Abstract
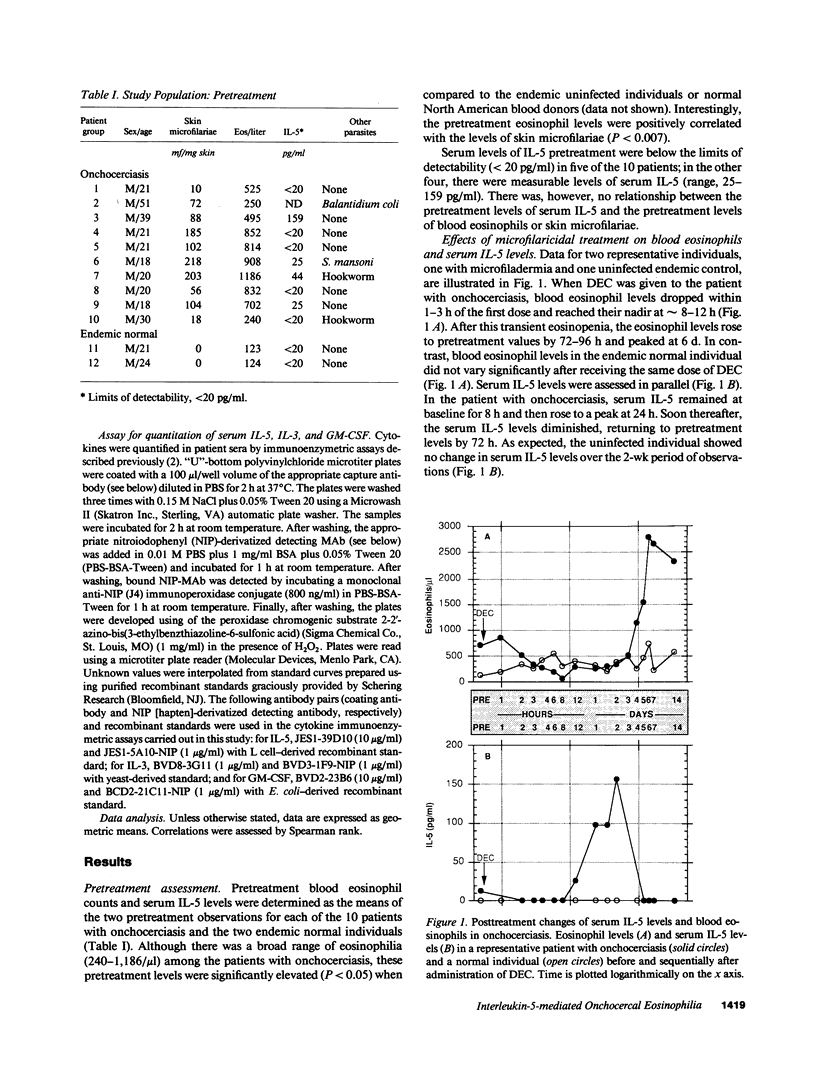
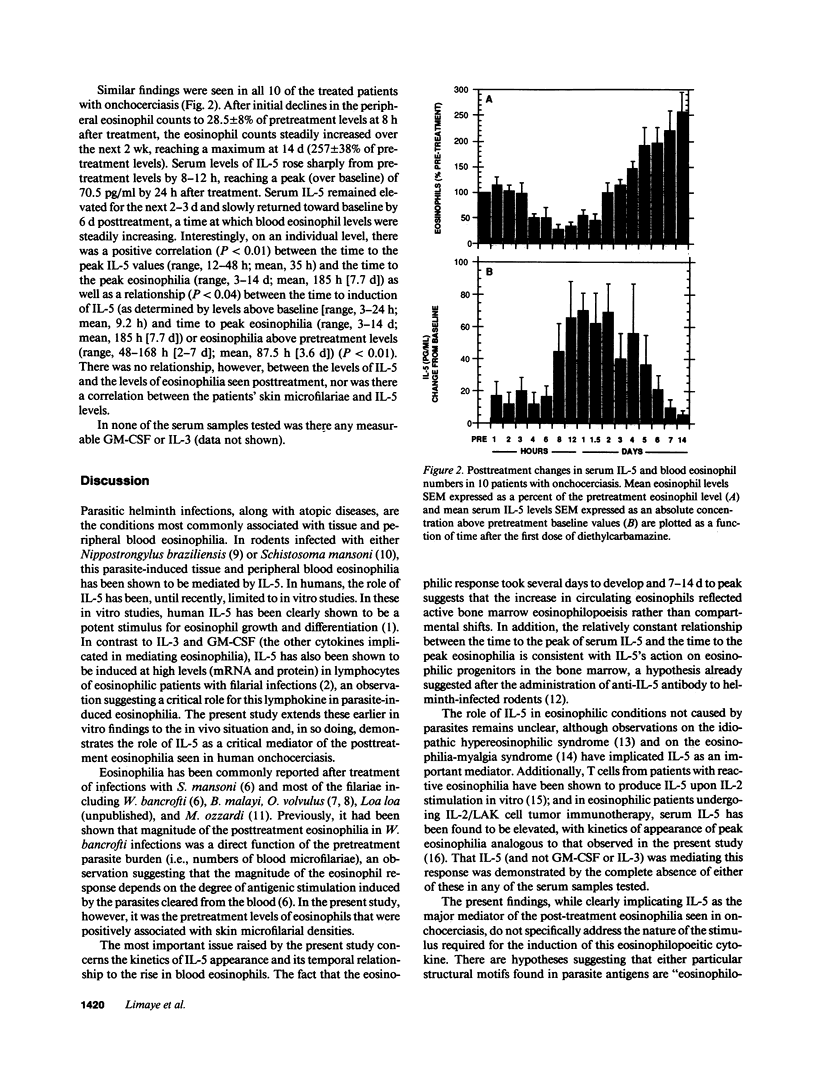
To understand the role of the eosinophilopoietic cytokine IL-5 in humans, the posttreatment eosinophilic response in a group of microfilaria (mf)-positive patients with onchocerciasis (n = 10) was examined before and after treatment with diethylcarbamazine (6 mg/kg for 7 d). Sequential blood samples were assessed at 24 and 1 h before treatment (baseline values), then at frequent intervals over the next 14 d. Symptom scores, skin microfilariae (mf), and peripheral blood eosinophil counts were recorded as a function of time after treatment, and serum levels of IL-5 were quantitated by a highly sensitive (sensitivity greater than or equal to 20 pg/ml) monoclonal-based ELISA. Pretreatment eosinophil counts ranged from 240 to 1,186 eosinophils/microliter (geometric mean, 675), and the mf counts from 10 to 218 per mg skin (geometric mean, 79). After an initial decline in the peripheral eosinophil count to 28 +/- 8% of pretreatment levels at 8 h after beginning treatment, the eosinophil counts steadily increased over the next 2 wk, reaching a maximum at 14 d (257 +/- 38% of pretreatment levels). Serum levels of IL-5 rose sharply from pretreatment levels to a peak of 70.5 +/- 11 pg/ml by 24 h after treatment. Serum IL-5 remained elevated over the next 2-3 d and declined toward baseline by approximately 6 d after treatment, at which time the eosinophil levels were steadily increasing. IL-3 and granulocyte macrophage colony-stimulating factor, two other cytokines implicated in eosinophilopoeisis, were not detectable in the serum at any time before or after treatment. The rise in serum IL-5 before the posttreatment eosinophilia seen in this group of patients with onchocerciasis demonstrates a temporal relationship between IL-5 and the subsequent development of eosinophilia and implicates IL-5 as an important mediator of eosinophilia in humans.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman S. J., Gleich G. J., Weller P. F., Ottesen E. A. Eosinophilia and elevated serum levels of eosinophil major basic protein and Charcot-Leyden crystal protein (lysophospholipase) after treatment of patients with Bancroft's filariasis. J Immunol. 1981 Sep;127(3):1093–1098. [PubMed] [Google Scholar]
