Abstract
Biodegradable scaffolds such as poly(lactic acid) (PLA), poly(lactic-co-glycolic acid) (PLGA) or poly(glycolic acid) (PGA) are commonly used materials in tissue engineering. The chemical composition of these scaffolds changes during degradation which provides a changing environment for the seeded cells. In this study we have developed a simple and relatively high-throughput method in order to test the physiological effects of this varying chemical environment on rat embryonic cardiac myocytes. In order to model the different degradation stages of the scaffold, glass coverslips were functionalized with 11-mercaptoundecanoic acid (MUA) and 11-mercapto-1-undecanol (MUL) as carboxyl- and hydroxyl-group presenting surfaces and also with trimethoxysilylpropyldiethylenetriamine (DETA) and (3-aminopropyl)triethoxysilane (APTES) as controls. Embryonic cardiac myocytes formed beating islands on all tested surfaces but the number of attached cells and beating patches was significantly lower on MUL compared to any of the other functionalized surfaces. Moreover, whole cell patch clamp experiments showed that the average length of action potentials generated by the beating cardiac myocytes were significantly longer on MUL compared to the other surfaces. Our results, using our simple test system, are in agreement with earlier observations that utilized the complex 3D biodegradable scaffold. Thus, surface functionalization with self-assembled monolayers combined with histological/physiological testing could be a relatively high throughput method for biocompatibility studies and for the optimization of the material/tissue interface in tissue engineering.
Keywords: Cardiomyocytes, cell culture, electrophysiology, cardiac tissue engineering, serum-free, SAM, Hydroxyl, Carboxyl, scaffolds, PLA, PLGA
INTRODUCTION
One of the most promising methods for the replacement of damaged cardiac muscle involves seeding cardiac myocytes or stem cells onto a bioresorbable scaffold. The tissue is allowed to maturate in vitro and then the construct is implanted at the appropriate location as a prosthesis [1-4].
Poly(lactic acid) (PLA), poly(lactic-co-glycolic acid) (PLGA) and poly(glycolic acid) (PGA) are among the few biodegradable scaffold materials which have been approved for human use [5]. They have been extensively used in cardiac tissue engineering [2, 6, 7]. Degradation of PLA, PLGA and PGA in vitro and in vivo was shown to be mediated by hydrolysis [8-12] producing degradation products with hydroxyl and carboxyl functional groups. Although PLA, PLGA and PGA have been extensively used in tissue engineering and considered biocompatible, bioactivity of their degradation products has been much less studied, due to technical difficulties. In a recent paper Sung and coworkers (2004) reported the different survival of cardiac myocytes in the different degradation stages of PGLA scaffolds [13]. The composition of the scaffold significantly influenced the survival of the cells. In addition, degradation rate and survival of the cardiac myocytes were inversely correlated.
There are several lines of evidence indicating that surface interactions are important in the development of cardiac myocytes. Clark (1998) reported that cell-cell contact was a major determinant in the development of spontaneous activity (beating) in adult feline cardiomyocyte-cultures [14]. The culture surface had a significant role not only in the attachment [15] of the cells, but also in cell signaling. Simpson et al. (1994) showed that the phenotype of cardiac myocytes was determined by the culturing substrate (type I collagen) through a signaling process, which involved the cardiac alpha (1) or beta (1) integrin chain [16].
The goal of our study was to create a simple in vitro test system where the chemical environment (both contact and soluble) of the cardiac myocytes could be controlled and systematically varied. We were especially interested in the physiological reaction of the cells to hydroxyl- and carboxyl-groups in the environment as a model of different degradation stages of PLA or PLGA scaffolds. Our defined system consisted of a functionalized surface to determine contact interactions between the cells and the culture-surface as well as the serum-free culture medium we had developed earlier [17, 18]. In the development of this test system we concentrated on the study of the effect of the chemical environment of the cells, although there are several lines of evidence indicating that topology of the surface / physical properties of the 3D scaffold are also important for the determination of growth and differentiation of cells [19-22].
For the functionalization of the surfaces we used self assembled monolayers (SAMs) because of the flexibility of the method [23-26]. Almost any surface can be equipped with functionalized monolayers, which possess a required specific electrical, optical or chemical property [24, 27-31]. A wide variety of functional groups are available for surface modification [32]. SAMs have already been used for: the study of cell-surface interactions [33-36], cell patterning [37], control of protein adsorption [38-41] and prosthetic device biocompatibility [42-44]. Fields (1999) suggested the use of SAMs to create bioactive surfaces with covalent protein-functionalization and controlled protein folding [45, 46].
For the physiological testing of the surfaces we utilized embryonic (vs. the more commonly used neonatal) rat cardiac myocytes, because in earlier studies we have developed the serum-free medium formulation which was supporting the growth and differentiation of embryonic cardiac myocytes on our defined surfaces [17]. Serum-free culture conditions were essential for the testing of surface effects on the physiology of the cells, because of the fast adsorption of serum proteins to the defined surfaces [47-52].
