Abstract
Here we have assessed the effects of extracellular matrix (ECM) composition and rigidity on mechanical properties of the human airway smooth muscle (ASM) cell. Cell stiffness and contractile stress showed appreciable changes from the most relaxed state to the most contracted state: we refer to the maximal range of these changes as the cell contractile scope. The contractile scope was least when the cell was adherent upon collagen V, followed by collagen IV, laminin, and collagen I, and greatest for fibronectin. Regardless of ECM composition, upon adherence to increasingly rigid substrates, the ASM cell positively regulated expression of antioxidant genes in the glutathione pathway and heme oxygenase, and disruption of a redox-sensitive transcription factor, nuclear erythroid 2 p45-related factor (Nrf2), culminated in greater contractile scope. These findings provide biophysical evidence that ECM differentially modulates muscle contractility and, for the first time, demonstrate a link between muscle contractility and Nrf2-directed responses.
Keywords: Oxidative stress, Nrf2, ECM, Airway smooth muscle, Airway hyperreactivity, Asthma
Introduction
Asthma is a complex respiratory disorder in which T helper-2 mediated inflammation interacts with a number of structural changes in the conducting airway to cause variable airflow limitation, including airway hyperreactivity [1]. Airway hyperreactivity is the excessive narrowing of the airway lumen [2] and underlies the morbidity and the mortality that are attributable to the disease. But, the mechanism explaining this pathologic narrowing is incompletely understood.
One of the prominent structural changes associated with asthma fatality is thickening of the various airway wall compartments, including the reticular basement membrane, the submucosa, and even the adventitia [3–5]. This subepithelial remodeling is, in large part, due to accumulation of airway smooth muscle (ASM) [6], as well as alterations in the amount and composition of extracellular matrix (ECM) [7,8]. ECM comprises the physicochemical microenvironment surrounding ASM. However, the mechanistic link between altered deposition of ECM and muscle pathophysiology as they relate to airway hyperreactivity remains unclear.
There is agreement that increased muscle mass and/or muscle contractility would predispose toward this excessive airway narrowing in asthma [9]. In that connection, several ECM constituents have been shown to modulate not only synthetic [10], proliferative [11] and migratory [12] functions of the ASM cell in culture, but also biochemical pathways that are implicated in muscle maturation and contraction [11,13]. Findings reported thus far are limited to cellular expression of smooth muscle markers, however, and it remains unknown if the ASM cell in the synthetic, proliferative or migratory state might be less contractile than a similar cell differentiated into fully the contractile state [10–14]. Moreover, how the contractile state of a muscle might be affected by altered deposition and turnover of ECM remains to be elucidated.
To fill that gap of knowledge, we focused here upon mechanical properties of the human ASM cell and, in particular, the role of ECM in these processes. Under both growth and growth-arrested conditions, we measured the abilities of an individual ASM cell to stiffen and to generate contractile force. To measure cell stiffness we used optical magnetic twisting cytometry (OMTC) and to measure contractile force we used traction microscopy. Using these quantitative methods, we present evidence that ECM differentially affects mechanical reactivity of the human ASM cell and that the magnitude of such reactivity is closely correlated with the activity of a redox-sensitive transcription factor, Nrf2, which has been shown to play a critical role in allergen-induced oxidative stress and airway hyperreactivity in mice [15].
Materials and methods
Materials
Tissue culture reagents and laminin (LN) were obtained from Sigma (St. Louis, MO). Fibronectin (FN) and collagen (Col) types I, IV and V were purchased from Calbiochem (San Diego, CA). The synthetic arginine-glycine-aspartic acid (RGD) containing peptide was purchased from American Peptide Company (Sunnyvale, CA). L-Sulforaphane and N-acetyl cysteine (NAC) were purchased from LKT Laboratories (St. Paul, MN) and Sigma, respectively. Unless otherwise noted, all other reagents were obtained from Fisher Scientific (Newark, DE).