- Clutterbuck E. J., Hirst E. M., Sanderson C. J. Human interleukin-5 (IL-5) regulates the production of eosinophils in human bone marrow cultures: comparison and interaction with IL-1, IL-3, IL-6, and GMCSF. Blood. 1989 May 1;73(6):1504–1512. [PubMed] [Google Scholar]
- Coffman R. L., Seymour B. W., Hudak S., Jackson J., Rennick D. Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science. 1989 Jul 21;245(4915):308–310. doi: 10.1126/science.2787531. [DOI] [PubMed] [Google Scholar]
- Denburg J. A., Silver J. E., Abrams J. S. Interleukin-5 is a human basophilopoietin: induction of histamine content and basophilic differentiation of HL-60 cells and of peripheral blood basophil-eosinophil progenitors. Blood. 1991 Apr 1;77(7):1462–1468. [PubMed] [Google Scholar]
- Enokihara H., Furusawa S., Nakakubo H., Kajitani H., Nagashima S., Saito K., Shishido H., Hitoshi Y., Takatsu K., Noma T. T cells from eosinophilic patients produce interleukin-5 with interleukin-2 stimulation. Blood. 1989 May 15;73(7):1809–1813. [PubMed] [Google Scholar]
- Francis H., Awadzi K., Ottesen E. A. The Mazzotti reaction following treatment of onchocerciasis with diethylcarbamazine: clinical severity as a function of infection intensity. Am J Trop Med Hyg. 1985 May;34(3):529–536. doi: 10.4269/ajtmh.1985.34.529. [DOI] [PubMed] [Google Scholar]
- Groopman J. E. Status of colony-stimulating factors in cancer and AIDS. Semin Oncol. 1990 Feb;17(1 Suppl 1):31–41. [PubMed] [Google Scholar]
- Limaye A. P., Abrams J. S., Silver J. E., Ottesen E. A., Nutman T. B. Regulation of parasite-induced eosinophilia: selectively increased interleukin 5 production in helminth-infected patients. J Exp Med. 1990 Jul 1;172(1):399–402. doi: 10.1084/jem.172.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutman T. B., Nash T. E., Ottesen E. A. Ivermectin in the successful treatment of a patient with Mansonella ozzardi infection. J Infect Dis. 1987 Oct;156(4):662–665. doi: 10.1093/infdis/156.4.662. [DOI] [PubMed] [Google Scholar]
