Abstract
Objective
To evaluate Vγ2Vδ2 T cells in a group of HIV-infected patients who suppress HIV replication without antiretroviral therapy (natural viral suppressors, NVSs).
Design
It is a cross-sectional study.
Methods
We compared Vγ2Vδ2 T-cell frequency, T-cell repertoire, and responses to isopentenyl pyrophosphate stimulation between NVSs (n = 21) and HIV-uninfected controls (n = 27) and between NVSs and HIV-infected patients taking HAART with suppressed viral replication (HIV-P; n = 25).
Results
NVSs had a mean frequency of 1.06 ± 0.82% CD3+Vδ2+ cells among total lymphocytes, which was significantly higher than both control groups (HIV-negative: 0.50 ± 0.53%, P = 0.042; HIV-P: 0.34 ± 0.37%, P = 0.002). The proportion of Vγ2 chains correlating with the Vγ2-Jγ1.2 rearrangement was reduced among NVSs compared with HIV-negative controls (0.57 ± 0.06 vs. 0.32 ± 0.04; P = 0.016) but was increased compared with HIV-P patients (0.32 ± 0.04 vs. 0.22 ± 0.03; P = 0.03). NVSs had a similar baseline frequency of CD27–/CD45RA– effector cells (19.6 ± 4.2%) compared with HIV-negative controls (20.8 ± 12.9%; P = 0.35).
Conclusion
The altered γδ T-cell receptor repertoire among NVS was consistent with the known effect of HIV-1 on these cells. Uniquely among all HIV-infected groups, NVS reconstituted the γδ T-cell population, eventually reaching levels significantly above controls. This capacity to recover γδ T-cell numbers and function distinguishes individuals who control HIV-1 with and without HAART.
Keywords: elite controller, elite suppressor, gamma delta, HIV, natural viral suppressor
Introduction
A small proportion of individuals with HIV-1 infection maintain durable suppression of viremia in the absence of antiretroviral therapy. Variously termed elite controllers [1], elite suppressors [2–4], HIV controllers [5], or, as in this study, natural viral suppressors (NVSs) [6,7], these individuals have confirmed HIV infections yet fail to show disease progression for up to 2 decades [6,7]. It is of great interest to define host or viral properties or both that contribute to this favored clinical status.
Several recent studies have described HLA-B, HLA-C, or killer immunoglobulin-like receptors (KIR) alleles present at increased frequency among elite suppressors/elite controllers [3,8,9] suggesting a role for cellular immunity in viral control [10–12]. Virological studies provided little evidence for defective HIV-1 strains [4], suggesting that infection occurred initially with virulent, nonattenuated virus. Humoral immune responses may be associated with controlling viremia in NVSs or elite controller/elite suppressor individuals [13–15], though no single factor has been found in all individuals [16]. A recent report suggested that elevated levels of regulatory T cells may dampen HIV replication by suppressing T-cell activation during the acute phase of HIV infection [17]. To date, no clear pattern has emerged regarding the immune response, host genetics, or viral diversity that explains the NVS condition.
We were interested to learn whether NVS individuals had experienced a typical early disease course or whether their disease was attenuated from the time of infection. Without access to preinfection or acute infection samples from NVS patients, we sought to examine markers of HIV infection that are linked to early phases of disease. A candidate marker for early HIV-1 disease is the loss of Vγ2Vδ2 T cells and their functional responses to phosphoantigens [18].
Vγ2Vδ2 T cells represent an antigen-experienced population of lymphocytes that are highly biased in healthy adults, with a large proportion expressing the Vγ2-Jγ1.2 rearrangement [19]. The abundance of Vγ2-Jγ1.2+ clones in healthy adults is the product of chronic positive selection [19]. Virulent HIV-1 infection leads to selective depletion of the Vγ2-Jγ1.2 subset [20], accounting for quantitative loss of these cells from peripheral blood [21]. Substantial declines in Vγ2Vδ2 T-cell count and function were noted in HIV-infected individuals with CD4 cell counts still in the normal range, arguing that the specific depletion was occurring early in disease [22]. As most human Vγ2Vδ2 T cells are CD4-negative, they are not susceptible to direct HIV-1 infection, and the cell depletion patterns noted above represent indirect effects of the virus.
