Abstract
Following myocardial infarction, the prognosis for females is better than males. Estrogen is thought to be protective, but clinical trials with hormone replacement failed to show protection. Here, we sought to identify novel mechanisms that might explain this sex-based difference. By diverging from the traditional focus on sex hormones, we employed a conceptually novel approach to this question by using a non-biased approach to measure global changes in gene expression following infarction. We hypothesized that specific gene programs are initiated in the heart following infarction that might account for this sex-based difference. We induced small, medium, and large infarcts in male and female mice and measured changes in gene expression by microarray following infarction. Regardless of infarct size, survival was better in females, while mortality occurred 3–10 days following infarction in males. Two days following infarction, males developed significant ventricular dilation, the best predictor of mortality in humans. Three days following infarction, we measured gene expression by microarray, comparing male versus female and sham versus surgery/infarction. In general, our results indicate a higher relative level of gene induction in females versus males and identified programs for angiogenesis, extracellular matrix remodeling, and immune response. This pattern of gene expression was linked to less pathologic remodeling in female hearts, including increased capillary density and decreased fibrosis. In summary, our results suggest an association between improved survival and less pathologic remodeling and the relative induction of gene expression in females following myocardial infarction.
Keywords: Myocardial Infarction, Microarray, Heart Failure, Sex/Gender
INTRODUCTION
In humans, cardiovascular disease, including myocardial infarction (MI), is a predominantly male disease until 65–70 years of age. Statistics from the American Heart Association indicate that in 2004 the overall death rate from cardiovascular diseases was 335.1/100,000 for white males, but only 238.0/100,000 for white females with similar trends in black men and women [1]. In general, women have a lower age-adjusted incident rate and death rate for coronary heart disease (CHD). For people 45–64 years of age the CHD incident rate was three-fold higher in men versus women and the death rate for CHD was 1.7-fold higher in men [1]. Although women have a higher one-year mortality rate following MI (age ≥ 40, 18% in men, 23% in women [1]), comparing outcomes following MI in men and women is confounded by the fact that: women have MIs at older ages, women are 55% less likely to participate in cardiac rehabilitation, and women are less likely to recognize the signs and symptoms of MI and therefore more likely to delay treatment [1]. However, once diagnosed with heart failure, a common outcome following MI, survival rates are significantly higher in women than men [1]. The lower death rates in women initially suggested that estrogen might protect against heart disease, but the results of the Heart and Estrogen/Progestin Replacement Study follow-up (HERS II) and Women’s Health Initiative (WHI) clinical trials found either no protection or an increased risk of cardiovascular disease with hormone replacement therapy [2, 3].
To date, most animal studies have focused on sex hormones, estrogen and testosterone, to address the issue of sex-based differences following MI. In male and female mice subjected to gonadectomy with hormone replacement (either testosterone or estrogen in both males and females), surgically induced MI produced higher mortality in mice receiving testosterone (male and female) due to cardiac rupture 3–5 days following MI [4–7], as well as significantly more contractile dysfunction and ventricular dilation [4–7]. Cumulatively, these studies generally indicated that estrogen protects the heart from long-term remodeling following MI, whereas testosterone increases the risk of cardiac rupture immediately following MI and adversely affects long-term remodeling [4–7].
A number of studies have addressed estrogen function in the heart and cardiac myocytes, attempting to identify the mechanisms leading to the better outcomes observed in females with cardiovascular disease. Female estrogen receptor-β deficient mice had worse outcomes following MI, suggesting that estrogen is required for adaptation to ischemic injury [8]. Others demonstrated that estrogen inhibits cardiac hypertrophy [9] and prevents myocyte apoptosis [10], suggesting possible protective mechanisms. Another study showed that estrogen lengthened cardiac myocyte repolarization and reducing automaticity in females following MI [11]. Conversely, some studies found that while estrogen inhibits apoptosis, it also increases ventricular remodeling following MI [12]. In total, experiments focused on the sex hormones have identified some potential mechanisms explaining estrogen mediated protection post-MI, but more work is needed.
Here, we examined the sex-based difference following MI to understand why females have better outcomes. Rather than focus on sex hormones, we employed a non-biased approach to measure global changes in gene expression following infarction. We hypothesized that specific gene programs are initiated in the heart following MI that might account for this sex-based difference. In summary, we found an association between improved survival and a higher level of gene induction, with specific programs for angiogenesis, extracellular matrix remodeling, and immune response, in female mice following MI.
MATERIALS AND METHODS
Male and female, C57BL/6 mice (12–15 weeks of age) from Jackson Laboratories were used for all experiments. The use of animals conformed to the PHS Guide for Care and Use of Laboratory Animals and was approved by Sanford Research/USD Institutional Animal Care and Use Committee.
Detailed methods describing coronary artery ligation surgery, measurement of cardiac function, microarray analysis, and measurements of capillary density, heart and myocyte size, and fibrosis are contained in the supplemental methods.
A complete, annotated version of the array data contained in this manuscript is available at GEO (http://www.ncbi.nlm.nih.gov/geo/query), accession number GSE23294
RESULTS
Survival was better in female versus male mice following myocardial infarction (MI)
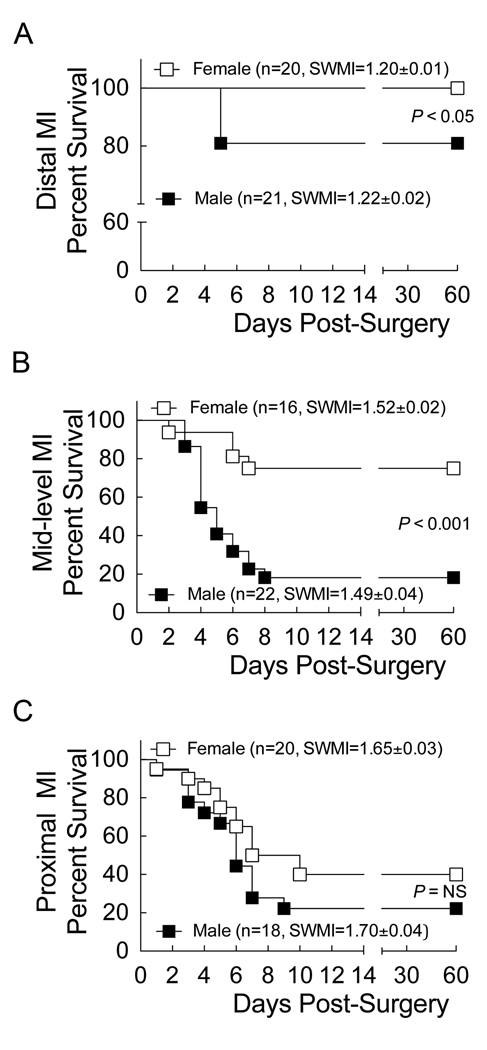
To examine sex-based differences in ventricular remodeling following ischemic injury, we induced MI in male and female C57BL/6 mice by ligation of the left anterior descending (LAD) coronary artery. Ligations of the LAD were made at distal, mid-level, or proximal sites to produce small, medium, and large infarcts respectively (Supplemental Figure 1). Two days following surgery infarct size was measured non-invasively by echocardiography using segmental wall motion index (SWMI) [13]. Regardless of infarct size, small (~15% of left ventricular area), medium (~22%), or large (~30%), survival was higher in females (Figure 1). Only one death was observed in all of the sham surgery groups (n = 39). For small, medium, and large infarcts, SWMI was not different between the sexes (Figure 1A–C) indicating that the initial infarct size was similar at each ligation site. Interestingly, mortality occurred in both groups only between 3–10 days post-infarction, suggesting the rapid development of a non-compensatory acute heart failure leading to death.
