Abstract
Changes in mechanical properties are an essential characteristic of the aging process of human skin. Previous studies attribute these changes predominantly to the altered collagen and elastin organization and density of the extracellular matrix. Here, we show that individual dermal fibroblasts also exhibit a significant increase in stiffness during aging in vivo. With the laser-based optical cell stretcher we examined the viscoelastic biomechanics of dermal fibroblasts isolated from 14 human donors aged 27 to 80. Increasing age was clearly accompanied by a stiffening of the investigated cells. We found that fibroblasts from old donors exhibited an increase in rigidity of ∼60% with respect to cells of the youngest donors. A FACS analysis of the content of the cytoskeletal polymers shows a shift from monomeric G-actin to polymerized, filamentous F-actin, but no significant changes in the vimentin and microtubule content. The rheological analysis of fibroblast-populated collagen gels demonstrates that cell stiffening directly results in altered viscoelastic properties of the collagen matrix. These results identify a new mechanism that may contribute to the age-related impairment of elastic properties in human skin. The altered mechanical behavior might influence cell functions involving the cytoskeleton, such as contractility, motility, and proliferation, which are essential for reorganization of the extracellular matrix.
Introduction
Aging is the result of a complex interaction of biological, physical, and biochemical processes that cause changes and damage to molecules, cellular function, and organs. To date, several studies have examined age-related alterations in cell division, biosynthesis (1–3), and cell migration (4), but there is little information about the relationship between aging and the mechanical properties of cells. Previous studies have shown that biomechanical properties are important for cell functions that are regulated by mechanical forces (5,6). Individual cells are able to sense mechanical signals and transduce them into a biochemical response. When the synthesis activity of cells is compared in stressed versus relaxed collagen gels, as well as for cells growing in collagen gels versus plastic dishes (7–10), a link is observed between the collagen production of fibroblasts and the mechanical forces acting on the cells. This is not surprising, since active and passive cellular biomechanics based on the stability provided by the cytoskeleton impacts many essential cellular functions. In addition to the role of the cytoskeleton in cellular motility and division (11,12), it has been shown that a functionally intact cytoskeleton is crucial to build up contraction forces in a three-dimensional collagen lattice (13,14). These cell functions are essential for the homeostasis of the extracellular matrix (ECM).
Considering the importance of cellular biomechanics for correct physiological functioning, we analyzed the mechanical behavior of dermal fibroblasts isolated from human donors of different ages. Changes in mechanical properties of the skin are generally referred to extracellular aspects such as alterations in polymerization and cross-linking of collagen and elastin (15). In this context, age-related changes have been observed for protein synthesis and production of ECM components (1,16,17). Further, atomic force microscopy studies indicate that individual epithelial cells show a considerable increase in stiffness in vitro at higher passage numbers (18,19). Even though comparisons of cells of early and late passage have yielded important insights into the aging process in vitro, such models cannot substitute for studies of cells isolated from aged human donors (aged in vivo). Thus far, no study has compared the viscoelastic properties of dermal fibroblasts from young and advanced-age donors. To address this issue, the optical stretcher technique was applied to examine the deformability of fibroblasts isolated from 14 human donors.
The microfluidic optical stretcher is an optical trap that allows trapping and controlled deformation of suspended cells using two counterpropagating laser beams (20). Cellular extension is caused by a momentum transfer acting on the cell surface. The global stress applied by the optical stretcher permits the measurement of whole-cell elasticity that characterizes the integral effects of molecular changes on the cytoskeleton. The use of optical deformability as a sensitive cell marker has already been demonstrated for characterization of individual cancer cell lines (21), as well as for cancer diagnosis by mechanical phenotyping of oral keratinocytes (22). We suggest that optical deformability can also serve as a sensitive biomarker of aging on the level of individual dermal cells. To date, cellular senescence as a measure of aging has been characterized by molecular markers based on an altered pattern of gene and protein expression. Typical known biomarkers are senescence-associated β-galactosidase (23), telomere dysfunction (24,25), expression of activated p53 and p16 (25,26), and nuclear accumulation of globular actin (27).
Since viscoelastic properties are determined by the cytoskeletal polymers, and actin is supposed to be a key element (28,29), we further quantified the amount of polymerized actin using FACS measurements. In addition, possible age-related changes in vimentin and microtubule content were analyzed. In vivo, there are many cells able to perform mechanical work, for example, muscle cells or cells of connective tissues that are connected to the proteins of the ECM. Three-dimensional collagen lattices seeded with fibroblasts have been used to analyze the effect of actin or microtubule disruption on the mechanical activity of cells (13,14). The mechanical properties of cells and the state of the cytoskeleton affect the interaction between fibroblasts and the ECM (30–32), so mechanical changes may reveal new aspects of the age-related degradation of the elastic properties of the dermis. Rheological measurements were used to characterize fibroblast-populated collagen gels as dermis equivalents. These experiments determine the influence of changes in the mechanical properties of cells and the cytoskeleton on the organization and mechanical behavior of the collagen matrix.
Materials and Methods
Cell culture
Biopsies were isolated from plastic surgery procedures. We adhered to the recommendations of the current version of the Declaration of Helsinki as applied to a non-drug study. All donors provided written, informed consent. Human dermal fibroblasts (HDFs) were isolated by outgrowth from skin biopsy samples obtained from healthy female donors of different ages (20–80 years). Primary cells were enzymatically prepared using a dispase digestion technique. Square-cut biopsy pieces were washed, rinsed, and incubated in dispase (Boehringer Mannheim, Mannheim, Germany) for 2 h at 37°C and 7% CO2. The dermis and epidermis were then separated and the dermal fraction was cultured in six-well plates containing Dulbecco's modified Eagle's medium (Life Technologies, Eggenstein, Germany) supplemented with 10% fetal calf serum (Life Technologies) and penicillin/streptomycin (50 μg/ml, Life Technologies). Confluent fibroblasts were seeded into appropriate flasks. For the experiments, cells were used at passage 3 grown to 90% confluency. Generally, the two age groups were defined as young donors <42 years and old donors >60 years. Due to limited biopsy material, donor ages can vary between different experiments.
Refractive index measurements
The refractive indices of fibroblast populations from different-aged donors were measured using a phase-matching technique (33–35). Cells are suspended in bovine serum albumin (BSA) phosphate-buffered saline solutions with different concentrations of BSA. Since the refractive index of the solution depends on the protein concentration, the refractive index of the surrounding medium is varied.
