Abstract
It is a consensus that familiarity and recollection contribute to episodic recognition memory. However, it remains controversial whether familiarity and recollection are qualitatively distinct processes supported by different brain regions, or whether they reflect different strengths of the same process and share the same support. In this review, I discuss how adapting standard human recognition memory paradigms to rats, performing circumscribed brain lesions and using receiver operating characteristic (ROC) methods contributed to solve this controversy. First, I describe the validation of the animal ROC paradigms and report evidence that familiarity and recollection are distinct processes in intact rats. Second, I report results from rats with hippocampal dysfunction which confirm this finding and lead to the conclusion that the hippocampus supports recollection but not familiarity. Finally, I describe a recent study focusing on the medial entorhinal cortex (MEC) that investigates the contribution of areas upstream of the hippocampus to recollection and familiarity.
Keywords: ROC, recollection, familiarity, episodic memory, hippocampus, MEC
The contribution of two memory types to recognition memory function has been discussed since the time of Aristotle. One of these processes is described as a vague feeling of familiarity or ‘reminiscence’; for example when you recognize somebody, but cannot identify this person by name. The second, the recollection process, involves additional dimensions of memory; for example the spatial or temporal context in which this person was encountered (for a review see Yonelinas, 2002). Interestingly, the recollection and the familiarity processes are differentially affected in aging and in patients with amnesia. Indeed, the recollection process is strongly impaired in aging and amnesia, while the familiarity process is relatively spared (Yonelinas et al 1998; Duezel et al, 2001; Quamme et al, 2004; Barbeau et al, 2005; Brandt et al, 2008; Vann et al, 2009, Daselaar et al., 2006; Howard et al., 2006; Prull et al., 2006; Duverne et al., 2007; Turriziani et al, 2008; Peters and Daum, 2008; but see Knowlton and Squire 1995, Schacter et al, 1996). Hence, uncovering the neural substrates of the recollection and the familiarity processes could contribute to the characterization of new targets to rescue at least part of these deficits. A standard method to analyze human recognition memory performance is to use receiver operating characteristic (ROC) functions (for a review see Yonelinas and Parks, 2007). In humans, episodic recognition memory is usually assessed by presenting a study list of items to a subject (for example a list of words appearing on a screen one at a time), and after a delay, presenting a longer list of items composed of the same items intermixed with an equal number of new items, also appearing one at a time. The probability of correct recognition of a study list item (p‘hit’) is plotted as a function of the probability of incorrect recognition of a ‘new’ item (p‘false alarm’) across confidence or bias levels, and the best fitting curve is defined to generate an ROC function (Yonelinas et al, 1999; see figure 1A and C for idealized human ROC curves for item and associative recognition memory, respectively).
Figure 1.
ROC functions for humans and rats recognition memory. Recollection (R) and familiarity (F) indices are shown as bar diagrams (see Yonelinas and Parks, 2007 for detailed calculations). A. Ideal human ROC curve for single item recognition memory: the ROC curve is asymmetrical and curvilinear, reflecting the contribution of recollection and familiarity to recognition memory. B. Similar ROC function observed in rats for single odour recognition memory (graph from Fortin et al., 2004). C. Ideal human ROC curve for associative recognition memory: the ROC is asymmetrical and linear suggesting that recognition performance is based essentially on recollection. D. Comparable ROC function observed in rats (graph from Sauvage et al, 2008). E. ROC function observed in rats for single odour recognition with a speeded response deadline demand (data from Sauvage et al., 2010a). The ROC is symmetrical and curvilinear, suggesting recognition relies primarily on familiarity.
Multiple models of recognition memory are based on the contribution of the familiarity and the recollection processes to recognition memory function (for a review see Yonelinas, 2002). Among them, two have been used extensively to study episodic recognition memory in humans: the dual-process model and the one-process model (for reviews see Yonelinas and Parks, 2007; Wixted et al, 2007 respectively). The dual-process model describes familiarity and recollection processes as qualitatively distinct processes. In light of this model, the familiarity process is described as a rapid and continuous signal detection process sensitive to perceptual manipulations, while the recollection process is described as a slower and conceptually driven threshold process (Atkinson 1974; Mandler, 1980; see for a review Yonelinas, 2002). Studies using the dual-process model of recognition memory also report that familiarity and recollection have different neural substrates: the hippocampus for recollection, the parahippocampal region for familiarity (Yonelinas et al, 2002; Aggleton et al, 2005; Bowles et al, 2007; Yonelinas et al, 2007; see for reviews Eichenbaum et al, 2007; Diana et al, 2007). According to this model, two distinct indices can be generated from the analysis of ROC functions. The recollection index (R), y-intercept of the ROC function, which reflects the contribution of the recollection process to recognition memory performance, and the familiarity index (F), reflected by the degree of curvilinearity of the function, which reveals the contribution of familiarity to recognition memory performance (see Yonelinas, 1994 and Yonelinas and Parks, 2007 for calculation of the indices). Hence, within the frame of the dual-process model, ROC functions are asymmetrical and curvilinear when familiarity and recollection contribute to recognition memory performance (figure 1A), asymmetrical and linear when the recollection process is primarily involved (figure 1C), or symmetrical and curvilinear when recognition memory is essentially based on the familiarity process (figure 5; Aged group).
Figure 5.
Item recognition ROC in aging (graph from Robitsek et al, 2008). Aging reduces the contribution of recollection to recognition performance while sparing familiarity.
A major alternative to this model is the one-process theory that describes familiarity and recollection as qualitatively similar processes differing only in the strength of memory they reflect (see for a review Wixted et al, 2007). Familiarity would reflect weak memory, while recollection would reflect stronger memory or memory involving more information. As a consequence, recollection and familiarity would have a unique neural substrate, the hippocampus and could not vary independently (Stark et al, 2000; Manns et al 2003; Wais et al, 2006; see for a review Squire et al, 2004). According to this view, the degree of curvilinearity of the ROC function reflects the sum of the strengths of memory components, and its asymmetry reflects greater variability in strength for the ‘old’ than for ‘new’ items. Among human ROC studies, some report that damage restricted to the hippocampus impairs specifically the recollection process (Yonelinas et al, 2002; Quamme et al, 2004; Aggleton et al, 2005), while others report that both processes are affected in patients with damage thought to be circumscribed to the hippocampus (Reed et al, 1997; Manns et al, 2003; Stark 2000, Wais et al, 2006). This discrepancy resides mainly in the fact that the hippocampus and the parahippocampal region are adjacent brain structures. Indeed, identifying the precise extent of brain damage in patients with amnesia, or the precise source of brain activity within adjacent brain regions is beyond the spatial and neuropsychological resolution of standard techniques used currently in humans (e.g. functional and structural MRI imaging, and psychological tests). Thus, it has been suggested that the familiarity impairments accompanying the recollection deficits reported in some studies in patients with damage to the hippocampus resulted from additional damage to areas adjacent to the hippocampus (e.g. the parahippocampal region), rather than from damage to the hippocampus per-se.
Given the medial temporal lobe is exceptionally conserved across species (Manns and Eichenbaum, 2006), one way to clearly define whether the hippocampus supports familiarity as well as recollection is to perform lesion restricted to the hippocampus in animals, and assess the effect of this circumscribed lesion with behavioural paradigms that allow for the generation of distinct recollection and familiarity indices. We discuss this approach in the present review.