Figure 1. Survival was better in female versus male mice following myocardial infarction (MI).
Survival curves following induction of (A) small, (B) medium, and (C) large infarcts in male and female C57BL/6 mice, 12–15 weeks of age. Two days after surgery, cardiac function and segmental wall motion index (SWMI) were measured by echocardiography. Survival data were analyzed using Log-rank (Mantel-Cox) Test with a median survival.
Segmental wall motion index (SWMI) predicted infarct size and functional impairment
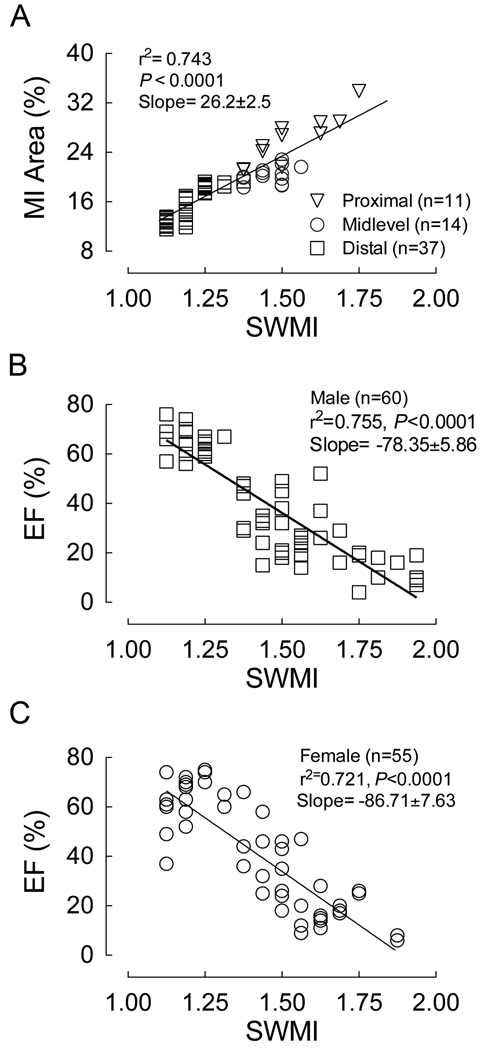
To confirm our non-invasive measurements of infarct size (SWMI) and further validate the survival benefit observed in females following MI, we correlated SWMI scores with infarct size. SWMI determined two days following MI correlated significantly with infarct size measured 60 days following MI (Figure 2A). Our results also indicated that ejection fraction, measured by echocardiography two days following MI, was inversely correlated with SWMI (Figure 2B–C).
Figure 2. Segmental wall motion index (SWMI) predicted infarct size and functional impairment.
Two days following MI, cardiac function and SWMI were measured by echocardiography. (A) The graph shows a significant correlation between SWMI and MI area (measured at 60 days), validating SWMI as a non-invasive measurement of infarct size. (B and C) The graphs show a significant inverse correlation between SWMI (infarct size, MI area) and functional impairment (ejection fraction, EF) in both male and female mice following MI respectively.
Left ventricular dilation correlated with increased mortality in male mice following MI
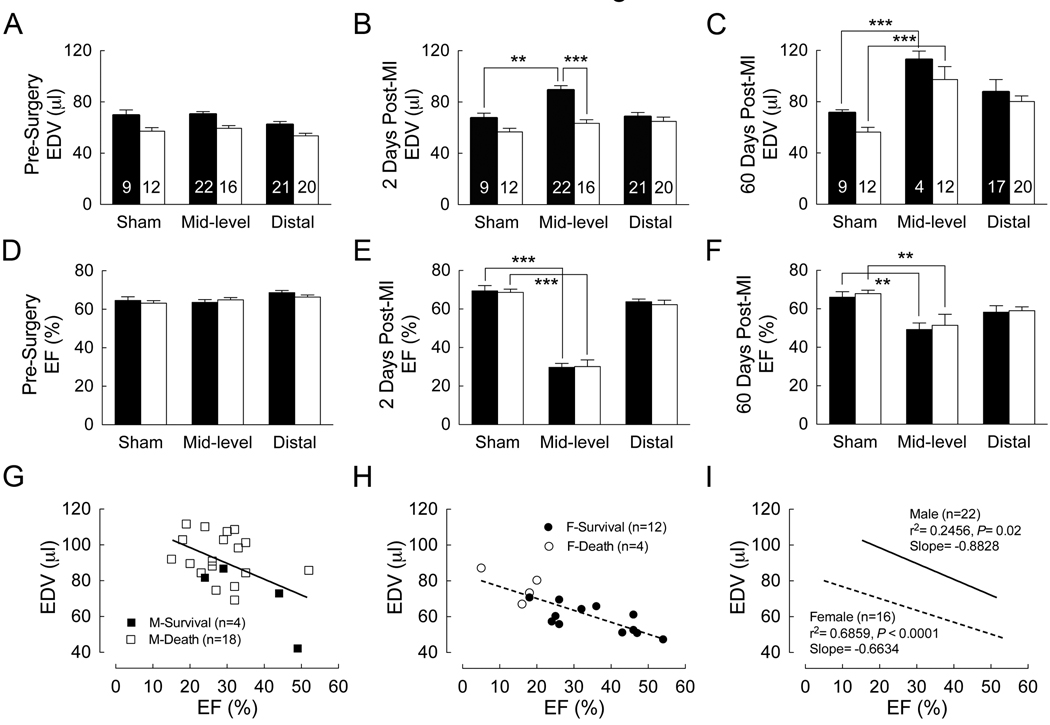
Two days following MI, significant left ventricular dilation was observed in males but not females with medium sized infarcts (Figure 3A–B), whereas ejection fraction was reduced similarly between the sexes (Figure 3D–E). No significant functional differences were observed in mice with small infarcts (Figure 3 and Supplemental Table 1, mice with large infarcts were not included in the rest of the study due to the high mortality observed in both sexes). A correlation between ejection fraction and end-diastolic volume two days following MI (Figure 3G–I) showed that for a given decrease in ejection fraction there was a proportionally larger increase in end-diastolic volume in males. These data were distributed by mice that survived versus those that ultimately died and indicated that ventricular dilation predicted mortality (Figure 3G–H). By 60 days, significant compensatory ventricular remodeling was evident in surviving mice and significant ventricular dilation and slight improvements in ejection fraction were observed in both sexes (Figure 3C and 3F and Supplemental Tables 1–2).
Figure 3. Left ventricular dilation correlated with increased mortality in male mice following MI.
Cardiac function was measured by echocardiography before, two days after, or 60 days after surgery in male (black bars) and female (white bars) mice. End-diastolic volume (EDV) (A, B and C) and ejection fraction (EF) (D, E and F) were measured. Data are presented as mean ± SEM with the number of mice shown in A–C. For each time point, groups were compared by two-way ANOVA with Bonferroni post-test to determine the contribution of sex or infarct size to EDV or EF (** P < 0.01, *** P < 0.001). (G–I) The graphs show the correlation between EDV and EF two days after surgery for male (G) and female (H) mice with mid-level LAD ligation (open symbols indicate death, closed symbols indicate survival).