Animals and care
Nrf2-deficient CD1:ICR mice (SLC Japan Inc.) were generated by replacing the b-ZIP region of the Nrf2 gene with the SV40 nuclear localization signal and β-galactosidase gene as described [15] and provided by Dr. Masayuki Yamamoto (Tohoku University, Japan). All experimental protocols conducted on the mice were performed in accordance with the standards established by the US Animal Welfare Acts, as well as the Policy and Procedures Manual of the Johns Hopkins University Animal Care and Use Committee.
Cell isolation and culture
Mouse ASM cells were prepared from the trachealis of Nrf2-wild type (Nrf2+/+) and Nrf2-deficient (Nrf2−/−) mice (male; 6–8 weeks old) using an enzymatic digestion [16]. Human ASM cells were provided by Dr. Reynold A. Pannettieri, Jr. (University of Pennsylvania). Cells were grown until confluence at 37°C in humidified air containing 5% CO2 and passaged with 0.25% trypsin–0.02% EDTA solution every 10–14 days. Unless otherwise specified, serum-deprived post-confluent cells were plated at 30,000 cells/cm2 on substrates coated with 500 ng/cm2 type I collagen.
Surface coating with ECM proteins
Using standard, well-established coating procedures [11–13], both the tissue culture plastic (96- and 24-well plates) and the inert polyacrylamide elastic (gel blocks) substrates were coated with FN, LN, Col I, Col IV or Col V; proteins (100 μg/ml) were diluted in PBS. In addition, as described by Engler and colleagues [17,18], a mixture of acrylamide (5–10%) and bis-acrylamide (0.03–0.3%) was used to vary the rigidity of gel blocks (~1–20 kPa) in the physiological range [19].
Optical magnetic twisting cytometry (OMTC)
Stiffness of the adherent ASM cell was measured as described by us in detail elsewhere [16,20]. In brief, an RGD-coated ferrimagnetic microbead (4.5 μm in diameter) bound to the surface of the cell was magnetized horizontally and then twisted in a vertically aligned homogenous magnetic field that varied sinusoidally in time. Here we defined the ratio of specific oscillatory torque on the bead to lateral bead displacements as the complex elastic modulus G* = G′ + iG″, where G′ is the storage modulus (cell stiffness), G″ is the loss modulus (cell friction), and i2 = −1 [20]. Cell stiffness and friction are expressed in units of Pa/nm.
Fourier transform traction microscopy
The contractile stress arising at the interface between each adherent cell and its substrate was measured with traction microscopy [21]. Cells were plated sparsely on elastic gel blocks (1500 cells/block), and allowed to adhere and stabilize for 24 h. For each adherent cell, the traction field was computed using Fourier transform traction cytometry as described [16,21,22]. The computed traction field was used to obtain net contractile moment, which is a scalar measure of the cell’s contractile strength [22]. Net contractile moment is expressed in units of pico-Newton meters (pNm).
Quantitative real-time RT-PCR
For each well of adherent ASM cells (~200,000 cells per 24-well or elastic gel), Nrf2-regulated transcript levels of γ glutamate cysteine ligase catalytic subunit (GCLc), glutamate cysteine ligase modifier subunit (GCLm), and heme oxygenase 1 (HO-1) were measured [15]. The mRNA expression levels for all samples were repeated in duplicates, and normalized to the level of the housekeeping gene GAPDH.
Statistical analysis
To satisfy the distributional assumptions associated with the Analysis of Variance (ANOVA), cell stiffness data were converted to log scale prior to analyses. For baseline stiffness, the comparisons among treatments were assessed by ANOVA; P-values from pair-wise comparisons were adjusted for multiple comparisons by applying the Tukey’s method. For agonist-induced changes in stiffness, the comparisons were tested using linear mixed-effects model by taking into account the repeated measurements after adjusting for baseline stiffness. For normally distributed data, the comparisons were performed with ANOVA followed by t-test. All analyses were performed in SAS V.9.1, and the 2-sided P-values less than 0.05 were considered significant.