Our method for testing of the physiological effects of the degradation products of PLA and PLGA scaffolds (modeled by the hydroxyl and carboxyl functionalized surfaces) can be generalized for in vitro biocompatibility testing. The interface between the engineered material and the tissue is the surface of the material. This surface can be mimicked by specific surface functional groups and the biological effects of these surfaces can be tested independently of the biomaterial's physical properties. In other applications an effective approach in the development of a clinically applicable biomaterial is to make it biocompatible through the surface modification of a material which already has excellent biofunctionality and bulk properties [53, 54].
MATERIALS AND METHODS
Surface modification
The following surfaces were used in this study: 1. Trimethoxysilylpropyldiethylenetriamine (DETA) 2. (3-aminopropyl)triethoxysilane (APTES) 3. 11-mercaptoundecanoic acid (MUA, HS(CH2)10COOH) and 4. 11-mercapto-1-undecanol (MUL, HS(CH2)11OH).
DETA surface modification: Briefly, glass coverslips (Thomas Scientific, Swedesboro, NJ, 6661F52, 22×22 mm No. 1) were cleaned using an O2 plasma cleaner (Harrick, Ithaca, NY, PDC-32G) for 30 min at 400 mTorr. The DETA (United Chemical Technologies Inc., Bristol, PA, T2910KG) SAM was formed by the reaction of the cleaned surface with a 0.1% (v/v) mixture of the organosilane in freshly distilled toluene (Fisher, Pittsburgh, PA, T2904). The coverslips with the mixture were heated to 70°C, rinsed with toluene, reheated to 70°C, and then oven dried. Surfaces were characterized by contact angle measurements using an optical contact angle goniometer (KSV Instruments, Monroe, CT, Cam 200) and by X-ray photoelectron spectroscopy (XPS) (Fissons Escalab 200i XL) by monitoring the N 1 s peak [55].
APTES: Briefly, after O2 plasma cleaning, the glass cover slips were immersed in a mixture of 1% APTES (Sigma, St Louis, MO) in freshly distilled and dried toluene for 60 minutes inside a nitrogen purged glove box. They were then rinsed with fresh dried toluene three times and cured in the oven at 110°C for 30 minutes. The APTES modified SAMs were immersed into absolute ethanol (Sigma) until they were used.
The carboxyl and hydroxyl modified SAMs were prepared using the bifunctional cross-linker N-succinimidyl 4-maleimidobutyrate (GMBS, Pierce, Rockford, IL): The APTES SAMs were immersed in a solution of 2mM GMBS in absolute ethanol after dissolving it with a minimum amount of dry dimethy sulfoxide (DMSO, Pierce) for about 60 minutes. They were then rinsed with absolute ethanol three times. The intermediate GMBS (a cross linker) on the APTES SAMs was immersed immediately in 10 mM MUA (Aldrich 45,056-1, 95%) with a minimum amount of dry DMSO, and 10 mM MUL (Aldrich 44,752-8, 97%) with a minimum amount of dry dimethylforamide (DMF, Pierce) in absolute ethanol for at least 1hr [56] followed by a final rinsing in ethanol.
Surface characterization
Surfaces were characterized by contact angle measurements using an optical contact angle goiniometer (KSV Instruments, Cam 200) and by XPS (Fissons Escalab 200i XL). XPS survey scans, as well as high-resolution N1 s and C1 s scans, utilizing monochromatic Al Kα excitation, were obtained.
Embryonic rat cardiac myocyte culture
Cardiomyocytes were obtained from rat embryos on the 14th day of the embryonic development (E14) [57] according to a protocol approved by the Institutional Animal Care and Use Committee of University of Central Florida. Briefly, rats were euthanized by inhalation of an excess of CO2. The hearts were removed from the embryos in Hibernate E medium (BrainBits, Springfield, IL) and dissociated using type II Collagenase (Worthington, Lakewood, NJ, LS004174, 125 units/g, 1 g/5 ml) in L-15 medium. The hearts in the collagenase solution were placed in the water bath (37 °C, 90 rpm) for 20 minutes followed by gentle manual trituration. The cell suspension was then centrifuged on a 4 % Bovine Serum Albumin (BSA, Sigma, A-3058) cushion at 300 g, 4°C for 10 minutes. The cell pellet was then resuspended in the culture medium and plated on the surface-modified coverslips at a density of 1000 cells/mm2. The culture medium consisted of 100 ml of Ultraculture medium (Cambrex, East Rutherford, NJ), 5 ml of B-27 (Invitrogen, Carlsbad, CA), 1 ml of non-essential Amino Acids (Invitrogen, 11140-050), 1 ml of L-Glutamine (Invitrogen, 25030-164), 1 ml of 1 M Hepes Buffer (Sigma), and 0.375 g of dextrose (Sigma). The medium was changed on the first day after culture and thereafter every 3 days.
Histology
The coverslips were fixed in 3.7% formaldehyde in phosphate-buffered saline (PBS) for 10 min and permeabilized with 0.1% Triton X-100 in PBS solution for 5 min. The fixed cells were preincubated with 1% BSA for 20-30 min. Then fluorescent phallotoxin was added to the coverslips (BODIPY 581/591 phalloidin, Invitrogen, B3416, 1:40 dilution in PBS). The coverslips with the staining solution were then kept at room temperature for 20 min and mounted with Citofluor mounting solution (Ted Pella) onto the slides. The immunostaining were visualized using a Zeiss LSM 510 confocal microscope [17].