A second major issue in human recognition memory is whether the parahippocampal region is functionally segregated in terms of its contribution to the recollection and the familiarity processes. Indeed, recent human and animal studies suggest that specific areas of the parahippocampal region, which are adjacent and strongly interconnected, contribute to different aspects of memory function (see Eichenbaum et al, 2007 for a review). The perirhinal cortex (PRc) and the lateral entorhinal cortex (LEC) would process information about the familiarity of individual items. In contrast, the postrhinal cortex (POR; parahippocampal cortex in humans) and the medial entorhinal cortex (MEC) would support recollection by representing the spatial and temporal context (whether the items are new or old) in which items have been experienced. However, this hypothesis could not be thoroughly tested in humans principally because of two reasons. One: it is not possible to determine the precise source of brain activity during recognition memory tasks in humans when areas are adjacent. Two: because cases showing restricted lesions to a single area of the parahippocampal region are extremely rare.
In this review I will show how we addressed these two controversial issues by developing behavioural animal ROC paradigms that allow for recognition memory performance to be evaluated in a similar manner to the way that it is in humans (e.g. translational paradigms). Moreover, given these tasks are performed with animals, they present a key advantage over human ROC studies in that they can be combined with stereotactic surgery, which allows for brain areas to be damaged in a very restricted manner, while preventing additional damage to the adjacent brain structures.
Using this approach, we first aimed at investigating the contribution of the hippocampus to recollection and familiarity by performing lesions circumscribed to the hippocampus, and defined whether the hippocampus supports recollection and familiarity, or recollection only. Second, we investigated the contribution of areas upstream of the hippocampus, more specifically the contribution of the MEC, to recollection and familiarity by performing lesion circumscribed to the MEC.
In the first part of this review, I will discuss how animal ROC paradigms contributed to bridging human and animal recognition memory, and brought evidence that recollection and familiarity are qualitatively distinct processes using intact rats. In a second part, I will show that this finding was confirmed in rats with impaired hippocampal function, and will show that animal ROC paradigms brought compelling evidence that the hippocampus supports recollection but not familiarity. In the last part of the review, I will report the first step of a series of studies investigating the functional segregation of the parahippocampal region, which focuses on the characterization of the contribution of the MEC to recollection and familiarity.
A translational model of episodic recognition memory
A prerequisite for animal ROC paradigms to be appropriate translational models is to yield results comparable to those observed in humans, e.g. the contribution of familiarity and recollection to recognition memory performance in rats would be expected to be comparable to that of humans under the same experimental conditions.
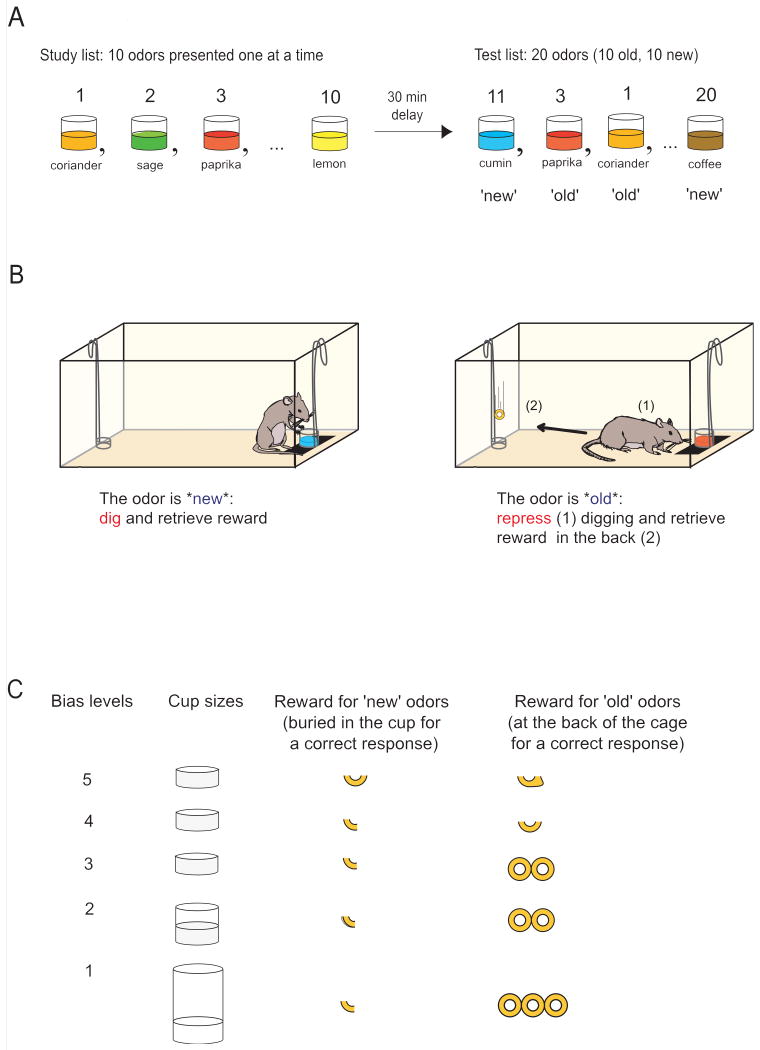
Within the frame of the dual-process model, a typical standard human ROC function for single item recognition memory reflects the contribution of the recollection and the familiarity processes, as shown by the asymmetry of the ROC function (y-intercept: R different from 0) and its curvilinearity (F different from 0) respectively (figure 1A; Yonelinas, 1994; see for a review Yonelinas and Parks, 2007). In 2004, Fortin and colleagues laid out the ground work for ROC studies in animals by readily adapting a standard human episodic recognition memory task to rats using their innate ability to discriminate odours and to forage (Fortin et al, 2004). The animal behavioural ROC paradigm was designed to be as similar as possible to the human paradigm to minimize the potential effect of methodological differences on the interpretation of the data. In human and rat ROC paradigms, items are presented during a study phase, and after a delay the same items are presented again intermixed with new items. Correct recognition of the odours presented during the study phase (a hit) and incorrect recognition of new odours (a false alarm; fa) are assessed across five decision criteria, and the probability of a hit (phit) is plotted as a function of the probability of false alarm (pfa). Subsequently, the exact same analytical methods are used to evaluate the shape of human or rat ROC functions, and to generate recollection and familiarity indices (see Yonelinas, 1999 and Yonelinas and Parks, 2007 for details). Understandably though, stimulus modalities differed as they were defined to yield an optimal performance for each species. In humans, stimuli used are usually visual, while in rats stimuli used are olfactory. In addition, human subjects reported that they recognized a stimulus item as ‘old’ or ‘new’ verbally or by pressing appropriate keyboard keys, while rats were trained on a non-matching to sample rule to ‘show’ that they recognized the test stimuli as being ‘new’ or ‘old’. In more detail, the animal protocol was performed as follows: every day, a unique study list of 10 odours mixed in sand contained in cups would be presented to a given animal. Odours were common household spices (cumin, coriander etc…) chosen from a pool of forty odours. Only twenty odours were used per session (ten ‘study’ odours, ten ‘new’ odours; one session a day), and the study list changed every day because episodic recognition memory was investigated. Sampling of the odours during the study phase was ensured by placing a small piece of cereal buried in the cup. After a 30 min delay, recognition memory was tested as animals were presented with the same odours (‘old’ odours) intermixed with additional odours that had not been presented that day (‘new’ odours), but which the animal was highly familiar with (figure 2A). Animals were trained on a delay non-matching to sample rule, following which they had to dig in the cup to retrieve a food reward if the odour presented was ‘new’, or repress digging if the odours were ‘old’ and go to the back of the cage to collect a food reward (figure 2B). To prevent that rats solve the task by smelling the presence of the reward buried in the cups containing the ‘new’ odours, cups containing ‘old’ odours were baited with food rewards that were not accessible to the animals. In addition, spatial information could not be used to solve the task because all stimulus cups were presented at the same location (in the front of the cage), and the reward location was experienced only after each trial ended, e.g. after the recognition judgment was completed. Once animals were trained on the delayed non-matching to sample rule, recognition performance was assessed across five bias levels, ranging from conservative to liberal, by manipulating the amount of reward and the cup sizes (each bias type once a week in a pseudorandom order; figure 2C). Recognition memory performance was assessed by collecting the number of hits and the number of false alarms (fa) over 20 trials per session. Subsequently, the probability of hits and fas were calculated, and data averaged over four sessions for each bias level to plot and generate ROC functions for odour recognition memory (Fortin et al, 2004).