Specific gene expression programs were induced in female mice following MI
We hypothesized that specific gene programs were initiated rapidly in the heart following MI that accounted for either the better survival in females, the worse survival in males, or both. To identify these sex-specific differences in gene expression, we induced medium-sized infarctions (where survival differences were greatest, Figure 1B), isolated RNA three days following surgery, and measured gene expression by microarray using the Illumina Mouse-6 v2 Expression BeadChip (48,000 gene transcripts). Based on the 2X2 design of the experiment, we analyzed our microarray expression data using a two-way ANOVA with a false discovery rate correction to test for an interaction between infarction and sex (see Supplemental Methods for more detailed description). We identified 5,574 genes with a statistically significant change (increased or decreased) induced by infarction, 187 genes with a statistically significant difference between males and females, but no genes showed a significant interaction between both variables. By applying a 1.6-fold change as a threshold, these numbers were reduced to 496 genes induced by infarction, 14 genes different between the sexes (Table 2 and Supplemental Table 3). These lists were further analyzed using Ingenuity Pathway Analysis software (Table 1). For genes changed by surgery, 23 clusters were identified with the top functions of cell cycle/cell proliferation, inflammation, metabolism and connective tissue disorders highly represented (Table 1). For genes changed by sex, 6 clusters were identified with the top functions of cardiovascular-specific functions and metabolism. In summary, this analysis initially suggested that infarction had a much greater effect than sex on gene expression, which might not be surprising, but fails to explain the observed sex-based differences in survival following infarction.
Table 2.
Sex-based differential gene expression.
| Differential Gene Expression by MI | |||
|---|---|---|---|
| Gene |
Female MI/Female Sh Male MI/Male Sh |
Gene Ontology | |
| Angiogenesis | |||
| Ddah1 | Dimethylarginine dimethylaminohydrolase 1 | 2.00 | arginine metabolism, NO synthesis, angiogenesis |
| Sphk1 | Sphingosine kinase 1 | 1.67 | activation of PKC, blood vessel development, brain development |
| Mmp14 | Matrix metallopeptidase 14 (membrane-inserted) | 1.67 | branching morphogenesis, cell migration, collagen catabolism |
| Plk1 | Polo-like kinase 1 (Drosophila) | 1.66 | cell cycle, cell division, mitosis |
| Angptl4 | Angiopoietin-like 4 | 1.59 | angiogenesis |
| Col8a1 | Collagen, type VIII, alpha 1 | 1.54 | angiogenesis, eye morphogenesis, cell adhesion, epithelial cell proliferation |
| Col18a1 | Collagen, type XVIII, alpha 1 | 1.52 | angiogenesis, cell adhesion |
| Srpx2 | Sushi repeat-containing protein, X-linked 2 | 1.52 | cell motility, cell-cell adhesion, cell migration and sprouting angiogenesis |
| Extracellular Matrix | |||
| Gpnmb | Glycoprotein (transmembrane) nmb | 1.75 | cell adhesion |
| Lgals3 | Lectin, galactoside-binding, soluble, 3 | 1.66 | cell differentiation, extracellular matrix organization, skeletal system development |
| Mmp12 | Matrix metallopeptidase 12 (macrophage elastase) | 1.63 | proteolysis, extracellular matrix |
| Loxl1 | Lysyl oxidase-like 1 | 1.58 | extracellular matrix, oxidation reduction |
| Loxl2 | Lysyl oxidase-like 2 | 1.55 | oxidation reduction, cell adhesion |
| Immune Response | |||
| Ccl4 | Chemokine (C-C motif) ligand 4 | 1.98 | chemotaxis, immune response, inflammatory response |
| Itgb1bp3 | Integrin beta 1 binding protein 3 | 1.75 | integrin (associated) negative regulation of myoblast differentiation |
| Cxcl10 | Chemokine (C-X-C motif) ligand 10 | 1.75 | chemotaxis, immune response, inflammatory response |
| Clec4n | C-type lectin domain family 4, member n | 1.62 | innate immune response |
| Rbp1 | Retinol binding protein 1, cellular | 1.53 | regulation of granulocyte differentiation |
| Nucleosome Assembly | |||
| Hist1h2af | Histone cluster 1, H2af | 1.71 | nucleosome assembly |
| Hist2h2ac | Histone cluster 1, H2ac | 1.70 | nucleosome assembly |
| Hist1h2ad | Histone cluster 1, H2ad | 1.65 | nucleosome assembly |
| Hist1h2ai | Histone cluster 1, H2ai | 1.63 | nucleosome assembly |
| Hist1h2ao | Histone cluster 1, H2ao | 1.61 | nucleosome assembly |
| Hist1h2ag | Histone cluster 1, H2ag | 1.59 | nucleosome assembly |
| Hist1h2an | Histone cluster 1, H2an | 1.54 | nucleosome assembly |
| Hist1h2ah | Histone cluster 1, H2ah | 1.52 | nucleosome assembly |
| Apoptosis | |||
| Cstb | Cystatin B (stefin B) | 1.57 | negative regulation of peptidase activity, regulation of apoptosis |
| Serpina3g | Serine peptidase inhibitor, clade A, member 3G | 1.51 | apoptosis, response to cytokine |
| Gadd45g | Growth arrest and DNA-damage-inducible, gamma | 1.50 | activation of MAPKK, apoptosis, cell differentiation, regulation of cell cycle |
| Other | |||
| Meox1 | Mesenchyme homeobox 1 | 1.83 | regulation of transcription |
| LOC380980 | 1.78 | ||
| Kcnv2 | Potassium channel, subfamily V, member 2 | 1.75 | voltage-gated potassium channel |
| Fkbp11 | FK506 binding protein 11 | 1.69 | protein folding |
| Ankrd1 | Ankyrin repeat domain 1 (cardiac muscle) | 1.67 | regulation of transcription |
| 2310057H16Rik | 1.66 | ||
| 2310050B05Rik | 1.64 | ||
| Hmgcs2 | 3-hydroxy-3-methylglutaryl-Coenzyme A synthase 2 | 1.63 | cholesterol and isoprenoid biosynthesis |
| Ms4a7 | Membrane-spanning 4-domains subfamily A | 1.62 | membrane protein |
| Fcrl3 | Fc receptor-like protein 3 | 1.62 | membrane protein |
| Crlf1 | Cytokine receptor-like factor 1 | 1.59 | ureteric bud development, cytokine binding |
| Slc15a3 | Solute carrier family 15, member 3 | 1.58 | peptide transport, membrane protein |
| Synpo | Synaptopodin | 1.58 | actin cytoskeleton assembly |
| Ppic | Peptidylprolyl isomerase C (cyclophilin C) | 1.57 | protein folding |
| LOC385699 | 1.56 | ||
| Sprr1a | Small proline-rich protein 1A | 1.53 | keratinocyte differentiation |
| Ces3 | Carboxylesterase 3 | 1.53 | carboxylesterase and hydrolase activity |
| Tyrobp | TYRO protein tyrosine kinase binding protein | 1.50 | cellular defense response, signal transduction |
| Mest | Mesoderm specific transcript homolog (mouse) | 1.50 | mesoderm development, regulation of lipid storage |
| Cdkn3 | Cyclin-dependent kinase inhibitor 3 | 1.50 | cell cycle arrest |
|
Differential Gene Expression by Sex | |||
| Gene |
Female MI Male MI |
Gene Ontology | |
| Extracellular Matrix | |||
| Mfap2 | Microfibrillar-associated protein 2 | 1.72 | microfibril, proteinaceous extracellular matrix |
| Immune Response | |||
| Cxcl14 | Chemokine (C-X-C) ligand 14 | 1.89 | chemotaxis immune response |
| Other | |||
| Eif2s3y | Eukaryotic translation initiation factor 2, subunit 3 | 17.52 | translation, GTP binding, GTPase activity |
| Ddx3y | DEAD (Asp-Glu-Ala-Asp) box polypeptide 3 | 5.64 | helicase activity |
| Xist | Inactive X specific transcripts | 3.41 | x-chromosome - unknown function |
| Jarid1d | Kdm5d lysine (K)-specific demethylase 5D | 2.97 | chromatin modification, oxidation reduction |
| Ptn | Pleiotrophin | 1.95 | bone mineralization, positive regulation of cell division |
| Hsd11b1 | Hydroxysteroid 11-beta dehydrogenase 1 | 1.93 | gluccocorticoid biosynthesis, lung development |
| 2610029G23Rik | 1.83 | ||
| Inmt | Indolethylamine N-methyltransferase | 1.83 | methyltransferase |
| Scn4b | Sodium channel subunit beta-4 | 1.78 | sodium ion transport |
| 4833426J09Rik | 1.72 | ||
| Kcne1 | Potassium voltage-gated channel subfamily E | 1.72 | potassium channel, muscle contraction |
| Lbh | Limb bud and heart | 1.72 | regulation of transcription |
Sex-specific differences in gene expression were assessed on the group of genes identified as significantly changed by surgery by calculating the fold difference in gene induction between males and females following surgery [female MI/female sham]/[male MI/male sham]. Forty-nine genes were changed 1.5 fold or more in the female than the male following MI, whereas no genes were changed 1.5 fold or more in the male than the female following MI (Genes down-regulated are italicized). The 49 genes that were identified are listed on top while the 14 genes changed by sex are listed on the bottom. Gene ontology was determine (geneontology.org) and classified by angiogenesis, extracellular matrix, immune response, nucleosome assembly (histone clusters), apoptosis, or other
Table 1.