The exact refractive index of each solution is measured using an Abbe-refractometer (AR4, Krüss Optronics, Hamburg, Germany) and the appearance of the cells in a phase-contrast microscope is analyzed. Cells with an index of refraction lower than the surrounding medium are brighter than the background, and cells with a higher refractive index appear dark (Fig. S8 A in the Supporting Material). Assuming a normal distribution for the refractive indices of the cells, the percentage of cells that appear brighter than the background can be fitted by an error function (Fig. S8 B):
| (1) |
Here, σ is the standard deviation of the distribution and μ is the mean value of the refractive index, which corresponds to the inflection point of the error function. Both parameters are determined by the fit of the data.
Optical stretcher
The optical stretcher is an optical trap consisting of two coaxially aligned, counterpropagating divergent laser beams that apply optical forces to the surfaces of suspended cells (20,21). Increasing the laser power will deform the whole cell along the laser-beam axis. The stretching is caused by transfer of momentum to the cell membrane pointing away from the medium of higher refractive index (21,36). Stress magnitude and distribution can be calculated by a ray optics approach. A schematic of a resulting stress profile acting on the cell surface is shown in Fig. 1 A.
Figure 1.
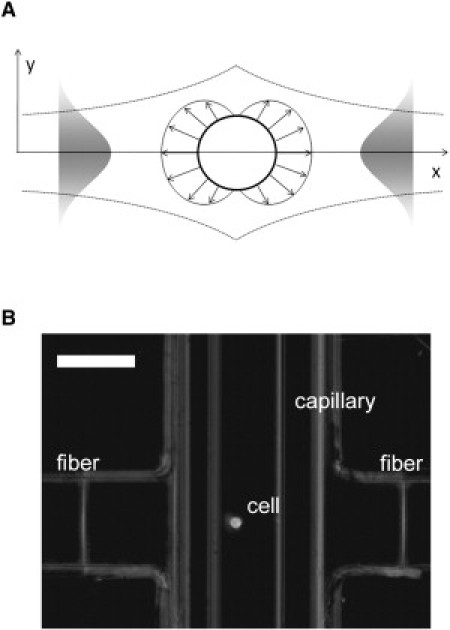
(A) Schematic of a stress profile acting on the cell surface due to momentum transfer caused by two divergent laser beams with Gaussian intensity profile. The peak stress along the laser beam axis depends on the laser power and refractive indices of the cell and the surrounding medium. (B) Optical stretcher setup. Two opposing optical fibers are aligned perpendicular to a glass capillary holding a suspended cell (37). Scale bar, 50 μm.
The setup consists of an ytterbium-doped fiber laser operating at a wavelength of 1064 nm (YLM-10-1064, IPG Photonics, Burbach, Germany) with a maximum output power of 10 W. The optical fiber was spliced to a 1 × 2 coupler, splitting the light in a 50:50 ratio. Cells were positioned between the two laser beams by a microfluidic delivery system (37) For this, a glass capillary tube (VitroCom, Mountain Lakes, NJ) was situated between the two fiber ends (Fig. 1 B). The optical fibers (PureMode HI 1060, Corning, Wiesbaden, Germany) were placed at a distance of ∼120 μm from the capillary wall. The whole microfluidic system was mounted on an inverted phase-contrast microscope (DMIL, Leica, Solms, Germany) and deformations of the cells were recorded at a rate of 30 frames/s using a CCD camera (A202k, Basler, Ahrensburg, Germany). The images obtained were analyzed automatically by a LabView routine that determines the cell boundaries (Fig. 1 A) and calculates the strain of the cells along the laser-beam axis (20,21). The laser power used was P = 0.2 W/fiber for trapping the cells and P = 1.2 W/fiber for the stretching. The resulting extension behavior was analyzed, and viscoelastic properties of the cells were calculated as described in Wottawah et al. (38). In the three-parameter model used, optical deformability, which corresponds to the compliance of the cells, is described by
| (2) |
when the stress is applied (0 < t < t1) and by
| (3) |
when the laser is off again (t > t1). Here, σ represents the applied stress and a1, a2, and b1 are parameters derived from the viscoelastic constitutive equation. Further information about calculation of the stress, σ, can be found in Guck et al. (20) and Wottawah et al. (38). The constants can be obtained by fitting the data to allow the calculation of rheological parameters like the plateau Young's modulus, Ep, which is a measure of the overall elastic behavior of cells:
| (4) |
FACS analysis
After incubation of fibroblasts to a confluence level of 90%, cells were trypsinized, centrifuged at 10,000 rpm, and washed twice with PBS. Cells were then resuspended in 3% paraformaldehyde and incubated for 30 min at room temperature. After two additional washing steps, the cells were permeabilized with 0.5% Triton-X100 (Sigma, St. Louis, MO) in PBS. Cells were then washed, blocked for 30 min with 3% BSA, and washed again. For actin staining, Alexa-488-labeled Dnase I and Alexa-647-labeled phalloidin (Invitrogen, Paisley, United Kingdom) were added at concentrations of 161.0 μM and 6.6 μM, respectively. Cells were incubated for 20 min at room temperature. After two washing steps with PBS, the fluorescence was measured using a FACSCanto flow cytometer (BD Biosciences, San Jose, CA) and analyzed by the software BD FACSDiva 4.0.
Concerning microtubule staining, the cells were incubated for 30 min with Oregon Green 488 conjugated paclitaxel (Invitrogen) at a concentration of 1.0 μM. Vimentin was detected using an antibody (sc-32322, dilution 1:200) purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Tissue rheology
For preparation of dermis equivalents, 100 mg Type I collagen (Sigma, St. Louis, MO) was dissolved at 4°C in 33.3 ml of 0.1% sterile acetic acid (c = 3 mg/ml). The used fibroblast populated gels consist of 80% collagen solution, 10% 10× Hanks' buffer salt solution (Biochrom, Berlin, Germany), and 10% fetal calf serum containing 3 × 106 suspended cells/ml. The final solution contained 2.4 mg/ml collagen and 3 × 105 fibroblasts/ml. NaOH (1 M) was added dropwise to neutralize the solution, whereof 2.5 ml was applied to six-well plates (Greiner, Germany) and incubated at 37°C and 7% CO2 for 1 h. The gels were covered with 2 ml medium (DMEM, 1% penicillin/streptomycin, 1% glutamax, and 10% FCS) and incubated for another 23 h at 37°C and 7% CO2. To study the specific effect of cytoskeletal stiffening, collagen gels were treated for 48 h with 0.5 μM jasplakinolide in DMEM. Jasplakinolide is described as promoting actin polymerization and stabilizes actin filaments, leading to increased cellular stiffness (38,39). A possible connection between actin stabilization and cellular aging has already been hypothesized by Gourlay et al. (41), who measured an increased reactive oxygen species production in connection with decreased actin turnover induced by jasplakinolide. Although the effect of jasplakinolide treatment is controversial in literature (42), optical stretcher experiments confirmed a higher cell stiffness after drug treatment (Fig. S10).