In a longitudinal study of patients starting HAART, we documented an inability to reconstitute the Vγ2 chain repertoire over 2.5 years of sustained viral suppression [23]. Coupled with our observation that the Vγ2 chain repertoire is highly stable for at least 7 years in healthy individuals [23], these data suggested that repertoire recovery after HIV-mediated depletion must be a slow process or it never occurs. Consequently, we concluded that the characteristics of Vγ2Vδ2 T cells in chronically infected individuals reflect damages during early infection that were not repaired.
By studying Vγ2Vδ2 cells in NVS patients, we hoped to distinguish between two possibilities that are as follows: disease was arrested early after infection, leaving the Vγ2Vδ2 population relatively intact or disease was arrested later in which case we should find evidence of substantial damage to Vγ2Vδ2 cells, and, according to studies with other HIV-infected patients, we would not expect recovery or reconstitution of the normal repertoire. The data reported here support the view that HIV disease in the NVS group damaged the Vγ2 repertoire but was arrested early enough after infection to partially spare the Vγ2Vδ2 T-cell compartment. However, levels of this T-cell subset were elevated significantly among NVS individuals that Vγ2Vδ2 T cells were recovered and actually exceed normal values.
Methods
Natural viral suppressors and control groups
The NVS cohort was defined previously [6]. Briefly, NVSs must have confirmed HIV infection [by both western blot and detection of proviral DNA in peripheral blood mononuclear cell (PBMC)]; at least four HIV RNA viral loads of less than 400 copies/ml for at least 2 years without the use of antiretroviral agents; one viral blip or viral load of more than 400 copies/ml was permitted if subsequent viral loads were less than 400 copies/ml. NVSs were naive to antiretroviral therapy, except for a provision allowing no more than 2 weeks of antiretroviral treatment for short-term pregnancy prophylaxis. Informed consent was obtained from all patients, and the study protocol was approved by the Institutional Review Board at the University of Maryland, Baltimore.
NVS specimens were compared to samples from two comparator groups. The first cohort included HIV-infected patients with normally progressing disease before receiving HAART (hereafter, HIV-P; n = 25). HIV-P patients were all on treatment and most had prolonged (i.e., at least 12 months) viral suppression with CD4 cell counts more than 300 cells/μl [24]. This group was predominantly composed of African–American (85%) and male patients (68%), had a mean age of 46.7 ± 6.0 years, and had a mean CD4 cell count of 512 + 144 cells per μl [24]. The second comparator group consisted of HIV-uninfected patients (n = 27); this group had a mean age of 46.4 ± 11.0 years, which had 37% male patients, and was entirely African–American [25]. The comparator groups were chosen specifically to illuminate differences between NVS and similar populations: the HIV-P group included patients optimally treated and achieving prolonged viral suppression, somewhat mimicking the natural viral suppression of NVSs; the HIV-uninfected group were racially matched because prior work has shown differences among racial populations in γδ T-cell frequencies [Cairo et al., unpublished observation].
Peripheral blood mononuclear cell isolation and Vγ2Vδ2 stimulation
Purified PBMCs were obtained from 21 NVS donors. For in-vitro stimulation, cells were thawed and resuspended in RPMI-1640 medium supplemented with 10% FBS, 2 mmol/l l-glutamine (Invitrogen, Carlsbad, California, USA), 1 U/ml penicillin/streptomycin (Invitrogen), and 100 U/ml recombinant human interleukin-2 (IL2, Tecin, Biological Resources Branch, National Institutes of Health, Bethesda, Maryland, USA). Isopentenyl pyrophosphate (IPP, Sigma) was added at a concentration of 15 μmol/l to trigger Vγ2Vδ2 T-cell proliferation. Cultures were incubated for 14 days at 37°C, 5% CO2 and were replenished every 3 days by adding IL2-supplemented medium. Viable cell counts were performed using trypan blue dye exclusion (Sigma, St. Louis, Missouri, USA).