Pathway analysis of gene identified as significantly changed by surgery or sex.
| IPA Analysis of Differential Gene Expression by MI | ||
|---|---|---|
| Top Functions in Identified Networks | Score | Molecules in Network |
| 53 | 32 | |
 |
39 | 26 |
| 32 | 25 | |
| 32 | 23 | |
| 32 | 23 | |
| 31 | 23 | |
 |
28 | 21 |
| 19 | 18 | |
 |
19 | 16 |
 |
16 | 16 |
 |
16 | 14 |
| Organismal injury and abnormalities, molecular transport, cell death | 16 | 14 |
| 15 | 14 | |
 |
14 | 13 |
| 14 | 13 | |
| 12 | 12 | |
 |
12 | 12 |
| Tumor morphology, cellular development, nervous system development/function |
11 | 11 |
| 10 | 10 | |
| 9 | 11 | |
| Molecular transport, protein synthesis, protein trafficking | 1 | 1 |
| Cell-to-cell signaling/interaction, cell morphology, cellular development | 1 | 1 |
| 1 | 1 | |
| IPA Analysis of Differential Gene Expression by Sex | ||
| Top Functions in Identified Networks | ||
| 14 | 6 | |
| Neuorological disease, opthalamic disease, dermatologic diseases and conditions |
3 | 1 |
| 3 | 1 | |
 |
2 | 1 |
| 2 | 1 | |
| 2 | 1 | |
Genes identified as significantly changed by surgery (500 genes, top) or sex (14 genes, bottom) were analyzed using Ingenuity Pathway Analysis (ingenuity.com). Twenty-three networks were identified from the surgery group and 6 networks were identified from the sex group. The top functions in each group are identified as well as the score (a rank ordering value, calculated by the IPA software) and number of molecules identified in the group. Commonalities in functions amongst the groups are color coded: blue for cell cycle/proliferation, red for inflammation, pink for metabolism, green for connective tissue disorders, orange for genetic disorder, and purple for cardiovascular disease.
However, a further analysis indicates that 49/496 of the genes induced by infarction were increased at least 1.5 fold more in females relative to males following infarction with 150/496 genes increased at least 1.3 fold more in females (Table 2). Conversely, no genes were increased at least 1.3 fold or more in males relative to females. Further analysis of the 49 genes increased 1.5 fold or more in females relative males following MI combined with the 14 genes changed by sex (63 in all) reveals some interesting patterns. Based on gene ontology (geneontology.org), 13% of the 63 genes were related to angiogenesis, 10% to extracellular matrix remodeling, 8% to immune response, and 13% to nucleosome assembly (histone clusters) (Table 2). This suggests a trend towards a higher level of gene induction in females relative to the males with patterns of gene induction that might be linked to the overall better outcomes in females.
To analyze more thoroughly the difference between males and females, we also performed less stringent statistical analysis, using t-tests to compare surgery versus sham for males and females separately. This analysis identified 138 genes in males and 303 genes in females changed (increased or decreased) at least two-fold versus sham (Supplemental Tables 4–6). Of these genes, 128 were common between both sexes. Therefore, the expression of only 10 genes was specifically altered in males, while the expression of 175 genes was altered specifically in females (See Supplemental Tables 4–6 for the full list). Interestingly, this analysis indentifies 313 total genes changed in males and/or females, of which 224 (72%) were also identified in the 514 genes indentified by the two-way ANOVA as specifically changed by surgery or sex. Further analysis of the 303 genes changed in females to identify those genes that showed the greatest fold increase relative to sham (at least 2.4 fold) or relative to males following MI, narrowed this list to 68 genes (See Supplemental Table 7 for the full list). Of the 68 genes increased most in females, 82% (56/68) were identified by the two-way ANOVA as specifically changed by surgery or sex, and 13% of the 68 genes were related to angiogenesis, 12% to extracellular matrix remodeling, and 16% to inflammation (Supplemental Table 7).
A small group of genes was analyzed using quantitative-PCR on the same RNA samples used for microarray analysis (Supplement Figure 2). The five genes; Aurora kinase A (Aurka/Styk6), collagen 18 (Col18a1), collagen 8 (Col8a1), Inner centromere protein (Incenp), and matrix metallopeptidase 12 (Mmp12), were all increased on the microarray more in the female relative to the male (Table 2 and Supplemental Table 3). In each case, changes in gene expression detected by the microarray were similar to the result with PCR (Supplement Figure 2). Although this is a small group of genes, the Illumina arrays used in these experiments have the same probe sequence for each gene is repeated ~30 times independently, making it much more accurate than first generation chips from 10 years ago. We feel that this provides a sufficient accuracy to reduce the need for PCR.
In summary, the results of two different statistical analyses suggested a higher level of gene induction in females relative to the males and identified programs for angiogenesis, extracellular matrix remodeling, and immune response that might be associated with better outcomes in females following MI.