Figure 2.
Example of ROC paradigm in rats. A. Sequence of odour presentations on the sample and test phases (Fortin et al, 2004). B. Non-matching-to-sample rule. C. Bias levels obtained by varying cup heights and payoff ratios of cereal rewards.
In this study, Fortin and colleagues showed for the first time that the recollection and the familiarity processes contributed to single item recognition memory in rats, as it is the case in humans (Yonelinas, 1994). Indeed, performance in rats resulted in an asymmetrical ROC function, reflecting the contribution of the recollection process. In addition, the ROC function for odour recognition memory in rats was curvilinear, reflecting the contribution of the familiarity process (figure 1B). This finding brought the first concrete evidence that analyzing single item recognition memory performance with ROC methods yielded comparable results in humans and rats with familiarity and recollection contributing to single item recognition memory, thereby suggesting that the animal ROC paradigm for odour recognition was an appropriate translational tool to study single item recognition memory. With this in mind, we pursued the validation of the animal ROC paradigms by testing whether rat ROC functions varied in a similar manner to human ROC functions when subjected to the same experimental conditions. In addition, this manipulation allowed us to test the hypothesis that recollection and familiarity were qualitatively difference processes.
Are recollection and familiarity distinct processes? evidence from intact rats
The first controversial issue that we addressed using animal ROC paradigms was whether recollection and familiarity were qualitatively distinct processes, or whether they reflected different strengths of the same process. Compelling evidence that these processes are distinct would be that one process could support recognition memory performance without a significant contribution of the other. To test this hypothesis, we investigated recognition memory performance of intact rats under memory demands reported to favour the contribution of the recollection process or the contribution of the familiarity process to recognition memory.
Can recognition memory performance solely rely on the recollection process?
In humans, episodic recognition memory for pairs relies primarily on the recollection process, as revealed by an ROC function which is asymmetrical, reflecting a strong contribution of the recollection process, and linear, which reflects the lack of contribution of the familiarity process to recognition performance (figure 1C; see Yonelinas, 2002 for a review). Although linear ROC functions have been reported in a number of independent studies focusing on associative recognition memory (Arndt and Reder, 2001; Kelley and Wixted, 2001; Slotnick et al, 2000; Rotello et al, 2000), the finding of linear ROCs remained controversial because they have been rarely reported within the frame of the one model process (Wais et al, 2006). To determine whether the main process contributing to associative recognition memory in rats is the recollection process, we examined recognition memory performance of rats in an associative ROC paradigm involving the recognition of odours paired with media (Sauvage et al, 2008). Given that rats can separately attend to odors and media as distinct dimension when presented as an odour-medium pair (Birrell et al, 2000), we adapted the ROC protocol described in the previous section (Fortin et al, 2004) by presenting odour-medium pairs instead of simple odours. For example, among the 10 study pairs, cumin was mixed with beads and thyme with cotton balls. During the recognition phase, the same study pairs (old pairs) would be presented intermixed with ‘new’ rearranged odour-medium pairs (for example: cumin with cotton balls, thyme with beads). Using model-independent parameters (polynomial and linear regression functions) to assess the shape of the ROC function, and subsequently analyzing the data within the frame of the dual process model, we reported that the rat ROC function for associative recognition memory was asymmetrical and linear, reflecting a recollection-based performance with no significant contribution of the familiarity process, as observed in humans (figure 1D; Sauvage et al, 2008). This result clearly showed that recognition memory performance could be achieved without a significant contribution of the familiarity process, supporting the claim that recollection and familiarity are qualitatively distinct processes. Note that partisans of the one process model suggested that the linearity of the ROC function for associative recognition memory stemmed from the use of differential reward payoffs to manipulate response biases, rather than from memory demands (Wixted and Squire, 2008). Indeed, Wixted and colleague suggested that using different reward payoffs led to a ‘differential outcomes effect’, which in turn, was responsible for the linearity of the ROC function. A differential outcomes effect occurs when animals learn faster (as measured by an increase in performance accuracy), after hundreds of repetitions, stimulus-response reward combinations that yield a large reward than those that yield a smaller one. There are multiple reasons why this effect is not relevant to our protocol (see Eichenbaum et al, 2008 for details). First, a simple visual inspection of the rat ROC function for single odor recognition memory reveals that it is possible to obtain a curvilinear ROC function using the exact same differential reward payoffs but different memory demands (compare the ROC for item recognition: figure 1B to the ROC for associative recognition: figure 1D). This clearly suggests that the shape of ROC functions is tied to memory demands and not to the use of differential payoff rewards. Second, the ‘differential outcomes effect’ requires hundreds of stimulus-response reward pairings for the preferential learning to take place. However, in our paradigm, stimuli are showed only once and the reward is received only after the recognition judgment is completed. Hence, there is no possibility for the animal to predict the amount of reward that will be received, and this to affect its performance. Last but not least, opposite to the predictions of the ‘differential outcomes effect’, accuracy is actually the lowest for the bias which yields the largest difference between rewards for the ‘new’ stimulus and the ‘old’ stimuli (Bias 1: ¼ compared to 3 froot loops), while it is the highest for the bias for which there is NO difference between rewards for ‘old’ and ‘new’ stimuli (Bias 5: 1/2 froot loop in both cases). In conclusion, the differential outcomes effect is clearly not at work in our study, and the linearity of the rat ROC function for associative recognition memory is tied to memory demands and not to the use of differential payoff rewards.
In the present study, we have reported that the ROC function for associative recognition in rats is linear, reflecting a strong contribution of the recollection process to the memory for pairs, without significant contribution of the familiarity process, which suggests that the recollection and the familiarity processes are qualitatively distinct processes. Moreover, given that linear ROC functions for associative recognition memory have been reported in the human literature (Yonelinas 1997; Arndt and Reder, 2001; Slotnick et al, 2000; Rotello et al, 2000), even by the principal detractor of the dual-process model (Kelley and Wixted, 2001; see ROC functions for associative recognition with rearranged pairs), obtaining comparable ROC functions for associative recognition memory in rats constitutes a second step in the validation of animal ROC paradigms as proper translational tools to investigate the contribution of recollection and familiarity to recognition memory. In the next section, we focused on the missing piece of the puzzle, and studied whether recognition memory performance in rats could be achieved on the basis of the familiarity process only.
Can recognition memory performance solely rely on the familiarity process?