- Ottesen E. A., Weller P. F. Eosinophilia following treatment of patients with schistosomiasis mansoni and Bancroft's filariasis. J Infect Dis. 1979 Mar;139(3):343–347. doi: 10.1093/infdis/139.3.343. [DOI] [PubMed] [Google Scholar]
- Owen W. F., Jr, Petersen J., Sheff D. M., Folkerth R. D., Anderson R. J., Corson J. M., Sheffer A. L., Austen K. F. Hypodense eosinophils and interleukin 5 activity in the blood of patients with the eosinophilia-myalgia syndrome. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8647–8651. doi: 10.1073/pnas.87.21.8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen W. F., Rothenberg M. E., Petersen J., Weller P. F., Silberstein D., Sheffer A. L., Stevens R. L., Soberman R. J., Austen K. F. Interleukin 5 and phenotypically altered eosinophils in the blood of patients with the idiopathic hypereosinophilic syndrome. J Exp Med. 1989 Jul 1;170(1):343–348. doi: 10.1084/jem.170.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennick D. M., Thompson-Snipes L., Coffman R. L., Seymour B. W., Jackson J. D., Hudak S. In vivo administration of antibody to interleukin-5 inhibits increased generation of eosinophils and their progenitors in bone marrow of parasitized mice. Blood. 1990 Jul 15;76(2):312–316. [PubMed] [Google Scholar]
- Saito H., Hatake K., Dvorak A. M., Leiferman K. M., Donnenberg A. D., Arai N., Ishizaka K., Ishizaka T. Selective differentiation and proliferation of hematopoietic cells induced by recombinant human interleukins. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2288–2292. doi: 10.1073/pnas.85.7.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher A., Coffman R. L., Hieny S., Scott P., Cheever A. W. Interleukin 5 is required for the blood and tissue eosinophilia but not granuloma formation induced by infection with Schistosoma mansoni. Proc Natl Acad Sci U S A. 1990 Jan;87(1):61–65. doi: 10.1073/pnas.87.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]