Results and discussion
Mechanics of the proliferating versus the growth-arrested living cells
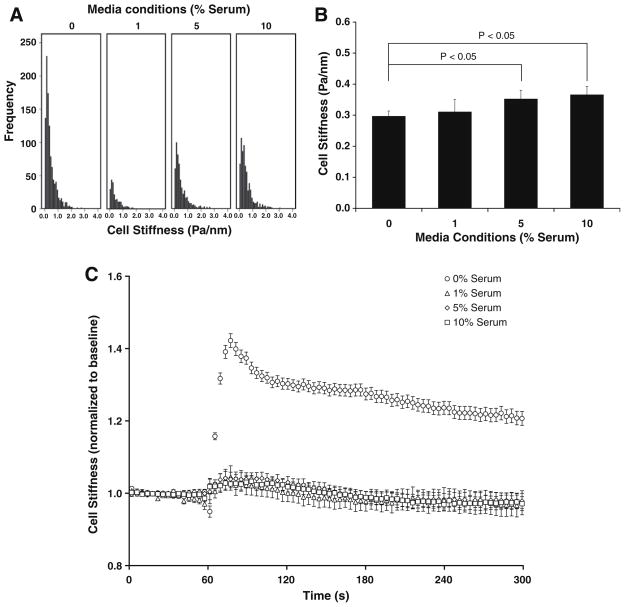
To assess the extent to which mechanical properties of the proliferating cells in culture differs from that of the growth-arrested cells, we measured stiffness of sub-confluent, human ASM cells treated for 48 h with media containing a range of serum (0%, 1%, 5%, or 10%). For each treatment, stiffness varied widely between individual adherent cells and approximated a log-normal distribution (Fig. 1A). There were no statistical differences between cells treated with 0% and 1% serum, but cells treated with 5% or 10% serum exhibited slightly higher stiffness (Fig. 1B).
Fig. 1.
(A) The histogram of stiffness data prior to histamine challenge. (B) Baseline stiffness of cells treated with a range of serum. (C) Changes in cell stiffness to 10 μM histamine: for each cell, changes were normalized to its baseline stiffness. Data are reported by descriptive statistics in the form of geometric means, and error bars indicate 95% confidence intervals (n = 229–1068 cells).
In response to the contracting agonist histamine, however, growth and growth-arrested conditions differed remarkably. Whereas ASM cells treated with 1%, 5%, and 10% serum showed no changes in stiffness to histamine, those treated with 0% serum exhibited large increases (Fig. 1C). Moreover, for the duration of histamine challenge, the stiffness of cells in a growth-arrested condition (0% serum) was quantitatively (P < 0.05) higher than that of cells in growth conditions (1%, 5%, and 10% serum). Hence, human ASM cells in a synthetic or proliferative state demonstrated no detectable mechanical responsiveness to histamine, whereas these same cells in culture under a growth-arrested condition were highly responsive. To our knowledge, this is the first biophysical evidence that corroborates the biochemical signature of muscle maturation in culture reported by others [14].
Effects of ECM composition on muscle mechanics
To further assess mechanical responsiveness of the human ASM cell, we contrasted the effects of several ECM constituents that have been shown to modulate the cellular expression of contractile proteins [11,13]. In particular, we focused on fibronectin, laminin and the several types of collagen that have been associated with subepithelial remodeling in asthma [3,7,8]. For this experiment, we used post-confluent cells that were serum-deprived for at least 48 h; cells were then harvested and allowed to adhere, for up to 5 days, on substrates coated with respective ECM protein.