Viscoelastic properties of the gels were measured by a shear rheometer (AR2000ex, TA Instruments, New Castle, DE) using a plate-plate geometry (diameter, 2 cm). The AR2000ex offers different modes of oscillatory experiments to determine the complex shear modulus, G∗ = G′ + iG″, which depends on shear frequency, stress, and strain. Fibroblast-populated collagen gels were characterized by measurement of the elastic storage modulus, G′, and the viscous loss modulus, G″, in the linear viscoelastic regime (γ = 2% and ω = 5 rad/s). At higher deformation, plastic effects were observed in the form of decreasing storage moduli.
Results
The microfluidic optical stretcher was used to determine the viscoelastic properties, i.e., stiffness, of dermal fibroblasts as a function of donor age. The optical stretcher uses two opposing divergent laser beams to trap and stretch a cell along the laser-beam axis with increasing laser power (20). To compare the optical deformability of cells, it has to be considered that the stretching forces acting on the cell surface crucially depend on the relative refractive index of the cell population and the surrounding medium. That means a cell with a higher refractive index gets more stretched. We determined the refractive indices of fibroblast populations from six different donors using a phase-matching technique described by Barer and Joseph (33–35). Briefly summarized, the percentage of bright and dark cells compared to the surrounding medium is evaluated by varying the optical density of the medium. Fitting an error function to the data provides the distribution of refractive indices of the cells. The differences in optical properties for the tested cell populations were within statistical error, with a mean refractive index of 1.368 (Supporting Material). Since fibroblasts isolated from different donors cannot be distinguished based on their refractive index, differences in optical deformability can be attributed solely to the mechanical properties of the cells.
To elucidate the relationship between aging and the viscoelastic properties of cells, basic rheologic-step stress experiments were performed with the optical stretcher. Suspended fibroblasts were trapped at a laser power of 200 mW and deformed for 2 s at a constant power of 1.2 W/fiber resulting in a peak stress of ∼1.8 Pa along the laser-beam axis. During the stretch, the relative radial extension, i.e., the strain, γ(t), is monitored. After the laser was switched back to trapping power (t > 3 s), the relaxation behavior was also observed. Fig. 2 A shows the typical viscoelastic extension behavior of two fibroblast populations from different-aged donors. The mean deformability of a cell population, together with the standard error of the mean as a function of time, was determined for every frame recorded. Cells of a 73-year-old donor showed a significantly higher stiffness, leading to decreased deformation compared to those of the 27-year-old donor. Sample sizes in this experiment were nyoung = 30 cells and nold = 24 cells. Assuming a normal distribution, histograms of the deformability at maximum strain were fitted as illustrated in Fig. 2 B. Student's t-test demonstrates that the two samples belong to different populations with a probability of 99%.
Figure 2.
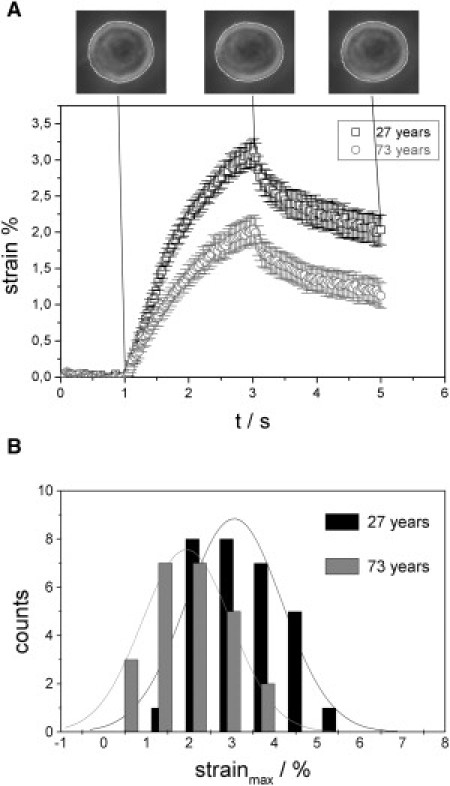
(A) Time-dependent deformation behavior of two fibroblast populations. Cells are stretched for 2 s at a laser power of 1.2 W/fiber in a step-stress experiment and the viscoelastic extension is measured. Error bars show the standard error of the mean. Fibroblasts of an old donor (73 years) show lower deformability resulting in a maximum strain γmax = 2.04% ± 0.19% along the laser beam axis compared to cells of a young donor (27 years; maximum strain, γmax = 3.12% ± 0.18%). (B) Histograms of the maximum strain. A Student's t-test (p < 0.01) indicates that the separation into two populations based on maximum deformability is statistically significant.
Since maximum deformation does not reflect the overall viscoelastic behavior of the cells and uses only a small part of the information provided by the stress-deformation experiments, we also calculated rheological parameters, namely the time- and frequency-dependent elastic moduli. As described in Wottawah et al. (38), these parameters can be derived from optical deformability, which is in general a function of time and corresponds to the compliance of the cells (see Supporting Material). The overall viscoelastic behavior can be characterized by the modulus functions E(t), the time-dependent Young's modulus, and E∗(ω) = E′(ω) + E″(ω), the frequency-dependent complex Young's modulus.
E′(ω) is a measure of the elastic energy stored in the sample during a periodic deformation and is also called the storage modulus. E″(ω) is the loss modulus, which represents the viscous dissipation of energy. E(t) describes the stress relaxation behavior of the cells. Typical graphs of these rheological properties are given in Fig. 3, A and B, and allow the calculation of characteristic material constants such as the elastic plateau modulus, Ep (Fig. 3 A), for short times and high frequencies. To compare the age-dependent elastic behavior of fibroblast populations of different donors, we determined the plateau modulus and found a significant increase in stiffness at higher ages (Fig. 3, C and D). Examining differences between the two age groups, 27–42 years and 61–80 years, the mean plateau modulus was found to range from 143.6 ± 38.3 Pa for the young cell populations to 235.2 ± 29.9 Pa in the elderly group, which is a considerable shift of ∼60% (p = 0.005, Mann-Whitney U-Test). To put this into perspective, the malignant transformation of a fibroblast causes a 30% higher compliance (38). This drastic effect is somewhat surprising and could not be anticipated from previous literature.