A counterpart to the previous experiment was to examine recognition memory performance under conditions that favour the familiarity process. Familiarity is usually described as a rapid process that is based on pattern matching, and is sensitive to perceptual modulations, whereas recollection is characterized as a slower and conceptually driven process (see Yonelinas, 2002; Mandler, 2008 for reviews). Indeed, studies involving process dissociation procedures (Yonelinas and Jacoby, 1994), response deadlines (Hintzman and Curran, 1994; 1998; Mc Elree, et al, 1999) and evoked response potentials or electroencephalograms (Smith, 1993; Curran, 2004; Duarte et al, 2006; Duzel et al, 1997; Woodruff et al, 2006) reveal a two-component temporal function that includes a rapid familiarity process and a slower recollective process. Consistent with this view, limiting appropriately the latency to respond in a recognition memory task should allow for the familiarity process to be fully completed but prevent a significant contribution of the recollection process to recognition memory performance. To test this hypothesis, we first assessed odour recognition memory performance in rats without a deadline, and obtained a typical ROC function for odour recognition memory: asymmetrical and curvilinear, reflecting the contribution of the recollection and the familiarity processes to recognition memory performance. However, when a deadline was subsequently applied, by giving the animals half the time to respond, the ROC function remained curvilinear, reflecting the contribution of the familiarity process to recognition memory, but became symmetrical (y-intercept not different from zero) suggesting that the recollection process did not contribute to recognition memory performance in a significant manner (figure 1E; Sauvage et al, 2010). These results suggested that under speeded conditions recognition memory performance relies essentially on the familiarity process, while the contribution of recollection is negligible, giving further support to the claim that recollection and familiarity are qualitatively distinct processes. Furthermore, this last experiment completed the validation of animal ROC paradigms as appropriate tools to study the contribution of familiarity and recollection to recognition memory in animals, given the animal ROC function under speeded conditions mirrored results obtained in the human literature under comparable conditions.
In conclusion, in this part of the review, we reported a double dissociation of the contribution of familiarity and recollection to episodic recognition memory in intact rats. This finding reveals that the contribution of each process depends on memory demands, and strongly supports the hypothesis that recollection and familiarity are qualitatively distinct processes. Moreover, we validated animal ROC paradigms as appropriate translational tools to investigate the contribution of recollection and familiarity to recognition memory, given that animal and human ROC studies yielded comparable results. In the following sections, we report findings that emerged from animal studies using ROC paradigms combined with lesions restricted to target areas to address issues that remained controversial in humans because of the difficulty of defining the extent of brain damage in patients with amnesia, or because of the impossibility of dissociating the precise source of activity in adjacent brain areas in healthy subjects.
Does the hippocampus support the recollection and the familiarity processes? evidence from rats with hippocampal dysfunction
A major controversy in recognition memory is whether the hippocampus supports the recollection process only or whether it also supports the familiarity process. Previous studies report a predominant role for the hippocampus in the recollection process while the parahippocampal region is suggested to primarily contribute to the familiarity process (Eichenbaum et al, 1994; Brown and Aggleton, 2001). However, conflicting results emerged from the ROC literature in humans. Evidence from studies on amnesic patients with damage restricted to the hippocampus suggests that the hippocampus specifically contributes to the recollection process but not to the familiarity process. Indeed, the y-intercept of the ROC function of patients with amnesia is significantly reduced compared to healthy subjects, while the curvilinear shape of the ROC function is maintained (Yonelinas et al, 1998; Yonelinas et al, 2002; Aggleton et al, 2005; Turriziani et al, 2008; see for a review Eichenbaum et al, 2007). In addition, studies focusing on specific areas of the parahippocampal region, the perirhinal cortex (PRc) and the entorhinal cortex (EC), report a preponderant role of these regions in the familiarity process (Bowles et al, 2007; Yonelinas et al, 2007; Haskins et al, 2008; for reviews see Diana et al, 2007; 2009). In striking contrast, other human studies report that both the recollection and the familiarity processes are affected following damage thought to be restricted to the hippocampus (Manns et al, 2003; see for a review Wixted and Squire 2004), reflected by an alteration in both the asymmetry and the curvilinear shape of the ROC function (Wais et al 2006). In addition, studies from the same group suggest that activity in the hippocampus and the PRc is correlated to the memory strength of remembered items, rather than to the specific contribution of these brain areas to the recollection or the familiarity processes to recognition memory (Shrager et al, 2008; for a review see Squire et al, 2007). These findings left the field of human recognition memory divided regarding the contribution of the hippocampus to the recollection and the familiarity processes. The controversy principally stems from the fact that using standard functional or structural MRI imaging techniques, it is impossible to determine with precision whether brain activity (or brain damage) is truly circumscribed to the hippocampus, or whether it also extends to areas adjacent to the hippocampus (the parahippocampal region). A clear advantage of animal studies over human studies is that brain damage can be generated in a very controlled and restricted manner using stereotactic surgery techniques. Hence, as a first step to study the contribution of the hippocampus to recollection and familiarity, we performed lesions unequivocally circumscribed to the hippocampus, and assessed the effect of this selective lesion on the contribution of the recollection and the familiarity processes to item and associative recognition memory using animal ROC paradigms. In addition, we report later animal ROC findings related to the ethological model of reduced hippocampal function that is aging. Of note, data from these experiments have been analyzed with model-independent parameters (linear and polynomial regressions) to evaluate the shape of the ROC functions, with the dual-process model and with the one-process model (see Fortin et al, 2004; Robitsek et al, 2008; Sauvage et al, 2008 for details). In each experiment, analysis with the one-process model confirmed the dual-process findings by revealing that reducing hippocampal function selectively eliminats one parameter of the ROC function, specifically the inequality of variances between ‘old’ and ‘new’ items. By contrast, no change was observed in the other parameter of the ROC function, d', which reflects the difference in memory strength between ‘old’ and ‘new’ items. In summary, analysis with the one-process model confirmed that hippocampal lesions and aging selectively eliminated one parameter supporting the identification of old items, which is comparable to altering the recollection process in the dual-process theory, and left intact another parameter comparable to familiarity.
Contribution of the hippocampus to recollection and familiarity in item recognition memory?
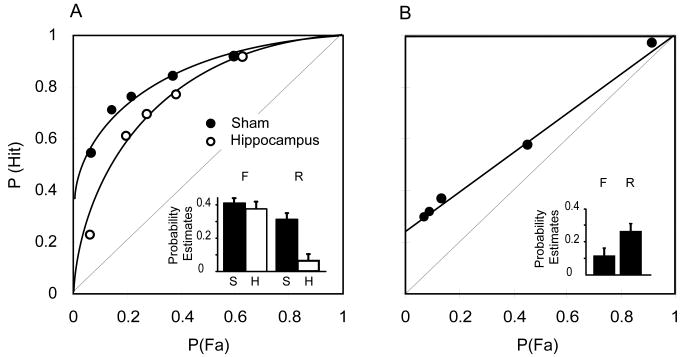
In 2004, Fortin and colleagues performed lesions restricted to the hippocampus in rats and reported that the ROC function of hippocampal-damaged rats remained curvilinear, reflecting the contribution of the familiarity process to recognition memory. In addition, the ROC function became fully symmetrical suggesting that the recollection process did not contribute significantly to recognition memory performance of hippocampal-lesioned rats (figure 3A, Hippocampus). This finding was the first evidence that damage unequivocally restricted to the hippocampus significantly impaired the recollection process without affecting the familiarity process, which suggested that the hippocampus supports recollection but not familiarity. Of note, opponents of the dual-process model argued that hippocampal lesions did not specifically affect the recollection process but rather reduced overall memory performance because the accuracy level of the hippocampus group was slightly but significantly reduced compared to that of sham rats. To test this hypothesis, Fortin and colleagues lowered the overall memory performance of sham rats by increasing the delay between study and recognition phases (Yonelinas et al, 2002), and studied the effect of this manipulation on the contribution of recollection and familiarity to recognition memory. Against the predictions of the one-process model, the ROC function of sham rats became linear and asymmetrical (figure 3B) instead of curvilinear and symmetrical as observed following hippocampal lesion (figure 3A, Hippocampus). This manipulation clearly stated that reducing overall recognition memory performance affected the familiarity process and not the recollection process, and therefore could not account for the recollection deficit observed after hippocampal lesion.