Electrophysiology
Whole-cell patch clamp recordings were performed in a recording chamber on the stage of a Zeiss Axioscope 2FS Plus upright microscope in L-15 medium at 22°C. Patch-clamp pipettes were prepared from borosilicate glass (BF150-86-10; Sutter, Novato, CA) with a Sutter P97 pipette puller, filled with intracellular solution (in mM: K-gluconate 140, EGTA 1, MgCl2 2, Na2ATP 5, Hepes 10; PH=7.2). The resistance of the electrodes was 6-8 MΩ. Action potentials were recorded in current clamp mode using a Multiclamp 700A amplifier (Axon, Union City, CA). Signals were filtered at 2 kHz and digitized at 20 kHz with an Axon Digidata 1322A interface. Data recording and analysis was performed with pClamp 9 software (Axon). Membrane potentials were corrected by subtraction of the 15 mV tip potential, which was calculated using Axon's pClamp 9 program.
RESULTS
Surface modification
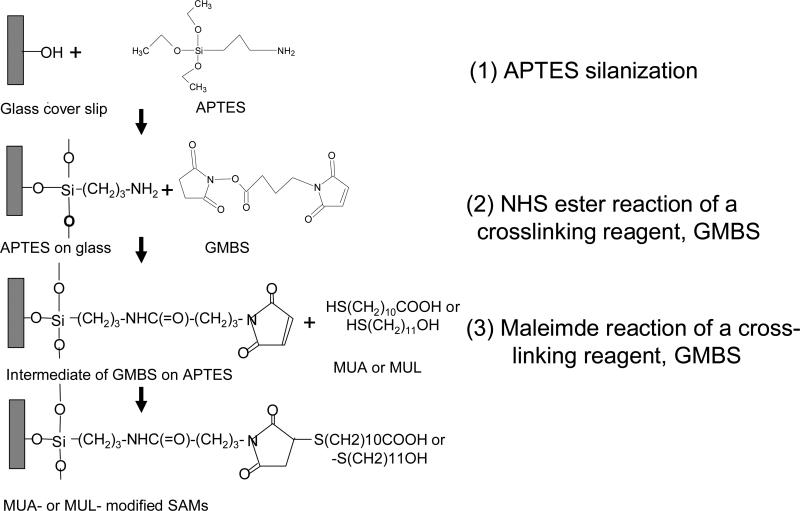
Glass coverslips were functionalized with: 1. Trimethoxysilylpropyldiethylenetriamine (DETA) 2. (3-aminopropyl)triethoxysilane (APTES) 3. 11-mercaptoundecanoic acid (MUA) 4. 11-mercapto-1-undecanol (MUL) according to the protocol outlined in Figure 1.
Figure 1.
Surface modification of the glass coverslips. First, glass coverslips were covalently modified with APTES self-assembled monolayer. MUA or MUL was covalently attached to the APTES monolayer with a bifunctional crosslinker (GMBS)
Contact angle and XPS measurements were used for the verification of the surface modification process. Contact angle for DETA, APTES, GMBS, MUA and MUL was 48 °±3, 45°±4, 60°± 2, 66°±3 and 56°± 3, respectively. The normalized intensity of nitrogen on APTES was 600±200 calculated as the area under the count (AUC) of the high resolution N 1s peak, divided with the internal reference peak, silicon (Si 2p).
Morphological characterization of cardiomyocytes on functionalized surfaces
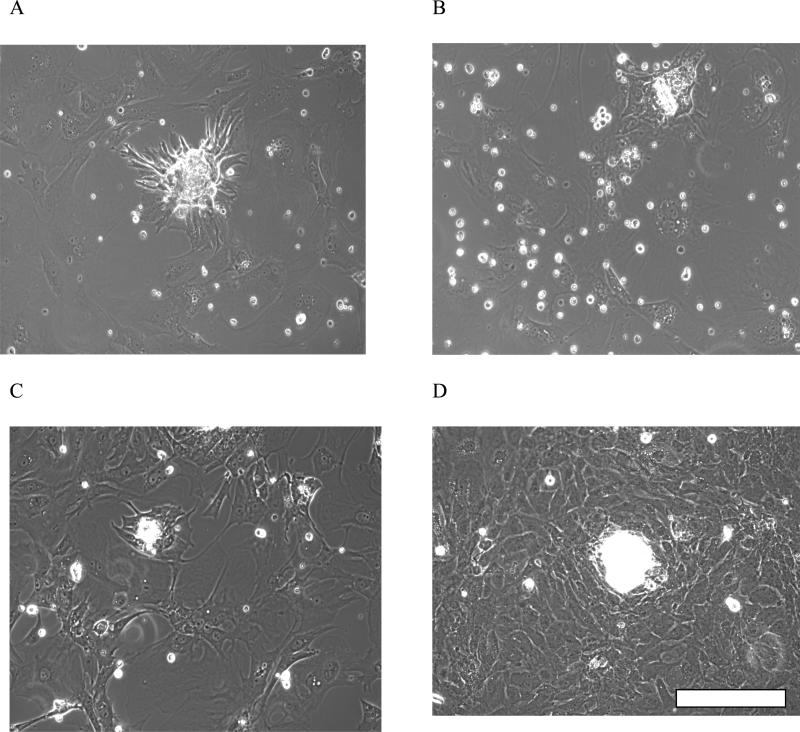
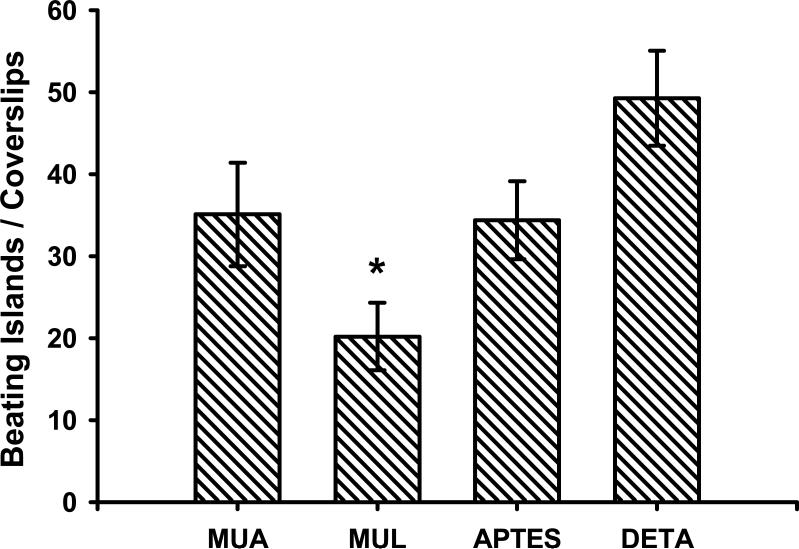