Left ventricular capillary density was increased in females following MI
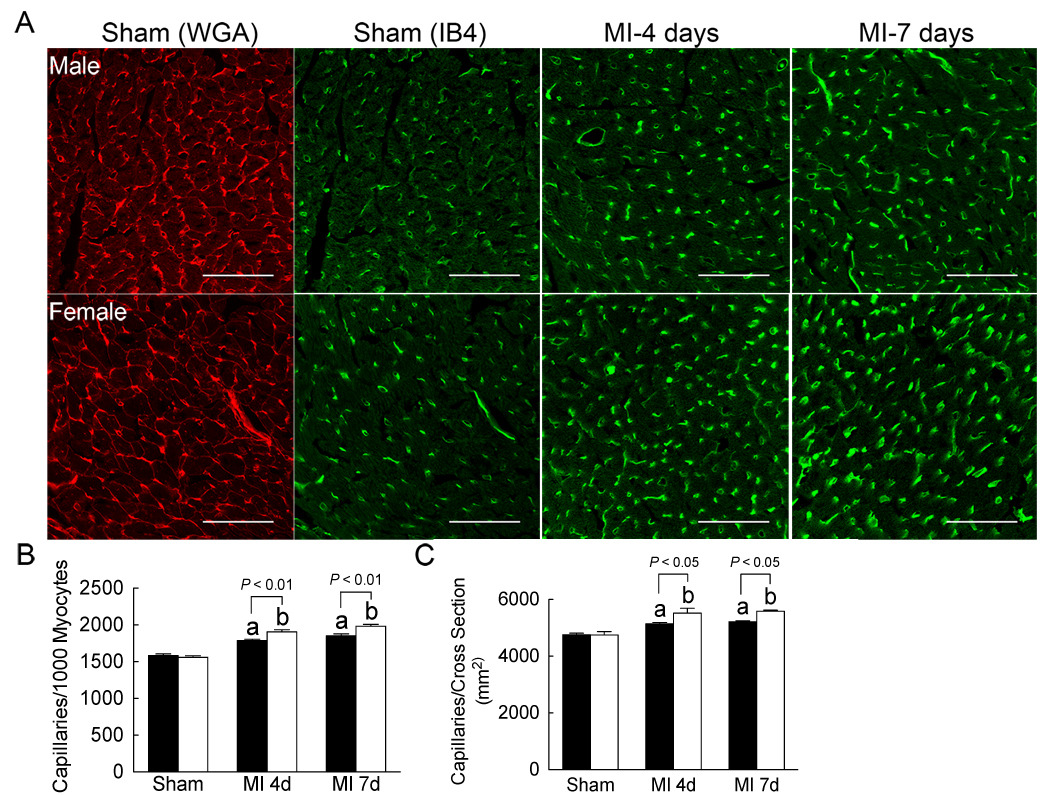
To determine if angiogenesis was increased in female mice following MI as suggested by our array analysis (Table 2), we measured capillary density in both sexes following MI. In sham-operated mice, capillary density was not different between the sexes (Figure 4A–B), but following MI, capillary density was increased proportionally more in females at both four and seven days (Figure 4A–B).
Figure 4. Left ventricular capillary density was increased in females following MI.
(A) Left ventricular (LV) capillary density was measured four and seven days following MI (medium-sized infarcts) in LV sections stained with wheat germ agglutinin (WGA) to identify cell membranes (red) and isolectin B4 (IB4) to identify capillaries (green). Scale bar = 60 µm. (B) Capillary density was measured and expressed as capillaries/1000 myocytes and capillaries/cross section area (mm2) from 4–5 regions per heart. The (a) and (b) indicate a significant increase versus the sex-specific sham. Black bars represent males and white bars represent females and data are presented as mean ± SEM for 5–6 samples per group. Groups were compared by two-way ANOVA with a Bonferonni post-test to determine the contribution of sex or surgery.
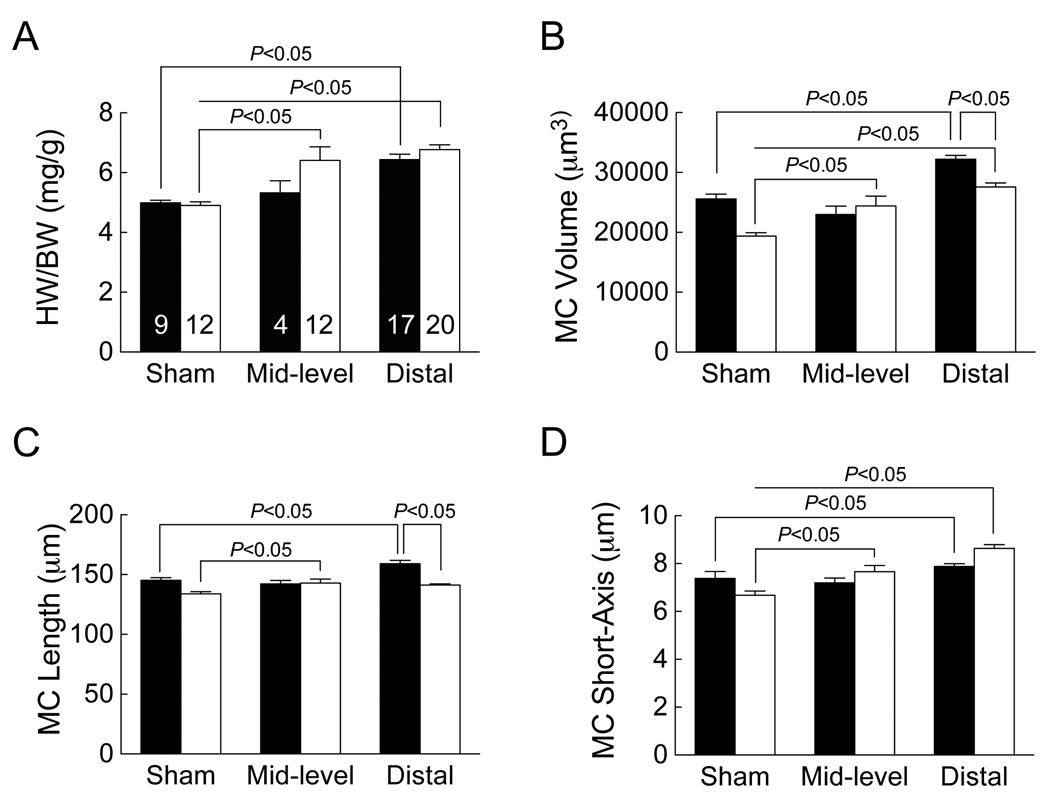
Ventricular remodeling following MI was characterized by myocyte lengthening in males but cross-sectional growth in females
Sixty days following MI, significant ventricular remodeling was evident in the surviving mice of both sexes. For the analysis of remodeling at 60 days following MI, we focused on mice with small infarcts, as survival in males with medium infarcts was low (Figure 1B). In mice with small infarcts, slightly greater hypertrophic responses (measured as heart weight-to-body weight ratio (HW/BW)) were observed in females (Figure 5A and Supplemental Table 2) with a corresponding increase in myocyte volume (Figure 5B and Supplemental Table 2), which would almost completely explain the increase in heart weight. Interestingly, myocyte shape (measured on isolated myocytes) was altered in a sex-specific manner. In males with small infarcts, the increase in myocyte volume was explained by an increase in myocyte length (Figure 5C and Supplemental Table 2), whereas in females with small infarcts (and medium-sized infarcts), the increase in myocyte volume was explained by an increase in the minor diameter of the cross-section (assuming an ellipsoid cross-section) (Figure 5D and Supplemental Table 2).
Figure 5. Ventricular remodeling following MI was characterized by myocyte lengthening in males but cross-sectional growth in females.
(A) Sixty days following MI (small infarcts), body weights were recorded, mice were euthanized, heart weight was measured, and HW/BW calculated. (B–D). Cardiac myocytes (MC) were isolated and myocyte volume was determined using a Coulter Channelyzer. Myocyte length was determined microscopically (40 cells/sample). Short axis length was then calculated assuming an ellipsoid cross-section. Data are presented as mean ± SEM for the number of mice shown in A (male, black bars; female, white bars). Groups were compared by two-way ANOVA with Bonferroni post-test to determine the contribution of sex or infarct size.