Figure 3.
Item recognition ROC in rats with circumscribed hippocampal lesion (graph from Fortin et al., 2004). A. Hippocampal lesion eliminates recollection-based performance while sparing the familiarity process. B. ROC function in sham rats with extended memory delay. Extending the delay significantly affects familiarity not recollection.
In summary, this study showed for the first time that the hippocampus supports recollection and not familiarity, given that lesions were specifically restricted to the hippocampus and only recollection was affected. Moreover, these data confirmed the results obtained in intact rats suggesting that recollection and familiarity are qualitatively distinct processes since the recollection and the familiarity processes were not affected in a similar manner following hippocampal lesion.
Contribution of the hippocampus to recollection and familiarity in associative recognition memory?
To study further the contribution of the hippocampus to the familiarity and the recollection processes, we studied recognition memory performance for pairs using the associative ROC paradigm described in Sauvage et al, 2008. Rats were presented with a study list of odours paired with media, and recognition memory performance was subsequently assessed by presenting the same pairs intermixed with rearranged odour-medium pairs. As previously described, the ROC function for associative recognition memory was asymmetrical and linear, reflecting a recollection-based memory performance with no significant contribution of the familiarity process (figure 4, Sham). In striking contrast, recollection was significantly impaired following hippocampal lesion as reflected by a significant drop of the y-intercept (index of recollection, R), suggesting that the hippocampus is critical for recollection in associative recognition memory. Even more interesting, the ROC function of hippocampal-lesioned rats was now curvilinear reflecting a significant contribution of the familiarity process to recognition memory following hippocampal damage (figure 4, Hippocampus). This finding was the first clear evidence in the human and animal literature that the hippocampus did not support the familiarity process since the contribution of the familiarity process to recognition memory increased following hippocampal damage. An alternative interpretation of these data, in line with the predictions of the one-process model, was recently voiced. It has been argued that if the hippocampus supports familiarity and recollection, and if recollection judgements are ‘more demanding’ than familiarity judgments, and the lesion of the hippocampus is only partial, it could be possible that hippocampal lesions significantly affect the recollection process, but that enough of the hippocampus remains to complete the ‘less demanding’ familiarity judgments. As much as this could theoretically be the case in humans, for example in mild cases of stroke (assuming that damage remains in the vicinity of blood vessels or brain arteries, and potentially leaves part of the hippocampus and its connections intact), it is highly improbable in our animal studies given the type of lesion we perform. Indeed, our surgery aims at lesioning the hippocampus by applying numerous small lesions (twenty-four total; twelve per hemisphere) spread along the rostro-caudal, dorso-ventral and medio-lateral axis. Hence, even if the lesion of the hippocampus is not total, the remaining hippocampal ‘bits’ are definitively disconnected and unlikely to be functional. Second, if in agreement with the single-process model, the hippocampus supports familiarity and recollection, the prediction of this model would be that hippocampal damage should also impair familiarity (even if it affects much more the recollection process), which is opposite to the results obtained in the present study, since a significant increase of the contribution of familiarity to recognition memory was reported. Finally, it is also important to underline that the specific recollection impairment observed in the Hippocampus group cannot be explained by a difference in memory strength between the Hippocampus and the Sham groups, since overall recognition memory performance did not significantly differ between the Hippocampus group and the Sham rats (Sauvage et al, 2008). Thus, the ‘memory strength hypothesis’ could not apply in the present study, and our data suggested that rats without a functional hippocampus recognized pairs to a similar level as sham rats but used an alternate strategy to solve the task. Interestingly, damage to the hippocampus in rats was reported to increase the tendency to unitize stimulus elements of a pair into a single stimulus; for example, lemon and sand could be encoded as lemon-scented sand by rats with a hippocampal lesion (Eichenbaum et 1994), and such a strategy was also observed in patients with amnesia. Indeed, amnesic patients who performed very poorly on memory tasks that use unrelated items as stimulus pairs, were reported to perform better on associative recognition memory tasks when given the possibility to rely more heavily on familiarity. For example, when compound words such as fireman, hardware or sleepwalk etc… were used as stimulus pairs instead of unrelated words, and patients could consider both elements of a pair as a single item (Giovanello et al, 2006; Quamme et al 2007).
Figure 4.
Associative recognition ROC of rats with restricted hippocampal lesion (graph from Sauvage et al., 2008). Hippocampal lesion affects recollection and familiarity in an opposite manner. Hippocampal damage decreases the recollection index (R: y-intercept) while increasing the familiarity index (F).
In summary, this study provided the first clear evidence that the hippocampus did not support familiarity, because the contribution of familiarity to recognition memory performance was enhanced following damage restricted to the hippocampus. It also revealed that the hippocampus supports the recollection process in associative recognition memory, as it is the case in item recognition memory. Furthermore, it strongly suggests that familiarity and recollection are qualitatively distinct processes because they can vary in an opposite manner.
Contribution of the hippocampus to recollection and familiarity in aging?
Finally, the finding of a selective contribution of the hippocampus to recollection and not familiarity was further supported by results from a ROC study with aged rats, used as a model of reduced hippocampal function (Rosenzweig and Barnes, 2003; Wilson et al., 2006; Daselaar et al, 2006). In this study, the ROC function for item recognition of memory-impaired rats was found to be symmetrical and curvilinear, as it was the case following hippocampal lesion, suggesting that reducing hippocampal function limits the contribution of recollection to recognition memory while sparing the familiarity process (figure 5, Robitsek et al, 2008). Interestingly, as observed in associative recognition memory, the contribution of the familiarity process to recognition memory performance was increased in a subset of the memory-impaired rats, confirming in an ethologically relevant model that the hippocampus supports recollection and not familiarity, and that the two processes are qualitatively distinct since they can vary in an opposite manner.
In conclusion, data from ROC studies in rats with reduced hippocampal function (lesion and aging studies) confirmed results in intact rats by showing that the recollection and the familiarity processes are qualitatively distinct, because they can vary independently when hippocampal function is compromised. In addition, these data brought convincing evidence that the hippocampus supports recollection but not familiarity, given the contribution of the recollection process to single item and associative recognition memory is significantly reduced following hippocampal lesion, whereas the contribution of familiarity is either unaltered or enhanced. In this section, I have discussed how animal ROC paradigms contributed to characterize the role of the hippocampus in recollection and familiarity in recognition memory by studying animals with hippocampal dysfunction. In the following section, I report how animal ROC paradigms were used to identify brain structures, upstream of the hippocampus, that provide information necessary to complete recollection based-judgments to the hippocampus.
What areas provide information required for recollection-based judgments to the hippocampus?
Here, I will address a second issue that considerably fuels the current debate in recognition memory, which is the investigation of the functional segregation of the parahippocampal region in terms of recollection and familiarity. More precisely, I report here the first step of a series of studies which aim at characterizing the specific contribution of each area of the parahippocampal region to recollection and familiarity, starting with the medial entorhinal cortex (MEC), which has recently drawn a lot of attention for its dedicated role in spatial navigation and path integration (Fyhn et al, 2004; Hafting et al 2005, see for reviews McNaughton et al, 2006; Moser and Moser, 2008).