The growth and differentiation of rat embryonic cardiomyocytes on the four functionalized surfaces (MUA, MUL, APTES and DETA) were studied over a two week period. At the early stage of the culture (1-3 days) the cells formed small, clearly separated, non-beating clusters [17]. By day 5 in vitro these clusters showed spontaneous beating on all surfaces. The best attachment and growth of cardiac cells was observed on DETA, followed by those on MUA surfaces. APTES was a close third with cell attachment and spontaneous activity almost similar to the MUA surface (Figure 2). The MUL surface showed comparatively poor attachment and a significantly lower presence of beating cell clusters compared to the other three surfaces (Figure 3).
Figure 2.
Morphology of cardiomyocyte beating clusters on the functionalized surfaces on day 5: A) MUA B) MUL C) APTES D) DETA. Scalebar=100 μm
Figure 3.
Beating cardiomyocyte-clusters / coverslip on the functionalized surfaces. There were significantly less beating clusters on MUL surface compared to either MUA, APTES or DETA. There was no significant difference between MUA, APTES or DETA. Two-sample Student's t-test, p<0.05. 5 experiments with 3 parallel coverslips in each. Mean±SEM.
Histological analysis using BODIPY-conjugated phallotoxin showed that the beating islands consisted of cardiac myocytes. At the single cell level we were not able to detect any differences in the morphology of cardiac myocytes grown on the functionalized surfaces. The measured parameters were cell size and shape quantified by the ratio of the longest and shortest axis of the cells measured by the NIH Image program (Data not shown).
Electrophysiology
Whole cell patch clamp experiments were performed on beating cardiac myocytes grown on the functionalized surfaces at day 7 in vitro. There was no significant difference in any of the measured parameters between the four groups except in the length of the action potentials (Figure 4 and (Table 1).
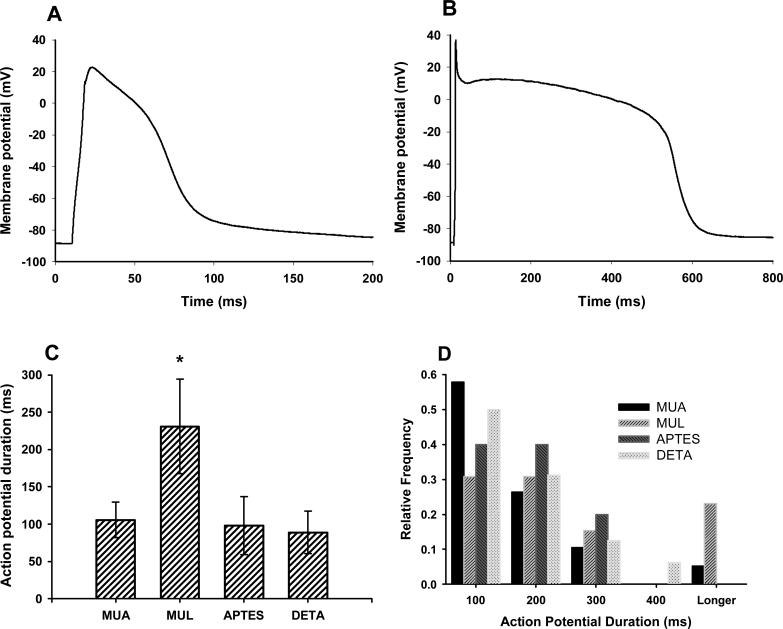
Figure 4.