Figure 3.
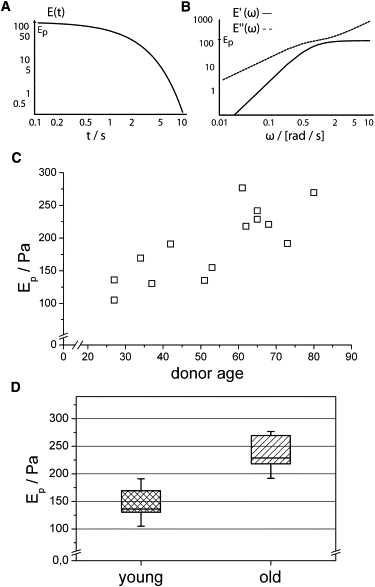
The relation between fibroblast elasticity and donor age. (A and B) The rheological behavior of cells can be characterized by time- (A) and frequency-dependent (B) modulus functions derived from the temporal development of strain (Fig. 2A) (21). (C) As a measure of the elastic strength of the cells, we calculated the plateau Young's modulus, Ep, for all optical stretcher experiments. We identified an age-related increase in cell stiffness. (D) The plateau modulus for the donor age group 27–42 years is significantly shifted to higher values in the age group 61–80 years, with a confidence level of >99% (Mann-Whitney U-test).
Since the state of actin polymerization and the organization of actin filaments is widely believed to be related to the mechanical properties of cells (28,29), the intracellular monomer (G-) and filamentous (F-) actin content was measured by FACS analysis. We compared the degree of polymerized actin for cells of young and old donors by dual fluorescence labeling using Alexa-647 phalloidin and Alexa-488 DNase I. Since the labeling of F-actin and G-actin with phalloidin and DNase I, respectively, is known to be specific and the staining patterns are thought to be spatially separate and distinct (43), this method allowed us to measure the relative amounts of G- and F-actin in the same cell simultaneously. As can be seen by confocal microscopy (Fig. 4 A), the F-actin polymer network forms a dense cortical layer underneath the plasma membrane even when the cells are in suspension. G-actin is distributed throughout the cytoplasm. Some cells also show a nuclear accumulation of G-actin, but the possible correlation with donor age described by Kwak et al. (27) was not observed. Fig. 4, B and C, shows typical fluorescence intensity distributions, obtained by FACS, for the fibroblast populations of a young and an old donor. An increase in the F-actin level is clearly visible for the older donor. To quantify the state of actin polymerization, we determined the G-actin/F-actin signal ratio for a total of 12 donors. The statistical results are given in Fig. 4, D–F. Comparison of the two age groups, 20–27 years (n = 6) and 61–72 years (n = 6), shows that the balance between F- and G-actin is altered in favor of F-actin by ∼31% (p = 0.007, Mann-Whitney U-test). This shift is basically due to an increased F-actin level. Due to its location just beneath the plasma membrane and its high mechanical strength, the actin cortex is thought to dominate the viscoelastic response of the cells to small deformations. The result confirms the hypothesis that the changes in mechanical properties observed are linked to an altered degree of actin polymerization in cells of old individuals.
Figure 4.
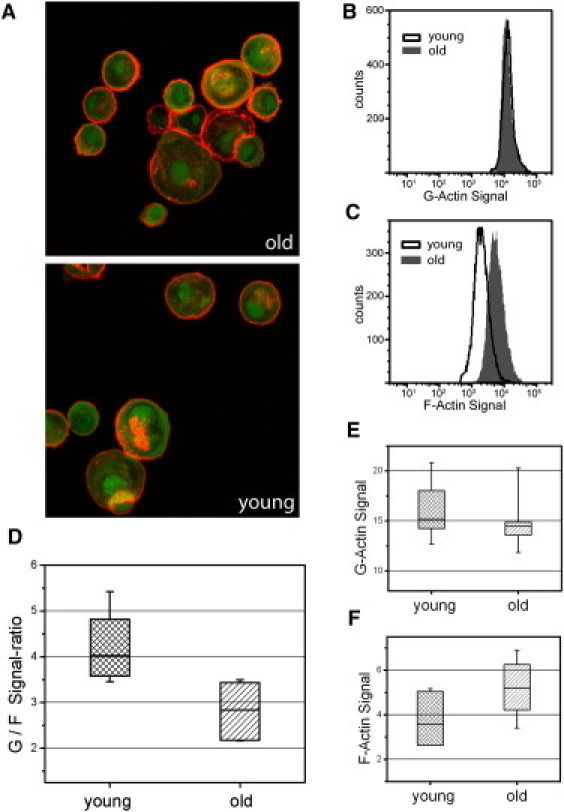
Analysis of actin polymerization in dermal fibroblasts. (A) For illustration of the actin staining, representative fluorescence confocal images of the F-actin cortex (red) and G-actin monomers (green) in suspended cells obtained from a young and an old donor are shown. Actin filaments form a homogeneous layer just beneath the plasma membrane. The fluorescence signals were quantified by FACS measurements to determine the state of actin polymerization. (B and C) Typical intensity distributions for the cells of an old and a young donor. Analyzing the G-/F-actin signal ratio in connection with donor age, we found a statistically significant shift to the polymerized state (p = 0.007, Mann-Whitney U-test) (D). Separate examination of G- and F-actin signals shows that this alteration results primarily from an increased F-actin fraction in fibroblasts of old donors.
To elucidate possible age-related changes of expression of the other cytoskeletal components (microtubules and vimentin), we compared the corresponding intracellular content of fibroblasts isolated from young (20–35 years) and old (60–76 years) donors. After fixation and permeabilization of suspended cells, microtubules and vimentin were stained and fluorescence intensities were quantified by FACS. For both cytoskeletal polymers, a statistically insignificant decrease of the FACS signals was observed at higher donor ages (Fig. 5). A possible correlation between increased cell stiffness and higher microtubule and vimentin content could not be confirmed. Nevertheless, we cannot rule out the possibility that some other events, such as changes in filament organization and cross-linking, contribute to the increased cell stiffness.
Figure 5.