Antibody staining and flow cytometry
Cells (3 × 105) were washed in Dulbecco's phosphate-buffered saline (D-PBS; Invitrogen) and labeled at 4°C for 15 min. The following monoclonal antibodies were used in this procedure: fluorescein isothiocyanate (FITC)-conjugated anti-Vδ1 (Pierce Biotechnology, Rockford, Illinois, USA); FITC-conjugated anti-Vδ2 (clone B6; Becton Dickinson Biosciences, San Diego, California, USA); phycoerythrin-conjugated anti-Vδ2 (clone B6; Pharmingen, San Diego, California, USA); phycoerythrin-conjugated anti-CD56 (clone N901; Beckman Coulter, Fullerton, California, USA); phycoerythrincyanine-5 (PC5)-conjugated anti-CD45RA (clone HI100; Becton Dickinson); PC5-conjugated anti-CD16 (clone 3G8, Becton Dickinson ) allophycocyanin (APC)-conjugated anti-CD27 (clone O323, eBioscience, San Diego, California, USA); APC-conjugated anti-CD3 (clone UCHT1, Becton Dickinson); and the appropriate isotype controls (Becton Dickinson).
After staining, cells were washed once with PBS and fixed with 1% paraformaldehyde. At least 3 × 104 cells were collected on a FACSCalibur flow cytometer (Becton Dickinson Biosciences). Flow cytometry data were analyzed using FlowJo software (Tree Star, San Carlos, California, USA).
The stimulation index represents the proportional increase in Vδ2+ cells following IPP stimulation compared with control with IL2 alone. Stimulation index is defined as the ratio of the absolute number of Vδ2+ lymphocytes on day 14 of the IPP expansion to the absolute number of Vδ2+ lymphocytes on day 14 without IPP expansion.
RNA extraction, reverse transcription-PCR and PCR
Total RNA was extracted from at least 106 cells (RNeasy Mini Kit, Qiagen, Valencia, California, USA), and 1 μg of total RNA was then converted into cDNA using the Reverse Transcription System (Promega, Madison, Wisconsin, USA). The following primers were used to amplify Vδ2 chain sequences: oligo-Vγ2 (5′-ATCAACGCTGGCAGTCC-3′), oligo-Cγ1 (5′-GTTGCTCTTCTTTTCTTGCC-3′), 5′ β-actin (5′-GTGGGGCGCCCCAGGCACCA-3′), and 3′ β-actin (5′-CTCCTTAATGTCACGCACGATTTC-3′), according to established methods [25].
Spectratype analysis
Primer extension reactions were performed as described previously [25] to generate run-off products that were diluted and mixed with GeneScan-500 Rox size standards. After a denaturation step (5 min at 95°C followed by immediate quenching on ice), products were loaded on an Applied Biosystems 3130 genetic analyzer (Applied Biosystems, Foster City, California, USA) and run for 27 min on a performance-optimized polymer (POP-7). Molecular size and relative frequency of extension products were determined using GeneMapper software (Applied Biosystems). To standardize the data irrespective of the run-off primer position, CDR3 length variation was expressed in terms of the total Vγ2 coding region lengths. Run-off product lengths were corrected by adding the lengths of known Vγ2 mRNA coding regions outside the run-off product. According to this calculation, the major peak for Vγ2 chains is 996 nucleotides based on a corresponding run-off product length of 447 nucleotides. Spectratype data were expressed with cumulative frequency plots.
Statistical analysis
We compared mean and median values for the percentage of lymphocytes that were identified as Vγ2Vδ2. After data analysis, it was clear that a few outlier values had significantly skewed the mean and median calculations. In order to better compare normally distributed data, we eliminated outlier values from each group using Tukey's definition of more than 1.5 times the mean plus the 75th percentile [26]. The correction eliminated one value from the NVS sample set and four from the controls. Without excluding outlier values, the significance of the comparisons was actually greater than after the correction. We elected the more conservative approach of excluding outlier values even though P values were slightly increased. Remaining values were compared by the Mann–Whitney test, with a P value of less than 0.05 considered significant. Other tests used the unpaired Student's t-test to compare differences in host human leukocyte antigen (HLA), response to IPP, spectratype and phenotype differences. Data for individuals in the outlier groups were not used in any subsequent analyses.
Results
Natural viral suppressor cohort characteristics
A full description of the NVS cohort has been published [6,7]. We obtained PBMC from 21 patients in the NVS cohort (Table 1). Two-thirds (67%) of the patients were of male gender, all were African–American, and most (86%) had been diagnosed with HIV infection for at least 10 years. The median duration of documented viral suppression was 8.3 years, and the median CD4 cell count was 845 cells/μl. Eight patients (38%) were positive for HLA-B57.