Electrophysiological characterization of cardiac myocytes grown on the functionalized surfaces on day 7 in vitro. A, B: Representative action potential shapes on MUA and MUL surfaces, respectively. Note the different timescales! C: Action potentials were significantly wider on MUL surface than on any of the other surfaces. Mean of 13 – 19 experiments ± SEM was shown. Two-sample Student's t-test, p<0.05. D: The distribution of the action potential width showed that on MUL surface there was a cell population generating unusually wide action potentials. Relative frequency was normalized to the total number of recorded cells on the given surface.
Table 1.
Electrophysiological characterization of 1 week old cardiac myocyte cultures grown on functionalized surfaces. n=13-19 experiments / group.
| MUA | MUL | APTES | DETA | |||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM |
| Rm (MΩ) | 35.0 | 4.00 | 34.9 | 2.77 | 51.6 | 11.32 | 28.3 | 8.30 |
| Vm (mV) | -44.3 | 3.26 | -49.8 | 4.27 | -44.9 | 5.63 | -43.2 | 6.04 |
| Cp (pF) | 111 | 22.20 | 77 | 23.38 | 76 | 13.23 | 135 | 25.8 |
| AP overshoot (mV) | 18.9 | 3.85 | 22.1 | 5.23 | 21.0 | 7.83 | 8.9 | 3.05 |
| AP1/2 Duration (ms) | 106 | 23.60 | 231* | 63.08 | 98 | 38.77 | 89 | 28.35 |
Significantly different from all of the other groups. P<0.05.
Action potentials were significantly longer on MUL compared to any of the other functionalized surfaces. The longer average action potential length in the MUL group was the result of the presence of a cell population generating unusually long action potentials (Figure 4). This cell population was not unique to the MUL surface, and, although less frequently, this type of cells could have been observed in the other groups. Longer action potentials in the MUL, and less frequently in the other groups were also observed in older (14 days in vitro) cultures (Data not shown).
DISCUSSION
The interaction of cardiac myocytes with the degradation products of commonly used scaffold materials such as poly(lactic acid) (PLA), poly(lactic-co-glycolic acid) (PLGA) and poly(glycolic acid) (PGA) were modeled by culturing embryonic rat cardiac myocytes on functionalized coverslips presenting carboxyl and hydroxyl groups on the surface. Contact angle measurements and XPS showed the successful functionalization of the glass coverslips with SAMs presenting the carboxyl and hydroxyl groups on the surface. Embryonic cardiac myocytes formed beating islands on all tested surfaces /Trimethoxysilylpropyldiethylenetriamine (DETA), (3-aminopropyl)triethoxysilane (APTES), 11-mercaptoundecanoic acid (MUA) and 11-mercapto-1-undecanol (MUL)/ but the number of attached cells and beating patches was significantly lower on MUL surface compared to any of the other functionalized surfaces. Moreover, whole cell patch clamp experiments showed that the average action potential length generated by the beating cardiac myocytes were significantly longer on MUL compared to other surfaces.
The result concerning the decreased number of beating cardiac myocytes on the hydroxylfunctionalized surface was in agreement with recent experiments by Sung and coworkers (2004) using 3D scaffolds. They have reported the decreased survival of cardiac myocytes in the advanced stages of degradation of PGLA scaffolds (higher hydroxyl / carboxyl ratio) compared to non-degraded scaffolds [13].
Our observation concerning the increased number of cardiac myocytes that generated long action potentials on MUL compared to the other functionalized surfaces might indicate that 1) these long action potentials are generated by non-healthy cells and the ratio of non-healthy / healthy cells was grater on MUL in agreement with the decreased viability or 2) the hydroxyl groups on the surface affected the differentiation of the cardiac myocytes by inducing the development of a phenotypically separate cell population or 3) surface properties selectively affected the survival of atrial vs ventricular myocytes, which have different electrophysiological properties or 4) hydroxyl groups on the surface slowed down the differentiation of cardiac myocytes, because the duration of the action potentials are shortened during the normal development of cardiac myocytes.
Further studies needed for the deeper understanding of the impact of the chemical environment on the physiology of cardiac myocytes (and also other cell types) because this information is essential for several tissue engineering applications. Our system, consisting of a serum-free culture medium and a defined surface offers a tool for these vital studies. As more and more cells will be available for culture in serum free conditions, our method can be extended to all of these new cell types. In this study we did not investigate the effect of substrate topography / texture on the physiology of the cells, only chemical composition, but those important effects can also be modeled in our defined system.
CONCLUSION
Our simple method that used hydroxyl- and carboxyl- functionalized surfaces to test the effect of the different chemical environments which surrounds cardiac myocytes at different stages of the degradation of PLA, PLGA or PGA scaffolds gave the same result, decreased viability in the case of hydroxyl-functionalization, than earlier experiments using the complex 3D scaffold. In our system the chemical environment affected not only the survival, but also the physiology (action potential duration) of cardiac myocytes. Surface functionalization with self-assembled monolayers combined with histological/physiological testing could be a relatively high throughput method for biocompatibility studies and optimization.