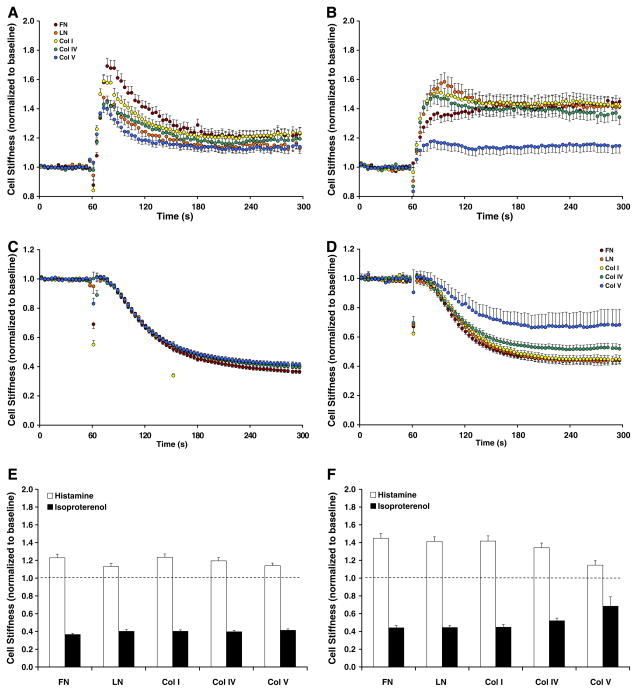
On all ECM protein substrates, baseline stiffness of adherent cells increased with days in culture but, by day 5, decreased to that of day 1 (data not shown). Most interestingly, however, the cell stiffening responses to histamine differed systematically with ECM composition (Fig. 2). On each ECM protein substrate, the stiffening responses also changed qualitatively with days in culture; cells adhered for 1 day showed transient increases (Fig. 2A), whereas those adhered for 5 days exhibited more prolonged and stable increases (Fig. 2B). In response to the relaxing agonist isoproterenol, cells adhered for 1 day exhibited a similar extent of cell stiffness decreases regardless of ECM composition (Fig. 2C). The extent of such decreases differed remarkably among cells adhered for 5 days, however (Fig. 2D).
Fig. 2.
Stiffness of cells adherent upon various ECM protein substrates for 1 day, (A,C); for 5 days, (B,D) were measured in response to 10 μM histamine (A,B) or isoproterenol (C,D). The steady-state, maximal cell stiffness responses to histamine (open bars) or isoproterenol (closed bars), for day 1 (E) and for day 5 (F). The data are presented by geometric means, and error bars indicate 95% confidence interval (n = 66–626 cells).
Considering this ability of the muscle to change stiffness from its most relaxed state to its most contracted state—corresponding to the cell contractile scope [16]—cells adherent upon FN, LN and Col I showed progressive increases whereas those adherent upon Col IV and Col V exhibited progressive decreases in contractile scope (Fig. 2E and F). These findings are consistent with phenotypic changes in the expression of contractile proteins reported by others [11,13,14] and, thereby, provide strong evidence that ECM composition differentially modulates mechanical properties of the human ASM cell.
Effects of ECM rigidity on muscle mechanics
ECM provides both structure and rigidity to the airway wall [19] and, as such, increased deposition of ECM may impose a stiffer cell microenvironment [5,23]. Tissue stiffness is a common critical factor for the differentiation of striated muscle [17], as well as the mesenchymal stem cell into different cell lineage [18]. To explore this physical aspect of cell–ECM interactions, we employed inert polyacrylamide gel substrates with varying rigidities and assessed changes in mechanical properties of the human ASM cell.
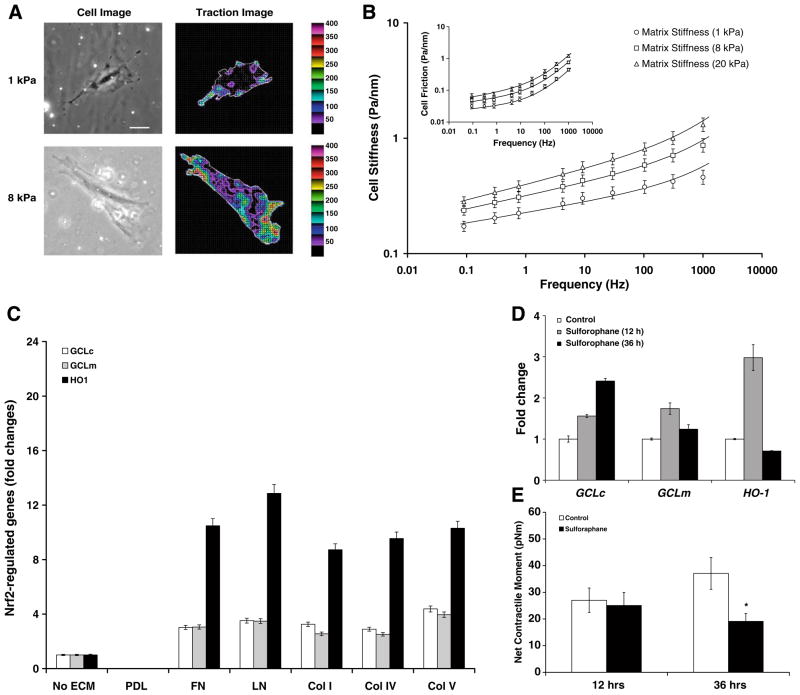
Consistent with the preferential cell spreading and migration toward more rigid substrates reported by others [17,24], ASM cells adherent upon a more rigid substrate, regardless of ECM composition, also spread more. In addition, upon adherence to a more rigid substrate, cells exerted greater contractile force (Fig. 3A). For example, compared with cells adherent upon a soft substrate [1 kPa gel; 38.9 ± 5.2 pNm (Mean ± SE, n = 28)], those adherent upon a stiff substrate [8 kPa gel; 70.3 ± 13.4 pNm (Mean ± SE, n = 39)] exhibited significantly higher (P < 0.05) net contractile moment—a scalar measure of the cell’s contractile strength [21,22].