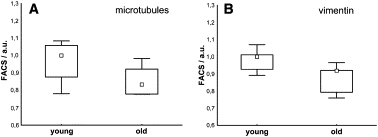
(A) Analysis of age-related changes of the microtubule cytoskeleton. Microtubules were stained using paclitaxel (Oregon Green 488 Taxol) and signals were quantified by FACS measurement. This comparison of cells obtained from young and old donors does not show statistically significant differences (p = 0.13, Mann-Whitney U-test) but does show a slight decrease in signal intensitiy. (B) Fluorescence signals of vimentin also show a nonsignificant decrease at higher donor age (p = 0.10).
The cytoskeleton is the source of mechanical activity of fibroblasts, which is thought to participate in the structural constitution of the dermis and its physiological tension. The impact of changing the cytoskeleton and cell elasticity on the constitution of the extracellular matrix was analyzed by tissue rheology. Dermal fibroblasts from different-aged donors or specifically modified actin cytoskeleton were seeded out in collagen gels to study the mechanical interaction between the cells and the ECM. Dermis equivalents were prepared as described in Materials and Methods and a shear rheometer was used to apply an oscillatory shear stress and measure the mechanical properties of the sample expressed by the complex shear modulus, G∗ = G′ + iG″. As a measure of the elastic response of fibroblast-populated collagen gels, the storage modulus, G′, was determined at a shear rate of ω = 5 rad/s and a maximum strain of γ = 2%. In the linear-viscoelastic regime, the storage modulus is independent of strain. At higher deformations (>4%), shear modulus values decline (data not shown), indicating measurement-generated irreversible plastic damage of the collagen gel, which should be avoided. The loss modulus, G″, reflects the viscous material properties associated with the energy loss in the sample. The results for six individual experiments are given in Fig. 6. In each experiment, cells of a young and an old donor were used, and the storage and loss moduli were measured for three to four gels. Fibroblast-populated collagen gels exhibit elastic rather than viscous behavior, observed as significantly higher values of G′ compared to G″. Gels containing cells of old donors have less mechanical strength, shown by lower values of G′ and G″. In summary, the median of the storage modulus, G′, decreases by ∼11.2% and that of the loss modulus, G″, by ∼11.7%. Since a direct comparison between individual experiments is not feasible because there is a new preparation of collagen solution in each case, a Wilcoxon test for paired samples was used for statistical analysis. Age-related differences in the mechanical properties of the gels are significant (p = 0.046 for G′ and 0.028 for G″). This result has not been described previously in the literature, to our knowledge, and it shows that reorganization of the collagen matrix according to the cells brought into the gel directly influences its mechanical properties.
Figure 6.
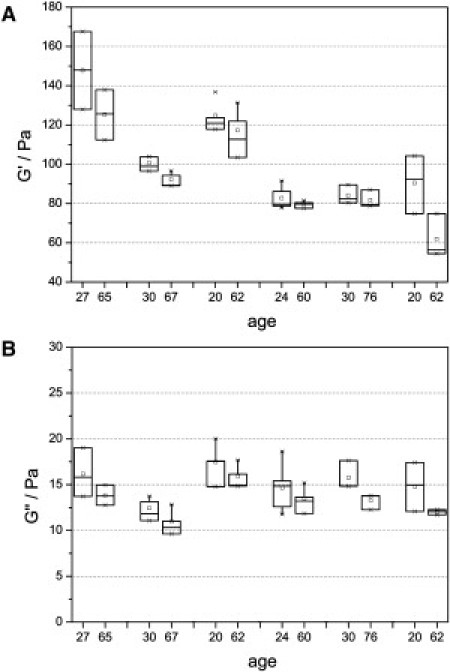
Rheological properties of fibroblast-populated collagen gels depending on donor age. (A) In six individual experiments, three to four gels with cells of a young and an old donor were analyzed using a shear frequency of 5 rad/s and a strain of 2%. A significant decrease of G′ was observed (p = 0.046, Wilcoxon test). (B) The loss modulus, G″, shows a statistically significant decrease with age (p = 0.028, Wilcoxon test). These measurements demonstrate that collagen gels reorganized by aged fibroblasts exhibit less mechanical strength compared to those reorganized by cells of younger individuals.
In addition to analyzing differences in gel properties depending on donor age, the effect of a specific modulation of the actin cytoskeleton of cells was measured. Treatment with jasplakinolide stabilizes actin filaments and was used to model the age-related increase in cell stiffness. Collagen gels were incubated for 24 h in cell culture medium. After that, 0.5 μM jasplakinolide was added, followed by another 48 h of incubation. Six individual experiments with cells of different-aged donors show that jasplakinolide treatment not only increases cell stiffness (40) but also influences the viscoelastic properties of the collagen matrix (Fig. 7). In each experiment, rheological behavior was compared between three treated and three control gels (shear frequency, ω = 5 rad/s; shear deformation, γ = 2%). The median of the storage modulus, G′, shows an average decrease of ∼12.8% in the case of jasplakinolide treatment. The loss modulus, G″, decreases by ∼15.0%. A Wilcoxon test confirms that the observed changes in mechanical properties are statistically significant (p = 0.028 for G′ and 0.028 for G″). The increased stiffness of the actin cytoskeleton caused by jasplakinolide results in altered rheological behavior of the collagen matrix, which reveals a connection between cell mechanics and the elasticity of dermal tissues. Decreased cell viability due to jasplakinolide treatment was excluded by an MTT vitality test.
Figure 7.
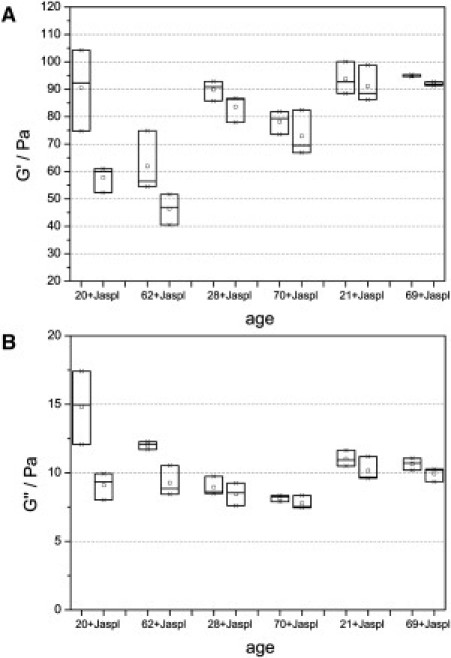
Storage (A) and loss (B) moduli of fibroblast-populated collagen gels measured at a shear frequency of 5 rad/s and a strain of 2%. In each of six individual experiments, six gels with cells of the same donor were analyzed, whereas three gels were treated for 48 h with 0.5 μM jasplakinolide. The increased cytoskeletal stiffness resulted in statistically significant decreases in the value of G′ and G″ (p = 0.028 for G′ and 0.028 for G″, Wilcoxon test), which clarifies that a specific modulation of the actin cytoskeleton has a direct effect on the mechanical behavior of the collagen matrix.