Acknowledgement
We would like to acknowledge that support for this research were provided by NIH grant R21 EB002307-02 as well as from the University of Central Florida.
References
- 1.Rabkin E, Schoen FJ. Cardiovascular tissue engineering. Cardiovascular Pathology. 2002;11:305–317. doi: 10.1016/s1054-8807(02)00130-8. [DOI] [PubMed] [Google Scholar]
- 2.Carrier RL, et al. Cardiac tissue engineering: Cell seeding, cultivation parameters, and tissue construct characterization. Biotechnology and Bioengineering. 1999;64:580–589. doi: 10.1002/(sici)1097-0290(19990905)64:5<580::aid-bit8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 3.Leor J, et al. Bioengineered cardiac grafts - A new approach to repair the infarcted myocardium? Circulation. 2000;102:56–61. doi: 10.1161/01.cir.102.suppl_3.iii-56. [DOI] [PubMed] [Google Scholar]
- 4.Evans HJ, Sweet JK, Price RL, Yost M, Goodwin RL. Novel 3D culture system for study of cardiac myocyte development. Am J Physiol Heart Circ Physiol. 2003;285:H570–578. doi: 10.1152/ajpheart.01027.2002. [DOI] [PubMed] [Google Scholar]
- 5.Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid polyglycolic acid copolymers. Biomaterials. 1996;17:93–102. doi: 10.1016/0142-9612(96)85754-1. [DOI] [PubMed] [Google Scholar]
- 6.Mann BK, West JL. Tissue engineering in the cardiovascular system: Progress toward a tissue engineered heart. Anatomical Record. 2001;263:367–371. doi: 10.1002/ar.1116. [DOI] [PubMed] [Google Scholar]
- 7.Papadaki M, et al. Tissue engineering of functional cardiac muscle: molecular, structural, and electrophysiological studies. American Journal of Physiology-Heart and Circulatory Physiology. 2001;280:H168–H178. doi: 10.1152/ajpheart.2001.280.1.H168. [DOI] [PubMed] [Google Scholar]
- 8.Gopferich A. Polymer degradation and erosion: Mechanisms and applications. European Journal of Pharmaceutics and Biopharmaceutics. 1996;42:1–11. [Google Scholar]
- 9.Gopferich A. Mechanisms of polymer degradation and erosion. Biomaterials. 1996;17:103–114. doi: 10.1016/0142-9612(96)85755-3. [DOI] [PubMed] [Google Scholar]
- 10.Lu L, et al. In vitro and in vivo degradation of porous poly(DL-lactic-co-glycolic acid) foams. Biomaterials. 2000;21:1837–1845. doi: 10.1016/s0142-9612(00)00047-8. [DOI] [PubMed] [Google Scholar]
- 11.Cai Q, Bei JZ, Wang SG. Relationship among drug delivery behavior, degradation behavior and morphology of copolylactones derived from glycolide, L-lactide and epsilon-caprolactone. Polymers for Advanced Technologies. 2002;13:105–111. [Google Scholar]
- 12.Cai Q, Shi GX, Bei JZ, Wang SG. Enzymatic degradation behavior and mechanism of poly(lactide-co-glycolide) foams by trypsin. Biomaterials. 2003;24:629–638. doi: 10.1016/s0142-9612(02)00377-0. [DOI] [PubMed] [Google Scholar]
- 13.Sung HJ, Meredith C, Johnson C, Galis ZS. The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials. 2004;25:5735–5742. doi: 10.1016/j.biomaterials.2004.01.066. [DOI] [PubMed] [Google Scholar]
- 14.Clark WA, Decker ML, Behnke-Barclay M, Janes DM, Decker RS. Cell contact as an independent factor modulating cardiac myocyte hypertrophy and survival in long-term primary culture. Journal of Molecular and Cellular Cardiology. 1998;30:139–155. doi: 10.1006/jmcc.1997.0580. [DOI] [PubMed] [Google Scholar]
- 15.Boateng S, et al. Peptides bound to silicone membranes and 3D microfabrication for cardiac cell culture. Advanced Materials. 2002;14:461–+. [Google Scholar]
- 16.Simpson DG, et al. Modulation of Cardiac Myocyte Phenotype in-Vitro by the Composition and Orientation of the Extracellular-Matrix. Journal of Cellular Physiology. 1994;161:89–105. doi: 10.1002/jcp.1041610112. [DOI] [PubMed] [Google Scholar]
- 17.Das M, et al. Long-term culture of embryonic rat cardiomyocytes on an organosilane surface in a serum-free medium. Biomaterials. 2004;25:5643–5647. doi: 10.1016/j.biomaterials.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 18.Natarajan A, Molnar P, Sieverdes K, Jamshidi A, Hickman JJ. Microelectrode array recordings of cardiac action potentials as a high throughput method to evaluate pesticide toxicity. Toxicology in Vitro. 2006;20:375–381. doi: 10.1016/j.tiv.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Berthiaume F, Moghe PV, Toner M, Yarmush ML. Effect of extracellular matrix topology on cell structure, function, and physiological responsiveness: Hepatocytes cultured in a sandwich configuration. Faseb Journal. 1996;10:1471–1484. doi: 10.1096/fasebj.10.13.8940293. [DOI] [PubMed] [Google Scholar]