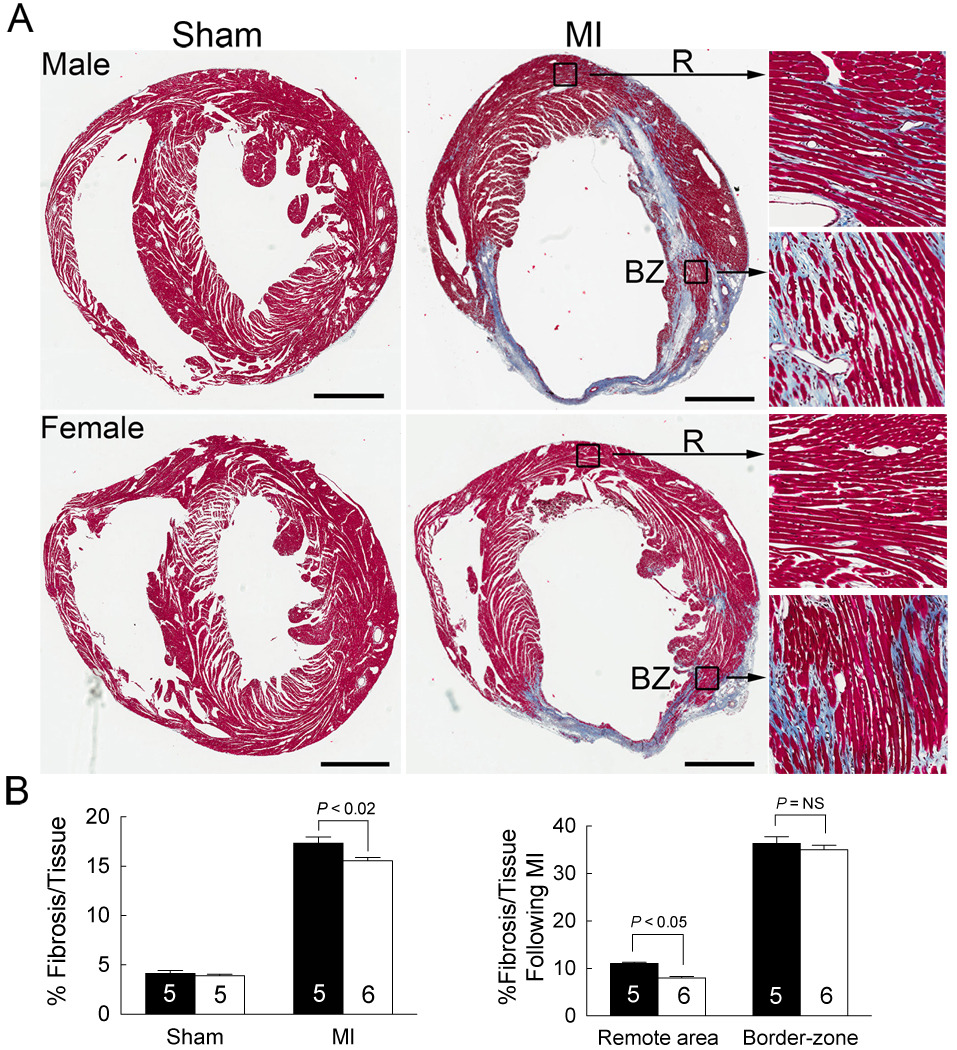
Left ventricular fibrosis was attenuated in females versus males following MI
To determine if the fibrotic response was different between the sexes following MI, as suggested by our array analysis (Table 2), we measured left ventricular fibrosis in both sexes 60 days following MI. In sham operated mice, left ventricular fibrosis was not different between the sexes, but following MI, interstitial fibrosis in the remote/spared myocardium increased to a greater degree in males, but was not different in the border zone (Figure 6A–B).
Figure 6. Left ventricle fibrosis was attenuated in females versus males following MI.
(A) Left ventricular (LV) fibrosis was measured 60 days following MI (small infarcts) in LV sections from male and female hearts stained with Masson’s Trichrome. Scale bar = 1 mm. LV interstitial fibrosis is shown by high magnification in both the remote area (R) and the border-zone (BZ) (B) LV fibrosis was quantified and expressed as the fibrosis area/total area of the whole ventricle or the infarct zone and spared myocardium. Black bars represent males and white bars represent females: the data are presented as mean ± SEM for the number of mice shown. Groups were compared by two-way ANOVA with a Bonferonni post-test to determine the contribution of sex or surgery.
DISCUSSION (completely re-written)
Here we identified an association between gene expression and better survival in female mice following MI. Confirming what others have observed [4–7], we found that following MI, survival was better in females. Interestingly, despite similar infarct size and functional impairment, males were more likely to develop left ventricular dilation. This left ventricular dilation, an indicator of diastolic dysfunction and the best predictor of mortality after MI in humans (better than either ejection fraction or SWMI) [14], was correlated with increased mortality in males observed 3–10 days following MI. We hypothesized that the sex-based differential response to MI might involve the induction of specific gene programs in the heart and account for either the better survival in females, the worse survival in males, or both. Using microarrays to examine gene expression three days following MI, we found that surgery had a far greater effect on gene expression, significantly inducing the expression of 5,574 genes, whereas sex had a much smaller effect, with only 187 genes different on the basis of sex, and no genes showed an interaction between both sex and surgery. These lists were reduced to 496 and 14 respectively when a 1.6 fold change threshold was applied. Closer examination of genes induced by surgery revealed that, in general, gene expression was increased relatively more in the female. Forty-nine genes from the list of 496 induced by surgery 1.6 fold or more showed a 1.5 fold greater induction in females relative to males, whereas none were induced 1.5 fold more in males relative to females (Table 2). Based on gene ontology, these 49 genes and the 14 that were different between the sexes (63 total) identified an increase in a set of gene programs related to angiogenesis, extracellular matrix remodeling, and immune response. A less stringent analysis, comparing surgery to sham in males and females separately, largely confirmed this finding. This pattern of relative induction of gene expression in females was associated with increased capillary density 4–7 days following MI, and with less pathologic myocyte remodeling and decreased fibrosis 60 days following MI. In summary, our results suggest an association between improved survival and less pathologic remodeling and the relative induction of gene expression in females following myocardial infarction.
Several studies have measured global changes in gene expression following infarction in animal models and in humans with ischemic cardiomyopathy. To date, the most complete study in mice included a genome-wide analysis of gene expression from 15 minutes to 48 hours following infarction in male C57BL/6 mice. This study compared approximately 37,000 transcripts from three regions of the heart, infarct, left ventricular free wall, and interventricular septum, in sham versus infarcted mice [15]. This study identified 515 unique transcripts regulated in the first 48 hours following infarction and the induction of two gene programs related to AP-1 transcription factors and nitric oxide production [15]. Comparison of this list of 515 genes induced in males following infarction to our 496 genes induced by surgery identified 131 genes in common. However, this study examined only males and further comparisons are difficult to make. To date, the most complete study in humans, examined over 22,000 transcripts in samples from 21 patients with end-stage non-ischemic heart failure, 10 patients with end-stage ischemic heart failure, and 6 non-failing controls [16]. This study identified 41 genes common to non-ischemic and ischemic, and 31 genes unique to ischemic versus non-failing hearts, but no overlap with our study. In summary, we saw good agreement with the previous study in male mice, but not the study in humans with end-stage ischemic cardiomyopathy, which we believe can be attributed to the differences in time points analyzed in the studies in mice versus human.