The parahippocampal region includes the perirhinal cortex (PRc), the parahippocampal cortex (PHc), the lateral entorhinal cortex (LEC) and the medial entorhinal cortex (MEC), which share intricate and bidirectional projections (see for a review Van Strien et al, 2009). Despite this complex network of connections, data emerging from the human literature suggest that some of the areas of the parahippocampal region support the recollection process, while others contribute the familiarity process (for reviews see Eichenbaum et al 2007; Diana et al, 2007; 2009). According to these studies, the PRc and the LEC would process information about the familiarity of individual items, whereas the PHc and the MEC would support recollection by representing spatial and temporal contexts (whether items are old or new) in which items have been experienced. Subsequently, both the item and contextual information would be combined within the hippocampus (see for a review Lipton and Eichenbaum, 2008). Thus, human fMRI studies report that activity in the PRc is correlated to familiarity-based judgments in recognition memory tasks (Davachi et al., 2003; Henson et al., 2003; Ranganath et al., 2003; Montaldi et al., 2006; Suchan et al, 2008; Haskins et al, 2008). In addition, ablation of the same structure, in a patient suffering from an intractable case of epilepsy, led to deficits specifically tied to the familiarity process (Bowles et al, 2007). In contrast, evidence from imaging studies points towards a preponderant role of the PHc and the hippocampus in the recollection of single items and associations (Eldridge et al, 2000; Cansino et al, 2002; Yonelinas et al, 2005; Woodruff et al, 2005; Aminoff et al 2008, Suchan et al, 2008). The contribution of the MEC and LEC to recollection and familiarity has been essentially extrapolated from their anatomical connections to the PHc and the PRc, because it is not possible with fMRI imaging techniques to dissociate the activity that occurs in the medial part of the EC during a memory task from that occurring in the lateral part of the EC. Moreover, no patients with damage restricted to the MEC or LEC have been reported to this date. Thus, as a part of the PHc-MEC complex, the MEC is suggested to play a preponderant role in the recollection process. Conversely, the LEC would contribute more specifically to the familiarity process, as a part of the PRc-LEC complex. However, direct evidence of these selective contributions is missing. To investigate the involvement of the MEC in the recollection process, we studied the effect of damage restricted to the MEC on the contribution of recollection and familiarity to odour recognition memory (Sauvage et al, 2010b). Following MEC lesions, the ROC function remained curvilinear, reflecting a strong contribution of the familiarity process (figure 6, MEC). However, in sharp contrast to the sham rats, the ROC function of MEC rats became symmetrical (y-intercept not different from 0), suggesting that the recollection process no longer significantly contributed to the recognition performance. These results provided the first evidence that the MEC contributes specifically to recollection based-judgments, whereas its contribution to the familiarity process is minimal. These findings are consistent with a broader view of the MEC function in recognition memory than its dedicated role in spatial representation, since MEC damage significantly altered performance in our non-spatial (odour) recognition memory task. In agreement with this finding, a recent electrophysiological study reported that dorsocaudal MEC (dcMEC) neurons had distinct activity patterns depending on whether rats turned left or right on a T-maze in an alternation working memory task (Lipton et al., 2007). This finding suggested that dcMEC cells signaled which of the two episodes was ongoing, and were encoding spatial and temporal contexts (Lipton and Eichenbaum, 2008). Similar findings regarding the representation of spatial and temporal contexts were reported for the PHc, which is adjacent and strongly connected to the MEC. Indeed, the PHc was commonly found to be activated during the viewing of spatial environments, of objects that evoke strong contextual associations and during the viewing of objects that have strong temporal associations (Epstein & Kanwisher, 1998; Aminoff et al., 2007; Bar et al., 2008). Thus, the PHc which is strongly connected with the MEC was also suggested to represent the spatial and temporal context of remembered objects.
Figure 6.
Item recognition ROC of rats with restricted lesion to the MEC (graph from Sauvage et al, 2010b). MEC lesion reduces the contribution of recollection to recognition memory while familiarity remained unaffected.
In light of previous studies focusing on the selective contribution of the PRc to the familiarity process, the present study brought further support to the hypothesis of a functional segregation within the parahippocampal region, by showing that MEC is not involved in familiarity for specific items but, is a part of the parahippocampal and medial entorhinal network that is essential to memory for the context in which events occur, a defining feature of episodic recollection (Eichenbaum et al., 2007). This perspective is consistent with the observations on the dcMEC in spatial representation (grid cells), but suggests a broader role in the representation of spatial and temporal contexts which is of importance to episodic memory.
In summary, results from this study suggested once more that recollection and familiarity are qualitatively distinct processes, since damage restricted to the MEC affected recollection while sparing familiarity. This study also allowed us to firmly conclude that the MEC contributes selectively to the recollection process, possibly by providing information regarding the temporal context of the remembered items to the hippocampus (whether items are ‘old’ or ‘new’). This study is the first of a series of ROC studies in animals focusing on the investigation of the functional segregation of the parahippocampal region in terms of recollection and familiarity, and calls for further studies to characterize the contribution of other areas of the parahippocampal region, for example the LEC, for which virtually no data are available.
In conclusion, developing ROC paradigms in animals contributed to bridging recognition memory in humans and animals by assessing and analyzing recognition memory performance in a comparable manner. In addition, combining circumscribed brain damage in animals to the use of animal ROC paradigms allowed for major controversies in human recognition memory to be addressed, and led consistently to the conclusion that familiarity and recollection are qualitatively distinct processes, and that the hippocampus supports recollection but not familiarity. Finally, we started to apply this approach to the characterization of the specific contribution of the parahippocampal areas to recollection and familiarity in recognition memory and are confident that this translational approach, combined to more standard approaches, will contribute to significant progress in the elucidation of the neural substrates of recognition memory.