Fig. 3.
(A) A representative phase contrast and traction field images of the single ASM cell adherent upon an elastic gel block (Young’s modulus of 1 or 8 kPa with a Poisson’s ratio of 0.48). Colors show the magnitude of the tractions in Pa, and arrows show the direction and relative magnitude of those tractions. Scale bar, 50 μm. (B) Stiffness and frictional modulus of the adherent cell across a spectrum spanning five decades of frequency. The solid lines are the fit of experimental data with a two-term power-law model [25], G* = G0(if)x−1 + G1(if)¾, where the first term accounts for slow glassy dynamics [20] and the second term for semiflexible polymer fluctuations [25]. Data are presented by geometric means, and error bars indicate 95% confidence intervals (n = 341–372 cells). (C) The basal transcript levels of GCLc, GCLm, and HO-1: cells were adherent upon plastic substrates coated without or with PDL, FN, LN, Col I, Col IV or Col V. (D) Induction of GCLc, GCLm, and HO-1 by Sulforaphane (20 μM): cells were adherent upon elastic gel (8 kPa) substrate coated with Col I. Data are presented as Mean ± SE (n = 3 separate experiments). (E) Net contractile moment of cells treated without or with 20 μM Sulforaphane. Data are presented as Mean ± SE (n = 24–31 cells).
Furthermore, ECM rigidity differentially affected dynamic stiffness of the cell over a wide frequency range (Fig. 3B). At a given substrate rigidity, nonetheless, cell stiffness increased with increasing frequency that followed a weak power-law behavior. Cell friction followed the same weak power-law behavior at lower frequencies but, at larger frequencies, exhibited a strong frequency dependence approaching a power-law exponent of 1 (Fig. 3B, inset): this is a characteristic of a Newtonian viscosity [20]. More strikingly, the power-law frequency dependence of cell stiffness at low frequencies varied with substrate rigidities (~1 kPa, f 0.09; ~8 kPa, f 0.11; ~20 kPa, f 0.13). Hence, as defined by us elsewhere [16,20,25], human ASM cells adherent upon increasingly rigid substrates exhibited higher effective temperature that represents physical malleability of the underlying CSK.
These findings, taken together, demonstrated that ECM rigidity not only influences the abilities of human ASM cells to spread, but also differentially affects their abilities to contract and to stiffen the CSK. As such, underlying CSK mechanics is intimately linked to physical stress that might be imposed by increased deposition of ECM.
Mechanistic link between ECM and Nrf2 activation
Tissue microenvironment of the lungs is not only the target of inflammation, but also plays a critical role in regulating inflammatory cells/mediators and oxidative stress [1,15,23]. Nuclear erythroid 2 p45-related factor 2 (Nrf2) is a basic leucine zipper transcription factor that confers protection against various forms of stress (i.e. oxidative/nitrosative and shear/mechanical) by regulating both basal and inducible expression of a battery of cytoprotective genes, including antioxidative enzymes [26,27]. Recently we have found that genetic deletion of Nrf2 leads to Th2 polarization in the airway microenvironment [28] and, renders the mice more susceptible to Th2-driven allergic inflammation, oxidative stress and airway hyperreactivity [15]. We examined here the role of Nrf2 in ASM.