Discussion
Our results show that cellular stiffness is significantly increased in dermal fibroblasts during aging in vivo. The mechanical properties were assessed by measurements of the optical deformability of suspended cells with the optical stretcher. The ability to actually measure significant differences among cells of individual donors depending on age shows how sensitive optical deformability is to changes in structural composition of cells and indicates its potential as a biomarker of aging on the cellular level.
The experiments performed are based on probing the global rheological properties of cells in suspension. Although this may be considered nonphysiological because of the lack of contact with the extracellular matrix, nevertheless, the symmetric shell-like structural design and continuous distribution of stress over the cell surface allow measurement of whole-cell elasticity, i.e., the integrated state of the cytoskeleton, at a high throughput. In addition, the adherence of cells to a hard substrate like culture dishes or microscope slides does not reflect the situation of cells in vivo either, and measuring the mechanical properties of adhered cells using methods like atomic force microscopy (AFM) can introduce artifacts due to mechanical contact and an inhomogeneous cell morphology. By contrast, the optical stretcher allows for capturing effects of molecular changes that are reflected in the entire state of the cytoskeleton. Thus, the measurements with the optical stretcher may not directly reflect the viscoelastic properties of cells as found in a tissue matrix. However, they are a sensitive measure of the state of the cytoskeleton after isolation, which in consequence is responsible for a cell's mechanical stability.
The cytoskeleton is composed of actin, microtubules, and intermediate filaments and is regulated by a set of accessory proteins that can polymerize, cap, sever, cross-link, and bundle the polymers. By adding specific cytoskeleton-disrupting drugs and measuring the effect on cell elasticity, Rotsch et al. (29) demonstrated that the actin cytoskeleton plays a dominant role in a cell's mechanical stability. This is consistent with results from polymer physics showing that a network formed by the semiflexible actin filaments exhibits a higher resistance to an applied shear stress than microtubule and intermediate filament networks (28). We found that depolymerization of F-actin using cytochalasin D leads to a significant increase in optical deformability up to 60% depending on donor age (Supporting Material). The crucial importance of the actin network for the mechanical properties of cells is also confirmed by our results showing that the degree of actin polymerization in fibroblasts of older individuals is shifted toward the filamentous form. This suggests that age-associated loss of cell flexibility is rooted in an altered polymerization of the actin cytoskeleton.
These are important findings, since the cytoskeleton and the mechanical properties of cells are essential for the organization of the collagen matrix, tissue development, and wound healing (15,44). The actin cytoskeleton is responsible for cell contraction, which could be shown by the inhibition of contraction force generation for cytochalasin-D-treated fibroblasts (13). Even concentrations of the F-actin disrupting drug as low as 2 nM lead to a significant alteration of the mechanical properties of a fibroblast-populated collagen matrix (45). Further, Reed et al. (4) have shown that the age-related impairment in cell motility, which is a possible reason for delayed wound repair in dermal tissues, is accompanied by changes in cytoskeletal organization. Rao and Cohen already proposed that alterations in the actin cytoskeletal function may be an important aspect of generalized decrease in cellular function associated with aging (46).
The changes found in the mechanical state and cytoskeletal organization of dermal fibroblasts reveal a new aspect of aging skin that could either directly or indirectly influence the viscoelastic behavior of the dermis. The age-associated impaired flexibility of the skin is generally attributed to a reduced density and modified architectural organization of collagen fibers (15,47), an alteration of the elastic fiber network, and a reduction of the amount of proteoglycans (48). The increased cell stiffness and higher actin polymerization could have a direct impact on cellular contraction capacity, as well as the organization and mechanical properties of the collagen matrix, since the actin cytoskeleton is an essential component of the interaction between fibroblasts and the ECM (31). Here, it was shown, for the first time we know of, that increased cell stiffness affects the mechanical activity of dermal fibroblasts within the collagen matrix, resulting in lower shear-modulus values as the rheological analysis of fibroblast-populated collagen gels has shown. As a model of cytoskeletal aging, the reorganization of the ECM was inhibited by jasplakinolide treatment, which specifically stabilizes actin filaments (39). This indicates a direct effect on skin structure and the mechanical properties of the dermis. The fact that collagen gels show an opposite trend compared to dermal cells, i.e., a decreased stiffness, seems nonintuitive and could be linked to a reduced contraction capacity of stiff fibroblasts (data not shown), leading to an altered structural organization and tension within the collagen matrix.
Besides this direct effect on the organization of the ECM, changes of the actin cytoskeleton could contribute to the altered gene expression in aged fibroblasts, leading to a reduced synthesis of ECM proteins. In recent years, it has become increasingly evident that the cytoskeleton and its mechanical state are key elements for mechanosensitivity and mechanotransduction (6,49), which play an essential role in cellular regulation of differentiation, proliferation (50), and gene expression (8). Lambert et al. (7) demonstrated that synthesis of collagen and other extracellular matrix proteins is regulated by mechanical forces when comparing expression patterns of skin fibroblasts in stressed and relaxed collagen gels. Future work could clarify how age-related changes in the mechanical properties of cells, as observed in this study, influence the expression of ECM proteins.
Other cell functions, like motility and proliferation, could be affected by cytoskeletal changes. We assume a possible link between age-related changes in cell proliferation and the observed higher cell stiffness in connection with changes in actin organization. It has been shown that high proliferative cancer cells show a reduced degree of actin polymerization and mechanical strength (21). Aging, on the other hand, leads to a high degree of actin polymerization, as we have shown, and decreased growth rate, as well as the replicative lifespan of a fibroblast culture (51). A possible link of impaired motility and proliferation to age-related diseases could be especially relevant in the context of wound healing.