Only a few previous studies have compared global changes in gene expression between the sexes in cardiovascular disease. One study examined gene expression in failing and non-failing human hearts and identified five genes that varied by sex regardless of heart failure status and 10 genes that specifically varied by sex in failing hearts [17]. Although a relatively small number compared to the current study, our study was performed three days following MI, when one might suspect transcriptional responses to be maximal, whereas the previous study examined gene expression in failing hearts long after the disease phenotype was firmly established [17]. Another recent study used microarray analysis to examine gene expression in mice two weeks following aortic constriction where differences in hypertrophy and function were observed after 6–9 weeks [18]. In this case, there was a more equal distribution in gene expression with 338 genes induced more in females relative to males and 315 genes induced more in males relative to females following TAC [18]. Although following aortic constriction the changes in gene expression seemed more similar between the sexes, our observation of a primarily female-specific transcriptional response might be related to the fact that we measured gene expression at an earlier time point (three days versus two weeks). In that regard, another study found that chronic pressure overload resulted in similar numbers of differentially expressed genes between males and females, but acute pressure overload resulted in more differentially expressed genes in males [19]. All of these studies, including ours, identify significant differences in transcriptional responses between the sexes, but additional research is required to draw more specific conclusions.
Angiogenesis is an important component of ventricular remodeling, and following injury a failure of angiogenesis can lead to a more pathologic response as shown in several studies [20–22]. Our microarray analysis suggested that angiogenesis-related genes were induced relatively more in females (Table 2). This group contained dimethylarginine dimethylaminohydrolase 1 (Ddah1), which inhibits NO production and promotes endothelial cell growth. Previous studies demonstrated that transgenic overexpression of Ddah promoted angiogenesis in a mouse model of hind-limb ischemia [23]. Another gene in this group, sphingosine kinase 1 (Sphk1), mediates the production of sphingosine-1-phosphate, which acting in autocrine/paracrine pathways mediates endothelial cell proliferation and migration essential for angiogenesis [24]. In addition, adenoviral gene transfer of Sphk1 protected against ischemia reperfusion injury and attenuated ventricular remodeling in rats [25]. In summary, the relative induction of angiogenesis-related genes correlated with an increase in capillary density, and might contribute to the better outcomes we observed in females following MI.
Our microarray analysis indicated that the immune response might be increased in females, although we did not see a difference in infiltrating cells into the infarct zone in either sex (data not shown). Interestingly, another report demonstrated a sex-based difference in the immune response following MI in mice [5]. In this report, the authors demonstrated that one day following MI, males showed a significant two-fold greater neutrophil infiltration into the infarct relative to females. Conversely, they also found that four days following MI, females showed a significant two-fold greater macrophage infiltration into the infarct relative to males. Infiltrating neutrophils are thought to release Mmp9 [26], which could adversely affect the early response to injury in the males, resulting in their higher rate of mortality. However, neutrophil accumulation, in both males and females, peaks at day 1, and our gene expression data is collected at three days, by which time neutrolphils are being cleared [5]. Macrophage accumulation in both sexes reaches a maximum around four days following infarction [5]. Following MI, macrophages mediate phagocytosis of necrotic cells, fibroblast activation and scar formation, and endothelial cell activation and angiogenesis [27]. Of note, our array analysis identified several genes important for recruitment of macrophages or secreted by macrophages that mediate extracellular matrix remodeling and angiogenesis. This list includes Cxcl10 (chemotractant) Il1β, Adamst4, Casp8 (required for macrophage activation), Mmp9, Mmp12, Rpb1, Mpeg, Tgfβ1, Msr. Interestingly, Cxcl10 and Mmp12 were increased in females mice relative to male mice following MI (Table 2).
Our results confirm that female mice adapt better than males to pathologic stress (in this case MI), which is generally true in humans until after menopause. It would seem that the most likely explanation for this sex-based protection in females would be estrogen. In humans, premenopausal women with cardiovascular disease have better outcomes, whereas postmenopausal women have an increase in morbidity and death (for a review see [28]). This correlation was one reason behind the push for hormone replacement therapy in women, but results from the two major trials, HERS II and WHI, failed to show a benefit [2, 3]. Both trials showed a significant increase in the risk of cardiovascular disease in the first year of therapy with decreased risk during later stages of the trial, despite an overall improvement in lipid profiles [29, 30]. While this might argue against a protective role for estrogen, a secondary analysis showed that initiation of hormone replacement therapy within 10 years of menopause versus 10 years after tended to show a reduced risk of cardiovascular disease [31]. Therefore, the protection afforded to women is likely more complex than just estrogen alone, indicating the need for more research to understand the cellular and molecular mechanisms responsible for the better outcomes in women with cardiovascular disease.
In summary, we observed better outcomes in female mice following MI. Using microarray analysis, we identified an association between improved survival and less pathologic remodeling and the relative induction of gene expression in females following myocardial infarction. Finally, the analysis presented here might constitute a platform for further study into sex-based differences following MI.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge David Wang, MD and the Physiology Core at Sanford Research/USD, and the Genomic-Microarray/qPCR Facility, at the Burnham Institute for Medical Research, La Jolla, CA for technical assistance. The authors also acknowledge Paul C. Simpson, MD, and William Harris, PhD, for critical discussion of the manuscript.
SOURCES OF FUNDING
This work was supported by grants from the NIH (F32 HL085980-02, CDW), the AHA (0435338Z, TDO), the South Dakota State Legislature (2010 Grant, TDO), and the NIH (P20 RR-017662, TDO).