Human ASM cells adherent upon substrates coated with various ECM proteins showed appreciable induction of classical Nrf2-regulated genes, including GCLc, GCLm, and HO-1 (Fig. 3C). Transcript levels of these genes were undetectable in cells adherent upon substrates coated with poly-D-lysine (PDL), however. In addition, regardless of ECM composition, a small molecule activator of Nrf2 signaling (Sulforaphane) caused further induction of these antioxidative and cytoprotective genes (Fig. 3D). Most interestingly, cells treated with Sulforaphane exhibited, in turn, time-dependent attenuation in contractile forces (Fig. 3E). These findings suggest a possible link between muscle contractility and a redox-sensitive transcription factor, Nrf2.
Nrf2 genotype and muscle phenotype
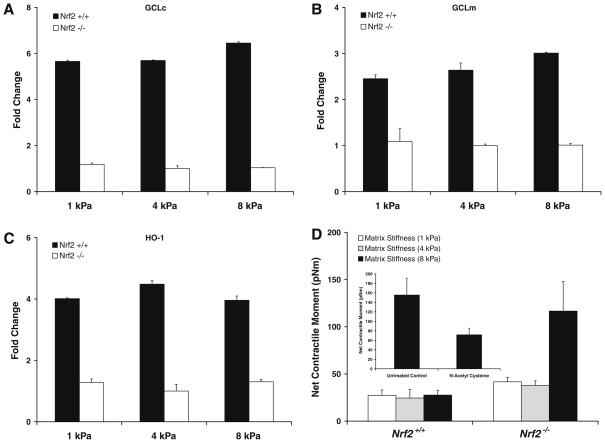
To probe deeper into the mechanistic role for Nrf2, we then contrasted mechanical properties of ASM cells isolated from Nrf2+/+ and Nrf2−/− mice. On all substrate rigidities, cells isolated from Nrf2−/− mice showed lower basal expression and no inducible changes in the transcript levels of GCLc, GCLm, and HO-1 (Fig. 4). In contrast, cells isolated from Nrf2+/+ mice showed appreciable induction of these classical Nrf2-regulated genes. Most interestingly, compared with cells isolated from Nrf2+/+ mice, those isolated from Nrf2−/− mice exhibited significant increases in contractile force (Fig. 4D). The greatest increases were observed upon adherence to the most rigid substrate, and such increases in contractile force were largely ablated with non-specific glutathione enhancer, N-acetyl cysteine (Fig. 4D, inset). These findings are consistent with the notion that increased deposition of ECM imposes physical stress to the embedded smooth muscle and that, under such stressful condition, the muscle adapts its mechanical properties by regulating Nrf2-directed responses—a failure to do so would lead to muscle hyperreactivity.
Fig. 4.
Transcript levels of GCLc (A), GCLm (B), and HO-1 (C) of ASM cells isolated from Nrf2+/+ and Nrf2−/− mice: cells were adherent upon elastic gel (1–8 kPa) substrates coated with Col I. Data are presented as Mean ± SE (n = 3 separate experiments). (D) Net contractile moment of cells isolated from Nrf2+/+ and Nrf2−/− mice. Data are presented as Mean ± SE (n = 9–14 cells). Inset: net contractile moment of Nrf2−/3−cells treated for 24 h without or with 10 mM NAC. Data are presented as Mean ± SE (n = 15–17 cells).