- 20.Ito Y. Surface micropatterning to regulate cell functions. Biomaterials. 1999;20:2333–2342. doi: 10.1016/s0142-9612(99)00162-3. [DOI] [PubMed] [Google Scholar]
- 21.Lin CY, Kikuchi N, Hollister SJ. A novel method for biomaterial scaffold internal architecture design to match bone elastic properties with desired porosity. Journal of Biomechanics. 2004;37:623–636. doi: 10.1016/j.jbiomech.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 22.Dityatev AE, et al. Comparison of the topology and growth rules of motoneuronal dendrites. Journal of Comparative Neurology. 1995;363:505–516. doi: 10.1002/cne.903630312. [DOI] [PubMed] [Google Scholar]
- 23.Whitesides GM, Mathias JP, Seto CT. Molecular self-assembly and nanochemistry: a chemical strategy for the synthesis of nanostructures. Science. 1991;254:1312–9. doi: 10.1126/science.1962191. [DOI] [PubMed] [Google Scholar]
- 24.Wink T, vanZuilen SJ, Bult A, vanBennekom WP. Self-assembled monolayers for biosensors. Analyst. 1997;122:R43–R50. doi: 10.1039/a606964i. [DOI] [PubMed] [Google Scholar]
- 25.Prime KL, Whitesides GM. Self-assembled organic monolayers: model systems for studying adsorption of proteins at surfaces. Science. 1991;252:1164–7. doi: 10.1126/science.252.5009.1164. [DOI] [PubMed] [Google Scholar]
- 26.Laibinis PE, Hickman JJ, Wrighton MS, Whitesides GM. Orthogonal Self-Assembled Monolayers: Alkanethiols on Gold and Alkane Carboxylic Acids on Alumina. Science. 1989;245:845–847. doi: 10.1126/science.245.4920.845. [DOI] [PubMed] [Google Scholar]
- 27.Bain CD, Whitesides GM. Molecular-Level Control over Surface Order in Self-Assembled Monolayer Films of Thiols on Gold. Science. 1988;240:62–63. doi: 10.1126/science.240.4848.62. [DOI] [PubMed] [Google Scholar]
- 28.Mrksich M, et al. Controlling cell attachment on contoured surfaces with self- assembled monolayers of alkanethiolates on gold. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:10775–10778. doi: 10.1073/pnas.93.20.10775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostuni E, Yan L, Whitesides GM. The interaction of proteins and cells with self-assembled monolayers of alkanethiolates on gold and silver. Colloids and Surfaces BBiointerfaces. 1999;15:3–30. [Google Scholar]
- 30.Stenger DA, et al. Coplanar Molecular Assemblies of Aminoalkylsilane and Perfluorinated Alkylsilane - Characterization and Geometric Definition of Mammalian-Cell Adhesion and Growth. Journal of the American Chemical Society. 1992;114:8435–8442. [Google Scholar]
- 31.Ferguson GS, Chaudhury MK, Biebuyck HA, Whitesides GM. Monolayers on Disordered Substrates - Self-Assembly of Alkyltrichlorosilanes on Surface-Modified Polyethylene and Poly(Dimethylsiloxane). Macromolecules. 1993;26:5870–5875. [Google Scholar]
- 32.Ulman A. Introduction to Ultrathin organic Films. Academic Press, Inc.; San Diego: 1991. [Google Scholar]
- 33.Georger JH, et al. Coplanar Patterns of Self-Assembled Monolayers for Selective Cell-Adhesion and Outgrowth. Thin Solid Films. 1992;210:716–719. [Google Scholar]
- 34.Schaffner AE, Barker JL, Stenger DA, Hickman JJ. Investigation of the factors necessary for growth of hippocampal neurons in a defined system. Journal of Neuroscience Methods. 1995;62:111–119. doi: 10.1016/0165-0270(95)00063-1. [DOI] [PubMed] [Google Scholar]
- 35.Mrksich M. Tailored substrates for studies of attached cell culture. Cellular and Molecular Life Sciences. 1998;54:653–662. doi: 10.1007/s000180050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das M, Molnar P, Devaraj H, Poeta M, Hickman JJ. Electrophysiological and Morphological Characterization of Rat Embryonic Motoneurons in a Defined System. Biotechnol. Prog. 2003;19:1756–1761. doi: 10.1021/bp034076l. [DOI] [PubMed] [Google Scholar]
- 37.Ravenscroft MS, et al. Developmental neurobiology implications from fabrication and analysis of hippocampal neuronal networks on patterned silane- modified surfaces. Journal of the American Chemical Society. 1998;120:12169–12177. [Google Scholar]
- 38.Tengvall P, Lundstrom I, Liedberg B. Protein adsorption studies on model organic surfaces: an ellipsometric and infrared spectroscopic approach. Biomaterials. 1998;19:407. doi: 10.1016/s0142-9612(97)00110-5. [DOI] [PubMed] [Google Scholar]