Acknowledgments
We thank Jarret Frank for his help in Graphic designs and Zachery Beer for proof-reading. These experiments were funded by MH71702, MH51520, MH52090 and AG09973.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, Vann SD, Denby C, Dix S, Mayes AR, Roberts N, Yonelinas AP. Sparing of the familiarity component of recognition memory in a patient with hippocampal pathology. Neuropsychologia. 2005;43(12):1810–23. doi: 10.1016/j.neuropsychologia.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Aminoff E, Gronau N, Bar M. The parahippocampal cortex mediates spatial and nonspatial associations. Cereb Cortex. 2007;17(7):1493–503. doi: 10.1093/cercor/bhl078. [DOI] [PubMed] [Google Scholar]
- Arndt J, Reder LM. Word frequency and receiver-operating characteristic curves in recognition memory: Evidence for a dual-process interpretation. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2002;28(5):830–42. doi: 10.1037//0278-7393.28.5.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson RC, Juola JF. Factors influencing speed and accuracy of word recognition. In: Kornblum S, editor. Attention and performance. Vol. 4. new york: Academic Press; 1973. pp. 583–612. [Google Scholar]
- Atkinson RC, Juola JF. Search and decision processes in recognition memory. In: krantz DH, Atkinson RC, Luce RD, editors. Contemporary developments in mathematical psychology. San Francisco: W.H. Freeman; 1974. pp. 243–293. [Google Scholar]
- Bar M, Aminoff E, Schacter DL. Scenes unseen: the parahippocampal cortex intrinsically subserves contextual associations, not scenes or places per se. J Neurosci. 2008;28(34):8539–44. doi: 10.1523/JNEUROSCI.0987-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeau EJ, Felician O, Joubert S, Sontheimer A, Ceccaldi M, Poncet M. Preserved visual recognition memory in an amnesic patient with hippocampal lesions. Hippocampus. 2005;15(5):587–96. doi: 10.1002/hipo.20079. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles B, Crupi C, Mirsattari SM, Pigott SE, Parrent AG, Pruessner JC, Yonelinas AP, Köhler S. Impaired familiarity with preserved recollection after anterior temporal-lobe resection that spares the hippocampus. Proc Natl Acad Sci USA. 2007;104(41):16382–7. doi: 10.1073/pnas.0705273104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt KR, Gardiner JM, Vargha-Khadem F, Baddeley AD, Mishkin M. Impairment of recollection but not familiarity in a case of developmental amnesia. Neurocase. 2008;15(1):60–5. doi: 10.1080/13554790802613025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus. Nature Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Craik FI, Byrd M. Aging and cognitive deficits: the role of attentional resources. In: Craik FI, Trehub S, editors. Aging and cognitive processes. 1982. pp. 51–110. [Google Scholar]
- Curran T. Effects of attention and confidence on the hypothesized ERP correlates of recollection and familiarity. Neuropsychologia. 2004;42:1088–106. doi: 10.1016/j.neuropsychologia.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event related fMRI study. Cereb Cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007;11(9):379–86. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Medial Temporal Lobe Activity during Source Retrieval Reflects Information Type, not Memory Strength. J Cogn Neurosci. 2009 doi: 10.1162/jocn.2009.21335. online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Trujillo C, Knight RT. Intact recollection memory in high performing older adults: ERP and behavioral evidence. J Cogn Neurosci. 2006;18(1):33–47. doi: 10.1162/089892906775249988. [DOI] [PubMed] [Google Scholar]
- Duverne S, Habibi A, Rugg MD. Regional specificity of age effects on the neural correlates of episodic retrieval. Neurobiol Aging. 2008;29(12):1902–16. doi: 10.1016/j.neurobiolaging.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Duzel E, Yonelinas AP, Mangun GR, Heinzem HJ, Tulving E. Event-related brain potential correlates of two states of conscious awareness in memory. Proc Natl Acad Sci USA. 1997;94(11):5973–78. doi: 10.1073/pnas.94.11.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Düzel E, Vargha-Khadem F, Heinze HJ, Mishkin M. Brain activity evidence for recognition without recollection after early hippocampal damage. Proc Natl Acad Sci U S A. 2001;98(14):8101–6. doi: 10.1073/pnas.131205798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. In: Memory Systems. Schacter DL, Tulving E, editors. MIT Press; Cambridge, Massachusetts: 1994. pp. 147–202. Q5. [Google Scholar]
- Eichenbaum H, Otto T, Cohen NJ. Two functional components of the hippocampal memory system. Behav Brain Sci. 1994;17:449–472. 472–518. [Google Scholar]
- Eichenbaum H, Sauvage M, Fortin N, Yonelinas AP. ROCs in Rats? Response to Wixted and Squire. Learning and Memory. 2008;15(9):691–3. doi: 10.1101/lm.1133808. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AR, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin NJ, Wright SP, Eichenbaum H. Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature. 2004;431(7005):188–91. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyhn M, Molden S, Witter MP, Moser EI, Moser MB. Spatial representation in the entorhinal cortex. Science. 2004;305(5688):1258–64. doi: 10.1126/science.1099901. [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Keane MM, Verfaellie M. The contribution of familiarity to associative memory in amnesia. Neuropsychologia. 2006;44(10):1859–65. doi: 10.1016/j.neuropsychologia.2006.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436(7052):801–6. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Haskins AL, Yonelinas AP, Quamme JR, Ranganath C. Perirhinal cortex supports encoding and familiarity-based recognition of novel associations. Neuron. 2008;59(4):554–60. doi: 10.1016/j.neuron.2008.07.035. [DOI] [PubMed] [Google Scholar]
- Hintzman DL, Caulton DA, Levitin DJ. Retrieval dynamics in recognition and list discrimination: further evidence of separate processes of familiarity and recall. Mem Cognit. 1998;26(3):449–62. doi: 10.3758/bf03201155. [DOI] [PubMed] [Google Scholar]
- Hintzman DL, Curran T. Retrieval dynamics of recognition and frequency judgments:evidence for separate processes of familiarity and recall. J Mem Lang. 1994;331:1–18. [Google Scholar]
- Howard MW, Bessette-Symons B, Zhang Y, Hoyer WJ. Aging selectively impairs recollection in recognition memory for pictures: evidence from modelling and receiver operating characteristic curves. Psychol Aging. 2006;21:96–106. doi: 10.1037/0882-7974.21.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley R, Wixted JT. On the nature of associative information in recognition memory. J Exp Psychol Learn Mem Cogn. 2001;27(3):701–22. [PubMed] [Google Scholar]
- Knowlton BJ, Squire LR. Remembering and knowing: two different expressions of declarative memory. J Exp Psychol Learn Mem Cogn. 1995;21(3):699–710. doi: 10.1037//0278-7393.21.3.699. [DOI] [PubMed] [Google Scholar]
- Lipton PA, Eichenbaum H. Complementary roles of hippocampus and medial entorhinal cortex in episodic memory. Neural Plast. 2008;2008:258467. doi: 10.1155/2008/258467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandler G. Recognizing: The judgment of previous occurrence. Psychological Review. 1980;87:252–271. [Google Scholar]
- Mandler G. Familiarity breeds attempts: A critical review of dual process theories of recognition. Perspectives in Psychological Science. 2008;3(5):392–401. doi: 10.1111/j.1745-6924.2008.00087.x. [DOI] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H. Evolution of Declarative Memory. Hippocampus. 2006;16:795–808. doi: 10.1002/hipo.20205. [DOI] [PubMed] [Google Scholar]
- Manns JR, Hopkins RO, Reed JM, Kitchener EG, Squire LR. Recognition memory and the human hippocampus. Neuron. 2003;37:171–180. doi: 10.1016/s0896-6273(02)01147-9. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the ‘cognitive map’. Nat Rev Neurosci. 2006;7(8):663–78. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- McElree B, Dolan PO, Jacoby LL. Isolating the contributions of familiarity and source information to item recognition: a time course analysis. J Exp Psychol Learn Mem Cogn. 1999;253:563–82. doi: 10.1037//0278-7393.25.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaldi D, Spencer TJ, Roberts N, Mayes AR. The neural system that mediates familiarity memory. Hippocampus. 2006;16(5):504–20. doi: 10.1002/hipo.20178. [DOI] [PubMed] [Google Scholar]