Conclusion
In this study, we considered the mechanistic link between ECM and mechanical responsiveness of the human ASM cell. We showed that both ECM composition and rigidity differentially affect mechanical reactivity of the ASM cell and that the magnitude of such reactivity correlates closely with the activity of a redox-sensitive transcription factor, Nrf2. Moreover, in agreement with an earlier study in which disruption of the Nrf2 gene leads to severe allergen-induce oxidative stress and airway hyperreactivity in mice [15], our findings now point to new molecular pathway by which Nrf2-directed responses of the human ASM cell might contribute to airway hyperreactivity in asthma. As regards the healthy versus asthmatic human ASM cells, our unpublished work indicates fundamental differences in their abilities to regulate Nrf2-regulated antioxidant genes and to generate contractile forces. Future studies will test the efficacy of small molecule approach of targeting Nrf2 in modulating the contractility of asthmatic cells and the pathogenesis of airway hyperreactivity.
Acknowledgments
This work was supported by NIH Grants HL59682 (J.J.F.), HL33009 (J.J.F.), HL081205 (S.B.), and COPD SCCOR P50HL074945 (S.B.); by Children Asthma Center P50ES015903; by NIEHS Center grant pilot grant (S.A.); and by Faculty Research Initiative Fund from Johns Hopkins Bloomberg School of Public Health (S.A.).
References
- 1.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma: from bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161:1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 2.Woolcock AJ, Peat JK. Epidemiology of bronchial hyperresponsiveness. Clin Rev Allergy Immunol. 1989;7:245–256. doi: 10.1007/BF02914477. [DOI] [PubMed] [Google Scholar]
- 3.Wilson JW, Li X. The measurement of reticular basement membrane and submucosal collagen in the asthmatic airway. Clin Expr Allergy. 1997;27:363–371. [PubMed] [Google Scholar]
- 4.Bosse Y, Pare PD, Seow CY. Airway wall remodeling in asthma: from the epithelial layer to the adventitia. Curr Allergy Asthma Reports. 2008;8:357–366. doi: 10.1007/s11882-008-0056-0. [DOI] [PubMed] [Google Scholar]
- 5.Bai TR, Cooper J, Koelmeyer T, Pare PD, Weir TD. The effect of age and duration of disease on airway structure in fatal asthma. Am J Respir Crit Care Med. 2000;162:663–669. doi: 10.1164/ajrccm.162.2.9907151. [DOI] [PubMed] [Google Scholar]
- 6.Woodruff PG, Dolganov GM, Ferrando RE, Donnelly S, Hays SR, Solberg OD, Carter R, Wong HH, Cadbury PS, Fahy JV. Hyperplasia of smooth muscle in mild to moderate asthma without changes in cell size or gene expression. Am J Respir Crit Care Med. 2004;169:1001–1006. doi: 10.1164/rccm.200311-1529OC. [DOI] [PubMed] [Google Scholar]
- 7.Roche WR, Beasley R, Williams JH, Holgate ST. Subepithelial fibrosis in the bronchi of asthmatics. Lancet. 1989;1:520–524. doi: 10.1016/s0140-6736(89)90067-6. [DOI] [PubMed] [Google Scholar]
- 8.Altraja A, Laitinen A, Virtanen I, Kampe M, Simonsson BG, Karlsson SE, Hakansson L, Venge P, Sillastu H, Laitinen LA. Expression of laminins in the airways in various types of asthmatic patients: a morphometric study. Am J Respir Cell Mol Biol. 1996;15:482–488. doi: 10.1165/ajrcmb.15.4.8879182. [DOI] [PubMed] [Google Scholar]
- 9.An SS, Bai TR, Bates JHT, Black JL, Brown RH, Brusasco V, et al. Airway smooth muscle dynamics: a common pathway of airway obstruction in asthma. Eur Respir J. 2007;29:834–860. doi: 10.1183/09031936.00112606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan V, Burgess JK, Ratoff JC, O’Connor BJ, Greenough A, Lee TH, Hirst SJ. Extracellular matrix regulates enhanced eotaxin expression in asthmatic airway smooth muscle cells. Am J Respir Crit Care Med. 2006;174:379–385. doi: 10.1164/rccm.200509-1420OC. [DOI] [PubMed] [Google Scholar]
