Marzo A, Lefrancois L. Cutting edge: IL-7-independent regulation of IL-7 receptor alpha expression and memory CD8 T cell development. J Immunol. 2006;177:4247–4251. doi: 10.4049/jimmunol.177.7.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goldrath AW, Sivakumar PV, Glaccum M, Kennedy MK, Bevan MJ, Benoist C, et al. Cytokine requirements of acute and basal homeostatic proliferation of naive and memory CD8+ T cells. J Exp Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tan JT, Ernst WC, Kieper WC, LeRoy E, Sprent J, Surh CD. IL-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory-phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198:1797–1806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol. 2003;4:680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 64.Tumanov A, Kuprash D, Lagarkova M, Grivennikov S, Abe K, Shakhov A, et al. Distinct role of surface lymphotoxin expressed by B cells in the organization of secondary lymphoid tissues. Immunity. 2002;17:239–250. doi: 10.1016/s1074-7613(02)00397-7. [DOI] [PubMed] [Google Scholar]
- 65.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T cell differentiation: Implications for vaccine development. Nature Reviews Immunology. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 66.Cinamon G, Zachariah M, Lam O, Jr FF, Cyster J. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat Immunol. 2008;9:54–62. doi: 10.1038/ni1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferguson A, Youd M, Corley R. Marginal zone B cells transport and deposit IgM-containing immune complexes onto follicular dendritic cells. Intl Immunology. 2004;16:1411–1422. doi: 10.1093/intimm/dxh142. [DOI] [PubMed] [Google Scholar]
- 68.Phan T, Grigorova I, Okada T, Cyster J. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat Immunol. 2007;8:992–1000. doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- 69.Dawicki W, Bertram E, Sharpe A, Watts TH. 4-1BB and OX40 Act independently to facilitate robust CD8 and CD4 recall responses. J Immunol. 2004;173:5944–5951. doi: 10.4049/jimmunol.173.10.5944. [DOI] [PubMed] [Google Scholar]
- 70.Vidric M, Bladt A, Dianzani U, Watts TH. Role of inducible costimulator in control of Salmonella enterica Serovar typhimurium infection in mice. Infect Immun. 2006;74:1050–1061. doi: 10.1128/IAI.74.2.1050-1061.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hendriks J, Gravestein LA, Tesselaar K, van Lier RAW, Shumacher TNM, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nature Immunol. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 72.Hendriks J, Yanling X, Rossen J, van der Sluijs K, Sugamura K, Ishii N, et al. During viral infection of the respiratory tract, CD27, 4-1BB, and OX40 collectively determine formation of CD8+ memory T cells and their capacity for secondary expansion. J Immunol. 2005;175:1665–1676. doi: 10.4049/jimmunol.175.3.1665. [DOI] [PubMed] [Google Scholar]
- 73.Gondré-Lewis T, Moquin A, Drake J. Prolonged antigen persistence within nonterminal late endocytic compartments of antigen-specific B lymphocytes. J Immunol. 2001;166:6657–6664. doi: 10.4049/jimmunol.166.11.6657. [DOI] [PubMed] [Google Scholar]