NON-STANDARD ABBREVIATIONS
- MI
myocardial infarction
- CHD
coronary heart disease
- SWMI
segmental wall motion index
- HERS II
Heart and Estrogen/Progestin Replacement Study
- WHI
Women’s Health Initiative
- LAD
left anterior descending coronary artery
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None
REFERENCES
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008 Jan 29;117(4):e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) Jama. 2002 Jul 3;288(1):49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- 3.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. Jama. 2002 Jul 17;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 4.Cavasin MA, Sankey SS, Yu AL, Menon S, Yang XP. Estrogen and testosterone have opposing effects on chronic cardiac remodeling and function in mice with myocardial infarction. Am J Physiol Heart Circ Physiol. 2003 May;284(5):H1560–H1569. doi: 10.1152/ajpheart.01087.2002. [DOI] [PubMed] [Google Scholar]
- 5.Cavasin MA, Tao Z, Menon S, Yang XP. Gender differences in cardiac function during early remodeling after acute myocardial infarction in mice. Life Sci. 2004 Sep 17;75(18):2181–2192. doi: 10.1016/j.lfs.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 6.Cavasin MA, Tao ZY, Yu AL, Yang XP. Testosterone enhances early cardiac remodeling after myocardial infarction, causing rupture and degrading cardiac function. Am J Physiol Heart Circ Physiol. 2006 May;290(5):H2043–H2050. doi: 10.1152/ajpheart.01121.2005. [DOI] [PubMed] [Google Scholar]
- 7.Fang L, Gao XM, Moore XL, Kiriazis H, Su Y, Ming Z, et al. Differences in inflammation, MMP activation and collagen damage account for gender difference in murine cardiac rupture following myocardial infarction. J Mol Cell Cardiol. 2007 Nov;43(5):535–544. doi: 10.1016/j.yjmcc.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Pelzer T, Loza PA, Hu K, Bayer B, Dienesch C, Calvillo L, et al. Increased mortality and aggravation of heart failure in estrogen receptor-beta knockout mice after myocardial infarction. Circulation. 2005 Mar 29;111(12):1492–1498. doi: 10.1161/01.CIR.0000159262.18512.46. [DOI] [PubMed] [Google Scholar]
- 9.Pedram A, Razandi M, Lubahn D, Liu J, Vannan M, Levin ER. Estrogen inhibits cardiac hypertrophy: role of estrogen receptor-beta to inhibit calcineurin. Endocrinology. 2008 Jul;149(7):3361–3369. doi: 10.1210/en.2008-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patten RD, Pourati I, Aronovitz MJ, Baur J, Celestin F, Chen X, et al. 17beta-estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phosphoinositide-3 kinase/Akt signaling. Circ Res. 2004 Oct 1;95(7):692–699. doi: 10.1161/01.RES.0000144126.57786.89. [DOI] [PubMed] [Google Scholar]
- 11.Korte T, Fuchs M, Arkudas A, Geertz S, Meyer R, Gardiwal A, et al. Female mice lacking estrogen receptor beta display prolonged ventricular repolarization and reduced ventricular automaticity after myocardial infarction. Circulation. 2005 May 10;111(18):2282–2290. doi: 10.1161/01.CIR.0000164262.08004.BB. [DOI] [PubMed] [Google Scholar]
- 12.van Eickels M, Patten RD, Aronovitz MJ, Alsheikh-Ali A, Gostyla K, Celestin F, et al. 17-beta-estradiol increases cardiac remodeling and mortality in mice with myocardial infarction. J Am Coll Cardiol. 2003 Jun 4;41(11):2084–2092. doi: 10.1016/s0735-1097(03)00423-6. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Takagawa J, Sievers RE, Khan MF, Viswanathan MN, Springer ML, et al. Validation of the wall motion score and myocardial performance indexes as novel techniques to assess cardiac function in mice after myocardial infarction. Am J Physiol Heart Circ Physiol. 2007 Feb;292(2):H1187–H1192. doi: 10.1152/ajpheart.00895.2006. [DOI] [PubMed] [Google Scholar]
- 14.Moller JE, Whalley GA, Dini FL, Doughty RN, Gamble GD, Klein AL, et al. Independent prognostic importance of a restrictive left ventricular filling pattern after myocardial infarction: an individual patient meta-analysis: Meta-Analysis Research Group in Echocardiography acute myocardial infarction. Circulation. 2008 May 20;117(20):2591–2598. doi: 10.1161/CIRCULATIONAHA.107.738625. [DOI] [PubMed] [Google Scholar]
- 15.Harpster MH, Bandyopadhyay S, Thomas DP, Ivanov PS, Keele JA, Pineguina N, et al. Earliest changes in the left ventricular transcriptome postmyocardial infarction. Mamm Genome. 2006 Jul;17(7):701–715. doi: 10.1007/s00335-005-0120-1. [DOI] [PubMed] [Google Scholar]
- 16.Kittleson MM, Minhas KM, Irizarry RA, Ye SQ, Edness G, Breton E, et al. Gene expression analysis of ischemic and nonischemic cardiomyopathy: shared and distinct genes in the development of heart failure. Physiol Genomics. 2005 May 11;21(3):299–307. doi: 10.1152/physiolgenomics.00255.2004. [DOI] [PubMed] [Google Scholar]
- 17.Boheler KR, Volkova M, Morrell C, Garg R, Zhu Y, Margulies K, et al. Sex- and age-dependent human transcriptome variability: implications for chronic heart failure. Proc Natl Acad Sci U S A. 2003 Mar 4;100(5):2754–2759. doi: 10.1073/pnas.0436564100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witt H, Schubert C, Jaekel J, Fliegner D, Penkalla A, Tiemann K, et al. Sex-specific pathways in early cardiac response to pressure overload in mice. J Mol Med. 2008 Sep;86(9):1013–1024. doi: 10.1007/s00109-008-0385-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinberg EO, Mirotsou M, Gannon J, Dzau VJ, Lee RT, Pratt RE. Sex dependence and temporal dependence of the left ventricular genomic response to pressure overload. Physiol Genomics. 2003 Jan 15;12(2):113–127. doi: 10.1152/physiolgenomics.00046.2002. [DOI] [PubMed] [Google Scholar]
- 20.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, et al. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005 Aug;115(8):2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heineke J, Auger-Messier M, Xu J, Oka T, Sargent MA, York A, et al. Cardiomyocyte GATA4 functions as a stress-responsive regulator of angiogenesis in the murine heart. J Clin Invest. 2007 Nov;117(11):3198–3210. doi: 10.1172/JCI32573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Laan AM, Piek JJ, van Royen N. Targeting angiogenesis to restore the microcirculation after reperfused MI. Nat Rev Cardiol. 2009 Aug;6(8):515–523. doi: 10.1038/nrcardio.2009.103. [DOI] [PubMed] [Google Scholar]
- 23.Jacobi J, Sydow K, von Degenfeld G, Zhang Y, Dayoub H, Wang B, et al. Overexpression of dimethylarginine dimethylaminohydrolase reduces tissue asymmetric dimethylarginine levels and enhances angiogenesis. Circulation. 2005 Mar 22;111(11):1431–1438. doi: 10.1161/01.CIR.0000158487.80483.09. [DOI] [PubMed] [Google Scholar]
- 24.Limaye V. The role of sphingosine kinase and sphingosine-1-phosphate in the regulation of endothelial cell biology. Endothelium. 2008 May–Jun;15(3):101–112. doi: 10.1080/10623320802125342. [DOI] [PubMed] [Google Scholar]
- 25.Duan HF, Wang H, Yi J, Liu HJ, Zhang QW, Li LB, et al. Adenoviral gene transfer of sphingosine kinase 1 protects heart against ischemia/reperfusion-induced injury and attenuates its postischemic failure. Hum Gene Ther. 2007 Nov;18(11):1119–1128. doi: 10.1089/hum.2007.036. [DOI] [PubMed] [Google Scholar]
- 26.Lindsey M, Wedin K, Brown MD, Keller C, Evans AJ, Smolen J, et al. Matrix-dependent mechanism of neutrophil-mediated release and activation of matrix metalloproteinase 9 in myocardial ischemia/reperfusion. Circulation. 2001 May 1;103(17):2181–2187. doi: 10.1161/01.cir.103.17.2181. [DOI] [PubMed] [Google Scholar]
- 27.Lambert JM, Lopez EF, Lindsey ML. Macrophage roles following myocardial infarction. Int J Cardiol. 2008 Nov 12;130(2):147–158. doi: 10.1016/j.ijcard.2008.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Konhilas JP, Leinwand LA. The effects of biological sex and diet on the development of heart failure. Circulation. 2007 Dec 4;116(23):2747–2759. doi: 10.1161/CIRCULATIONAHA.106.672006. [DOI] [PubMed] [Google Scholar]
- 29.Mohandas B, Mehta JL. Lessons from hormone replacement therapy trials for primary prevention of cardiovascular disease. Curr Opin Cardiol. 2007 Sep;22(5):434–442. doi: 10.1097/HCO.0b013e328201cb7a. [DOI] [PubMed] [Google Scholar]
- 30.Herrington DM, Klein KP. Randomized clinical trials of hormone replacement therapy for treatment or prevention of cardiovascular disease: a review of the findings. Atherosclerosis. 2003 Feb;166(2):203–212. doi: 10.1016/s0021-9150(02)00202-2. [DOI] [PubMed] [Google Scholar]
- 31.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. Jama. 2007 Apr 4;297(13):1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.