- 11.Hirst SJ, Twort CHC, Lee TH. Differential effects of extracellular matrix proteins on human airway smooth muscle cell proliferation and phenotype. Am J Respir Cell Mol Biol. 2000;23:335–344. doi: 10.1165/ajrcmb.23.3.3990. [DOI] [PubMed] [Google Scholar]
- 12.Parameswaran K, Radford K, Zuo J, Janssen LJ, O’Byrne PM, Cox PG. Extracellular matrix regulates human airway smooth muscle cell migration. Eur Respir J. 2004;24:545–551. doi: 10.1183/09031936.04.00113103. [DOI] [PubMed] [Google Scholar]
- 13.Tran T, McNeill K, Gerthoffer W, Unruh H, Halayko A. Endogenous laminin is required for human airway smooth muscle cell maturation. Respir Res. 2006;7:117. doi: 10.1186/1465-9921-7-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halayko AJ, Camoretti-Mercado B, Forsythe SM, Vieira JE, Mitchell RW, Wylam ME, Hershenson MB, Solway J. Divergent differentiation paths in airway smooth muscle culture: induction of functionally contractile myocytes. Am J Physiol. 1999;276:L197–L206. doi: 10.1152/ajplung.1999.276.1.L197. [DOI] [PubMed] [Google Scholar]
- 15.Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Expr Med. 2005;202:47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.An SS, Fabry B, Trepat X, Wang N, Fredberg JJ. Do biophysical properties of the airway smooth muscle in culture predict airway hyperresponsiveness? Am J Respir Cell Mol Biol. 2006;35:55–64. doi: 10.1165/rcmb.2005-0453OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophys J. 2004;86:617–628. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 19.Kamm RD. Airway wall mechanics. Annu Rev Biomed Eng. 1999;1:47–72. doi: 10.1146/annurev.bioeng.1.1.47. [DOI] [PubMed] [Google Scholar]
- 20.Fabry B, Maksym GN, Butler JP, Glogauer M, Navajas D, Fredberg JJ. Scaling the microrheology of living cells. Phys Rev Lett. 2001;87:148102. doi: 10.1103/PhysRevLett.87.148102. [DOI] [PubMed] [Google Scholar]
- 21.Wang N, Tolic-Norrelykke IM, Chen JX, Mijailovich SM, Butler JP, Fredberg JJ, Stamenovic D. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am J Physiol. 2002;282:C606–C616. doi: 10.1152/ajpcell.00269.2001. [DOI] [PubMed] [Google Scholar]
- 22.Butler JP, Tolic-Norrelykke IM, Fabry B, Fredberg JJ. Traction fields, Moments, and strain energy that cells exert on their surroundings. Am J Physiol. 2002;282:C595–605. doi: 10.1152/ajpcell.00270.2001. [DOI] [PubMed] [Google Scholar]
- 23.Wagers S, Lundblad LKA, Ekman M, Irvin CG, Bates JHT. The allergic mouse model of asthma: normal smooth muscle in an abnormal lung? J Appl Physiol. 2004;96:2019–2027. doi: 10.1152/japplphysiol.00924.2003. [DOI] [PubMed] [Google Scholar]
- 24.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 25.Deng L, Trepat X, Butler JP, Millet E, Morgan KG, Weitz DA, Fredberg JJ. Fast and slow dynamics of the cytoskeleton. Nat Mater. 2006;5:636–640. doi: 10.1038/nmat1685. [DOI] [PubMed] [Google Scholar]
- 26.Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest. 2006;116:984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warabi E, Takabe W, Minami T, Inoue K, Itoh K, Yamamoto M, Ishii T, Kodama T, Noguchi N. Shear stress stabilizes NF-E2-related factor 2 and induces antioxidant genes in endothelial cells: role of reactive oxygen/nitrogen species. Free Rad Biol Med. 2007;42:260–269. doi: 10.1016/j.freeradbiomed.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 28.Williams MA, Rangasamy T, Bauer SM, Killedar S, Karp M, Kensler TW, Yamamoto M, Breysse P, Biswal S, Georas SN. Disruption of the transcription factor Nrf2 promotes pro-oxidative dendritic cells that stimulate Th2-like immunoresponsiveness upon activation by ambient particulate matter. J Immunol. 2008;181:4545–4559. doi: 10.4049/jimmunol.181.7.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]