To date, molecular markers based on an altered pattern of gene and protein expression have been used to characterize cellular senescence as a measure of aging. The objective of a biomarker of aging is to reflect physiological function and help to monitor treatment response. Since the mechanical properties of cells are linked to their cytoskeletal structure, which in turn is governed by cellular function, optical deformability as measured with the optical stretcher appears to be a sensitive marker for cellular aging. Guck et al. (21) have already demonstrated the potential of optical deformability as a diagnostic marker for cancerous cells. We suggest that measurement of the global deformation behavior of individual cells also offers a novel, to our knowledge, cytological alternative to the genomic and proteomic techniques characterizing cellular senescence.
Acknowledgments
This study was funded by the Federal Ministry of Education and Research (BMBF).
Supporting Material
References
- 1.Takeda K., Gosiewska A., Peterkofsky B. Similar, but not identical, modulation of expression of extracellular matrix components during in vitro and in vivo aging of human skin fibroblasts. J. Cell. Physiol. 1992;153:450–459. doi: 10.1002/jcp.1041530303. [DOI] [PubMed] [Google Scholar]
- 2.Cristofalo V.J., Allen R.G., Beck J.C. Relationship between donor age and the replicative lifespan of human cells in culture: a reevaluation. Proc. Natl. Acad. Sci. USA. 1998;95:10614–10619. doi: 10.1073/pnas.95.18.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rattan S.I. Theories of biological aging: genes, proteins, and free radicals. Free Radic. Res. 2006;40:1230–1238. doi: 10.1080/10715760600911303. [DOI] [PubMed] [Google Scholar]
- 4.Reed M.J., Ferara N.S., Vernon R.B. Impaired migration, integrin function, and actin cytoskeletal organization in dermal fibroblasts from a subset of aged human donors. Mech. Ageing Dev. 2001;122:1203–1220. doi: 10.1016/s0047-6374(01)00260-3. [DOI] [PubMed] [Google Scholar]
- 5.Ingber D.E. Tensegrity: the architectural basis of cellular mechanotransduction. Annu. Rev. Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- 6.Janmey P.A., Weitz D.A. Dealing with mechanics: mechanisms of force transduction in cells. Trends Biochem. Sci. 2004;29:364–370. doi: 10.1016/j.tibs.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Lambert C.A., Soudant E.P., Lapière C.M. Pretranslational regulation of extracellular matrix macromolecules and collagenase expression in fibroblasts by mechanical forces. Lab. Invest. 1992;66:444–451. [PubMed] [Google Scholar]
- 8.Chiquet M. Regulation of extracellular matrix gene expression by mechanical stress. Matrix Biol. 1999;18:417–426. doi: 10.1016/s0945-053x(99)00039-6. [DOI] [PubMed] [Google Scholar]
- 9.Kessler D., Dethlefsen S., Eckes B. Fibroblasts in mechanically stressed collagen lattices assume a “synthetic” phenotype. J. Biol. Chem. 2001;276:36575–36585. doi: 10.1074/jbc.M101602200. [DOI] [PubMed] [Google Scholar]
- 10.Flück M., Giraud M.-N., Chiquet M. Tensile stress-dependent collagen XII and fibronectin production by fibroblasts requires separate pathways. Biochim. Biophys. Acta. 2003;1593:239–248. doi: 10.1016/s0167-4889(02)00394-4. [DOI] [PubMed] [Google Scholar]
- 11.Bereiter-Hahn J., Lüers H. The role of elasticity in the motile behavior of cells. In: Akkas N., editor. Biomechanics of Active Movement and Division of Cells. Springer; Berlin: 1994. pp. 181–230. [Google Scholar]
- 12.Fletcher D.A., Mullins R.D. Cell mechanics and the cytoskeleton. Nature. 2010;463:485–492. doi: 10.1038/nature08908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolodney M.S., Wysolmerski R.B. Isometric contraction by fibroblasts and endothelial cells in tissue culture: a quantitative study. J. Cell Biol. 1992;117:73–82. doi: 10.1083/jcb.117.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown R.A., Talas G., Eastwood M. Balanced mechanical forces and microtubule contribution to fibroblast contraction. J. Cell. Physiol. 1996;169:439–447. doi: 10.1002/(SICI)1097-4652(199612)169:3<439::AID-JCP4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 15.Uitto J. The role of elastin and collagen in cutaneous aging: intrinsic aging versus photoexposure. J. Drugs Dermatol. 2008;7(2, Suppl):s12–s16. [PubMed] [Google Scholar]
- 16.Johnson B.D., Page R.C., Pieters H.P. Effects of donor age on protein and collagen synthesis in vitro by human diploid fibroblasts. Lab. Invest. 1986;55:490–496. [PubMed] [Google Scholar]
- 17.Varani J., Dame M.K., Voorhees J.J. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006;168:1861–1868. doi: 10.2353/ajpath.2006.051302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berdyyeva T.K., Woodworth C.D., Sokolov I. Human epithelial cells increase their rigidity with ageing in vitro: direct measurements. Phys. Med. Biol. 2005;50:81–92. doi: 10.1088/0031-9155/50/1/007. [DOI] [PubMed] [Google Scholar]
- 19.Sokolov I., Iyer S., Woodworth C.D. Recovery of elasticity of aged human epithelial cells in vitro. Nanomedicine. 2006;2:31–36. doi: 10.1016/j.nano.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Guck J., Ananthakrishnan R., Käs J. Optical deformability of soft biological dielectrics. Phys. Rev. Lett. 2000;84:5451–5454. doi: 10.1103/PhysRevLett.84.5451. [DOI] [PubMed] [Google Scholar]
- 21.Guck J., Schinkinger S., Bilby C. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys. J. 2005;88:3689–3698. doi: 10.1529/biophysj.104.045476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Remmerbach T.W., Wottawah F., Guck J. Oral cancer diagnosis by mechanical phenotyping. Cancer Res. 2009;69:1728–1732. doi: 10.1158/0008-5472.CAN-08-4073. [DOI] [PubMed] [Google Scholar]