- Moser EI, Moser MB. A metric for space. Hippocampus. 2008;18(12):1142–56. doi: 10.1002/hipo.20483. [DOI] [PubMed] [Google Scholar]
- Prull MW, Dawes LL, Martin AM, 3rd, Rosenberg HF, Light LL. Recollection and familiarity in recognition memory: adult age differences and. Psychology Human Learning and Memory. 2006;28(6):1499–1517. doi: 10.1037/0882-7974.21.1.107. [DOI] [PubMed] [Google Scholar]
- Quamme JR, Yonelinas AP, Widaman KF, Kroll NEA, Sauve MJ. Recall and recognition in mild hypoxia: Using covariance structural modeling to test competing theories of explicit memory. Neuropsychologia. 2004;42:672–691. doi: 10.1016/j.neuropsychologia.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Quamme JR, Yonelinas AP, Norman KA. Effect of unitization on associative recognition in amnesia. Hippocampus. 2007;17(3):192–200. doi: 10.1002/hipo.20257. [DOI] [PubMed] [Google Scholar]
- Reed JM, Squire LR. Impaired recognition memory in patients with lesions limited to the hippocampal formation. Behavioral Neuroscience. 1997;111:667–675. doi: 10.1037//0735-7044.111.4.667. [DOI] [PubMed] [Google Scholar]
- Robitsek RJ, Fortin NJ, Koh MT, Gallagher M, Eichenbaum H. Cognitive aging: a common decline of episodic recollection and spatial memory in rats. J Neurosci. 2008;28(36):8945–54. doi: 10.1523/JNEUROSCI.1893-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol. 2003;69:143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Rotello CM, Macmillan NA, Van Tassel G. Recall-to-reject in recognition: Evidence from ROC curves. Journal of Memory and Language. 2000;43(1):67–88. [Google Scholar]
- Sauvage M, Fortin NJ, Owens CB, Yonelinas AP, Eichenbaum H. Recognition memory opposite effects of hippocampal damage on recollection and familiarity. Nat Neurosc. 2008;11(1):16–8. doi: 10.1038/nn2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvage M, Beer Z, Eichenbaum H. Recognition memory: adding a response deadline eliminates recollection but spares familiarity. Learn Mem. 2010a;17(2):104–8. doi: 10.1101/lm.1647710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvage M, Beer Z, Ekovich M, Ho L, Eichenbaum H. The Medial Entorhinal Cortex: a selective role in recollection-based memory. J Neurosci. 2010b doi: 10.1523/JNEUROSCI.4301-10.2010. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Verfaellie M, Anes MD. Illusory memories in amnesic patients: conceptual and perceptual false recognition. Neuropsychology. 1997;11(3):331–42. doi: 10.1037//0894-4105.11.3.331. [DOI] [PubMed] [Google Scholar]
- Shrager Y, Kirwan CB, Squire LR. Activity in both hippocampus and perirhinal cortex predicts the memory strength of subsequently remembered information. Neuron. 2008;59(4):547–53. doi: 10.1016/j.neuron.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick SD, Klein SA, Dodson CS, Shimamura P. An analysis of signal detection and threshold models of source memory. Journal of Experimental Psychology: Human Learning and Memory. 2000;28(6):1499–1517. doi: 10.1037//0278-7393.26.6.1499. [DOI] [PubMed] [Google Scholar]
- Smith ME. Neurophysiological manifestations of recollective experience during recognition memory judgments. J Cog Neurosci. 1993;5(1):1–13. doi: 10.1162/jocn.1993.5.1.1. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Annu Rev Neurosci Review. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007;8(11):872–83. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchan B, Gayk AE, Schmid G, Köster O, Daum I. Hippocampal involvement in recollection but not familiarity across time: a prospective study. Hippocampus. 2008;18(1):92–8. doi: 10.1002/hipo.20371. [DOI] [PubMed] [Google Scholar]
- Stark CE, Squire LR. Functional magnetic resonance imaging (fMRI) activity in the hippocampal region during recognition memory. J Neurosci. 2000;20:7776–7781. doi: 10.1523/JNEUROSCI.20-20-07776.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turriziani P, Fadda L, Caltragirone C, Carlesimo GA. Recognition memory for single items and for associations in amnesic patients. Neuropsychologia. 2004;42:426–433. doi: 10.1016/j.neuropsychologia.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Turriziani P, Serra L, Fadda L, Caltagirone C, Carlesimo GA. Recollection and familiarity in hippocampal amnesia. Hippocampus. 2008;18(5):469–80. doi: 10.1002/hipo.20412. [DOI] [PubMed] [Google Scholar]
- Vann SD, Tsivilis D, Denby CE, Quamme JR, Yonelinas AP, Aggleton JP, Montaldi D, Mayes AR. Impaired recollection but spared familiarity in patients with extended hippocampal system damage revealed by 3 convergent methods. Proc Natl Acad Sci U S A. 2009;106(13):5442–7. doi: 10.1073/pnas.0812097106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Strien NM, Cappaert NL, Witter MP. The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci. 2009;10(4):272–82. doi: 10.1038/nrn2614. Review. [DOI] [PubMed] [Google Scholar]
- Wais PE, Wixted JT, Hopkins RO, Squire LR. The hippocampus supports both the recollection and the familiarity components of recognition memory. Neuron. 2006;49(3):459–66. doi: 10.1016/j.neuron.2005.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wixted JT, Squire LR. Recall and recognition are equally impaired in patients with selective hippocampal damage. Cogn Affect Behav Neurosci. 2004;4(1):58–66. doi: 10.3758/cabn.4.1.58. Review. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Gallagher M, Eichenbaum H, Tanila H. Neurocognitive aging: prior memories hinder new hippocampal encoding. Trends Neurosci. 2006;29:662–670. doi: 10.1016/j.tins.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wixted JT. Dual-process theory and signal-detection theory of recognition memory. Psychol Rev. 2007;114(1):152–76. doi: 10.1037/0033-295X.114.1.152. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Squire LR. Constructing receiver operating characteristics (ROCs) with experimental animals: cautionary notes. Learn Mem. 2008;15(9):687–90. doi: 10.1101/lm.1077708. [DOI] [PubMed] [Google Scholar]
- Woodruff CC, Johnson JD, Uncapher MR, Rugg MD. Content-specificity of the neuralcorrelates of recollection. Neuropsychologia. 2005;43:1022–32. doi: 10.1016/j.neuropsychologia.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Woodruff CC, Hayama HR, Rugg MD. Electrophysiological dissociation of the neural correlates of recollection and familiarity. Brain Res. 2006;1100(1):125–35. doi: 10.1016/j.brainres.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Receiver-operating characteristics in recognition memory: evidence for a dual-process model. J Exp Psychol Learn Mem Cogn. 1994;20(6):1341–54. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Jacoby LL. Dissociations of processes in recognition memory: effects of interference and of response speed. Can J Exp Psychol. 1994;48(4):516–35. doi: 10.1037/1196-1961.48.4.516. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Recognition memory ROCs for item and associative information: the contribution of recollection and familiarity. Mem Cognit. 1997;25(6):747–63. doi: 10.3758/bf03211318. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NE, Dobbins I, Lazzara M, Knight RT. Recollection and familiarity deficits in amnesia: convergence of remember-know, process dissociation, and receiver operating characteristic data. Neuropsychology. 1998;12:323–339. doi: 10.1037//0894-4105.12.3.323. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The contribution of recollection and familiarity to recognition and sourcememoryjudgments: a formal dual-process model and an analysis of receiver operating characteristics. J Exp Psychol Learn Mem Cogn. 1999;25:1415–34. doi: 10.1037//0278-7393.25.6.1415. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: a review of 30 years of research. J Mem Lang. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Kroll NE, Quamme JR, Lazzara MM, Sauvé MJ, Widaman KF, Knight RT. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nature Neurosci. 2002;5:1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Parks CM. Receiver operating characteristics (ROCs) in recognition memory: a review. Psychol Bull. 2007;133(5):800–32. doi: 10.1037/0033-2909.133.5.800. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Widaman K, Mungas D, Reed B, Weiner MW, Chui HC. Memory in the aging brain: doubly dissociating the contribution of the hippocampus and entorhinal cortex. Hippocampus. 2007;17(11):1134–40. doi: 10.1002/hipo.20341. [DOI] [PMC free article] [PubMed] [Google Scholar]