- 39.Liu VA, Jastromb WE, Bhatia SN. Engineering protein and cell adhesivity using PEO-terminated triblock polymers. Journal of Biomedical Materials Research. 2002;60:126–134. doi: 10.1002/jbm.10005. [DOI] [PubMed] [Google Scholar]
- 40.Vanderah DJ, Valincius G, Meuse CW. Self-assembled monolayers of methyl 1-thiahexa(ethylene oxide) for the inhibition of protein adsorption. Langmuir. 2002;18:4674–4680. [Google Scholar]
- 41.Bhatia SK, et al. Fabrication of surfaces resistant to protein adsorption and application to two-dimensional protein patterning. Anal Biochem. 1993;208:197–205. doi: 10.1006/abio.1993.1027. [DOI] [PubMed] [Google Scholar]
- 42.Puleo DA, Nanci A. Understanding and controlling the bone-implant interface. Biomaterials. 1999;20:2311–2321. doi: 10.1016/s0142-9612(99)00160-x. [DOI] [PubMed] [Google Scholar]
- 43.Schoenfisch MH, Ovadia M, Pemberton JE. Covalent surface chemical modification of electrodes for cardiac pacing applications. Journal of Biomedical Materials Research. 2000;51:209–215. doi: 10.1002/(sici)1097-4636(200008)51:2<209::aid-jbm9>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 44.Tosatti S, Michel R, Textor M, Spencer ND. Self-assembled monolayers of dodecyl and hydroxy-dodecyl phosphates on both smooth and rough titanium and titanium oxide surfaces. Langmuir. 2002;18:3537–3548. [Google Scholar]
- 45.Fields GB. Induction of protein-like molecular architecture by self- assembly processes. Bioorganic & Medicinal Chemistry. 1999;7:75–81. doi: 10.1016/s0968-0896(98)00216-8. [DOI] [PubMed] [Google Scholar]
- 46.Fields GB, et al. Proteinlike molecular architecture: Biomaterial applications for inducing cellular receptor binding and signal transduction. Biopolymers. 1998;47:143–151. doi: 10.1002/(SICI)1097-0282(1998)47:2<143::AID-BIP3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 47.Molnar P, Wang WS, Natarajan A, Rumsey JW, Hickman JJ. Photolithographic patterning of C2C12 myotubes using vitronectin as growth substrate in serum-free medium. Biotechnology Progress. 2007;23:265–268. doi: 10.1021/bp060302q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mrksich M, Whitesides GM. Using self-assembled monolayers to understand the interactions of man-made surfaces with proteins and cells. Annual Review of Biophysics and Biomolecular Structure. 1996;25:55–78. doi: 10.1146/annurev.bb.25.060196.000415. [DOI] [PubMed] [Google Scholar]
- 49.Jenney CR, Anderson JM. Adsorbed serum proteins responsible for surface dependent human macrophage behavior. Journal Of Biomedical Materials Research. 1999;49:435–447. doi: 10.1002/(sici)1097-4636(20000315)49:4<435::aid-jbm2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 50.Faucheux N, Schweiss R, Lutzow K, Werner C, Groth T. Self-assembled monolayers with different terminating groups as model substrates for cell adhesion studies. Biomaterials. 2004;25:2721–2730. doi: 10.1016/j.biomaterials.2003.09.069. [DOI] [PubMed] [Google Scholar]
- 51.Li LY, Chen SF, Zheng J, Ratner BD, Jiang SY. Protein adsorption on oligo(ethylene glycol)-terminated alkanethiolate self-assembled monolayers: The molecular basis for nonfouling behavior. Journal of Physical Chemistry B. 2005;109:2934–2941. doi: 10.1021/jp0473321. [DOI] [PubMed] [Google Scholar]
- 52.Goncalves IC, Martinsa MCL, Barbosa MA, Ratner BD. Protein adsorption on 18-alkyl chains immobilized on hydroxyl-terminated self-assembled monolayers. Biomaterials. 2005;26:3891. doi: 10.1016/j.biomaterials.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 53.Ikada Y. Surface Modification of Polymers for Medical Applications. Biomaterials. 1994;15:725–736. doi: 10.1016/0142-9612(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 54.Ratner BD. Surface Modification of Polymers - Chemical, Biological and Surface Analytical Challenges. Biosensors & Bioelectronics. 1995;10:797–804. doi: 10.1016/0956-5663(95)99218-a. [DOI] [PubMed] [Google Scholar]
- 55.Das M, et al. A defined system to allow skeletal muscle differentiation and subsequent integration with silicon microstructures. Biomaterials. 2006;27:4374–4380. doi: 10.1016/j.biomaterials.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 56.Ge B, Lisdat F. Superoxide sensor based on cytochrome c immobilized on mixed-thiol SAM with a new calibration method. Analytica Chimica Acta. 2002;454:53–64. [Google Scholar]
- 57.Harary I, Farley B. In vitro studies on single beating rat heart cells. Experimental Cell Research. 1963;29:451–465. doi: 10.1016/s0014-4827(63)80008-7. [DOI] [PubMed] [Google Scholar]