- 23.Dimri G.P., Lee X., Basile G., Acosta M., Scott G., Roskelley C., Medrano E.E., Linskens M., Rubelj I., Pereira-Smith O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang E., Harley C.B. Telomere length and replicative aging in human vascular tissues. Proc. Natl. Acad. Sci. USA. 1995;92:11190–11194. doi: 10.1073/pnas.92.24.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herbig U., Ferreira M., Sedivy J.M. Cellular senescence in aging primates. Science. 2006;311:1257. doi: 10.1126/science.1122446. [DOI] [PubMed] [Google Scholar]
- 26.Collado M., Serrano M. The power and the promise of oncogene-induced senescence markers. Nat. Rev. Cancer. 2006;6:472–476. doi: 10.1038/nrc1884. [DOI] [PubMed] [Google Scholar]
- 27.Kwak I.H., Kim H.S., Lim I.K. Nuclear accumulation of globular actin as a cellular senescence marker. Cancer Res. 2004;64:572–580. doi: 10.1158/0008-5472.can-03-1856. [DOI] [PubMed] [Google Scholar]
- 28.Janmey P.A. Mechanical properties of cytoskeletal polymers. Curr. Opin. Cell Biol. 1991;3:4–11. doi: 10.1016/0955-0674(91)90159-v. [DOI] [PubMed] [Google Scholar]
- 29.Rotsch C., Radmacher M. Drug-induced changes of cytoskeletal structure and mechanics in fibroblasts: an atomic force microscopy study. Biophys. J. 2000;78:520–535. doi: 10.1016/S0006-3495(00)76614-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grinnell F. Fibroblast-collagen-matrix contraction: growth-factor signalling and mechanical loading. Trends Cell Biol. 2000;10:362–365. doi: 10.1016/s0962-8924(00)01802-x. [DOI] [PubMed] [Google Scholar]
- 31.Walpita D., Hay E. Studying actin-dependent processes in tissue culture. Nat. Rev. Mol. Cell Biol. 2002;3:137–141. doi: 10.1038/nrm727. [DOI] [PubMed] [Google Scholar]
- 32.Petroll W.M. Dynamic assessment of cell-matrix mechanical interactions in three-dimensional culture. In: Coutts A.S., editor. Methods in Molecular Biology: Adhesion Protein Protocols. 2nd ed. Humana; Louisville, KY: 2007. pp. 67–81. [DOI] [PubMed] [Google Scholar]
- 33.Barer R., Joseph S. Refractometry of living cells, Part I. Basic principles. Q. J. Microsc. Sci. 1954;95:399–423. [Google Scholar]
- 34.Barer R., Joseph S. Refractometry of living cells, Part II. The immersion medium. Q. J. Microsc. Sci. 1955;96:1–26. [Google Scholar]
- 35.Barer R., Joseph S. Refractometry of living cells, Part III. Technical and optical methods. Q. J. Microsc. Sci. 1955;96:423–447. [Google Scholar]
- 36.Ashkin A. Acceleration and trapping of particles by radiation pressure. Phys. Rev. Lett. 1970;24:156–159. [Google Scholar]
- 37.Lincoln B., Wottawah F., Guck J. High-throughput rheological measurements with an optical stretcher. Methods Cell Biol. 2007;83:397–423. doi: 10.1016/S0091-679X(07)83017-2. [DOI] [PubMed] [Google Scholar]
- 38.Wottawah F., Schinkinger S., Käs J. Optical rheology of biological cells. Phys. Rev. Lett. 2005;94:098103. doi: 10.1103/PhysRevLett.94.098103. [DOI] [PubMed] [Google Scholar]
- 39.Holzinger A. Jasplakinolide. An actin-specific reagent that promotes actin polymerization. Methods Mol. Biol. 2001;161:109–120. doi: 10.1385/1-59259-051-9:109. [DOI] [PubMed] [Google Scholar]
- 40.Laudadio R.E., Millet E.J., Fredberg J.J. Rat airway smooth muscle cell during actin modulation: rheology and glassy dynamics. Am. J. Physiol. Cell Physiol. 2005;289:C1388–C1395. doi: 10.1152/ajpcell.00060.2005. [DOI] [PubMed] [Google Scholar]
- 41.Gourlay C.W., Carpp L.N., Ayscough K.R. A role for the actin cytoskeleton in cell death and aging in yeast. J. Cell Biol. 2004;164:803–809. doi: 10.1083/jcb.200310148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bubb M.R., Spector I., Fosen K.M. Effects of jasplakinolide on the kinetics of actin polymerization. An explanation for certain in vivo observations. J. Biol. Chem. 2000;275:5163–5170. doi: 10.1074/jbc.275.7.5163. [DOI] [PubMed] [Google Scholar]
- 43.Knowles G.C., McCulloch C.A.G. Simultaneous localization and quantification of relative G and F actin content: optimization of fluorescence labeling methods. J. Histochem. Cytochem. 1992;40:1605–1612. doi: 10.1177/40.10.1527379. [DOI] [PubMed] [Google Scholar]
- 44.Delvoye P., Wiliquet P., Lapière C.M. Measurement of mechanical forces generated by skin fibroblasts embedded in a three-dimensional collagen gel. J. Invest. Dermatol. 1991;97:898–902. doi: 10.1111/1523-1747.ep12491651. [DOI] [PubMed] [Google Scholar]
- 45.Wakatsuki T., Schwab B., Elson E.L. Effects of cytochalasin D and latrunculin B on mechanical properties of cells. J. Cell Sci. 2001;114:1025–1036. doi: 10.1242/jcs.114.5.1025. [DOI] [PubMed] [Google Scholar]
- 46.Rao K.M., Cohen H.J. Actin cytoskeletal network in aging and cancer. Mutat. Res. 1991;256:139–148. doi: 10.1016/0921-8734(91)90007-x. [DOI] [PubMed] [Google Scholar]
- 47.Pierard G.E., Lapière C.M. Physiopathological variations in the mechanical properties of skin. Arch. Dermatol. Res. 1977;260:231–239. doi: 10.1007/BF00561418. [DOI] [PubMed] [Google Scholar]
- 48.Lavker R.M., Zheng P.S., Dong G. Aged skin: a study by light, transmission electron, and scanning electron microscopy. J. Invest. Dermatol. 1987;88(3, Suppl):44s–51s. doi: 10.1111/1523-1747.ep12468934. [DOI] [PubMed] [Google Scholar]
- 49.Galli C., Guizzardi S., Scandroglio R. Life on the wire: on tensegrity and force balance in cells. Acta. Biomed. 2005;76:5–12. [PubMed] [Google Scholar]
- 50.Pavalko F., Chen N., Turner C. Fluid shear-induced mechanical signaling in MC3T3–E1 osteoblasts requires cytoskeleton-integrin interactions. Am. J. Physiol. Cell Physiol. 1998;275:1591–1601. [PubMed] [Google Scholar]
- 51.Schneider E.L., Mitsui Y. The relationship between in vitro cellular aging and in vivo human age. Proc. Natl. Acad. Sci. USA. 1976;73:3584–3588. doi: 10.1073/pnas.73.10.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