Table 1.
Demographic, clinical characteristics, CD3+Vδ2+ T cellsa and ratio of Vδ2:Vδ1 in natural viral suppressors patients.
| Samplea | Age | Sex | Year of HIV diagnosis | Years of documented HIV RNA VL suppression | Last CD4 (cells/μl) | Last CD4% (cells/μl) | Last CD8 (cells/μl) | Last CD4/CD8 ratio | HLA-B57 status | Vδ2+ frequency (%)b | Vδ2/Vδ1 (ex vivo) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 62 | M | 1991 | 5.8 | 956 | 27.0 | 2125 | 0.45 | – | 1.3 | 0.51 |
| 4 | 48 | F | 1993 | 11.1 | 845 | 48.0 | 361 | 2.34 | + | 0.3 | 0.25 |
| 5 | 50 | M | 1991 | 9.0 | 668 | 21.0 | 1985 | 0.34 | – | 0.1 | 0.04 |
| 6 | 48 | F | 1992 | 11.2 | 1422 | 52.0 | 615 | 2.31 | + | 0.9 | 0.58 |
| 7 | 60 | M | 1994 | 9.8 | 1323 | 44.0 | 1057 | 1.25 | – | 2.0 | 3.52 |
| 8 | 58 | M | 1989 | 9.1 | 722 | 37.0 | 674 | 1.07 | + | 0.3 | 0.33 |
| 9 | 53 | M | 2003 | 4.3 | 981 | 34.0 | 1084 | 0.90 | + | 5.3c | 2.22 |
| 10 | 57 | F | 1995 | 7.1 | 990 | 50.0 | 348 | 2.11 | + | 0.7 | 0.26 |
| 11 | 60 | M | 1997 | 9.8 | 1163 | 34.0 | 845 | 1.38 | + | 1.1 | 0.72 |
| 12 | 40 | M | 1997 | 5.5 | 994 | 44.0 | 713 | 1.39 | – | 1.2 | 0.59 |
| 13 | 54 | M | 1993 | 9.7 | 821 | 57.0 | 179 | 4.59 | – | 2.5 | 1.53 |
| 15 | 44 | F | 1988 | 10.8 | 1305 | 52.0 | 724 | 1.80 | + | 0.8 | 0.38 |
| 16 | 58 | M | 1997 | 8.3 | 696 | 54.0 | 274 | 2.91 | – | 0.5 | 0.37 |
| 18 | 60 | M | 1994 | 6.2 | 630 | 22.0 | 1418 | 0.44 | – | 0.2 | 0.25 |
| 21 | 55 | M | 2000 | 7.9 | 638 | 42.0 | 376 | 1.70 | – | 1.5 | 0.59 |
| 23 | 49 | M | 1992 | 11.0 | 696 | 28.0 | 1122 | 0.63 | – | 0.7 | 0.31 |
| 24 | 50 | F | 2000 | 6.8 | 689 | 30.0 | 682 | 0.81 | – | 1.7 | 1.11 |
| 25 | 53 | M | 1997 | 7.8 | 555 | 33.0 | 790 | 0.71 | – | 0.4 | 0.08 |
| 26 | 30 | F | 1995 | 10.3 | 1210 | 46.0 | 900 | 1.34 | + | 3.3 | 1.51 |
| 27 | 35 | F | 1995 | 8.3 | 1232 | 35.0 | 1451 | 0.85 | – | 1.0 | 0.35 |
| 29 | 57 | M | 1990 | 6.0 | 647 | 32.0 | 674 | 0.96 | NA | 1.1 | 1.30 |
| Median | 54 | 67% M | 1994 | 8.3 | 845 | 37.0 | 724 | 1.25 | 38% + | 1.0 | 0.5 |
F, female; M, male; NA, not available; PMBCs, peripheral blood mononuclear cells; VL, viral load.
Sample numbering system as in prior work.
Vδ2+ frequency as a percentage of total lymphocytes.
Outlier excluded from median calculations.
Both control groups have been described previously [24]; demographic data for these groups were provided in the Methods section.
High frequency of Vδ2 T cells and inverted ratio of Vδ2:Vδ1 in natural viral suppressor patients
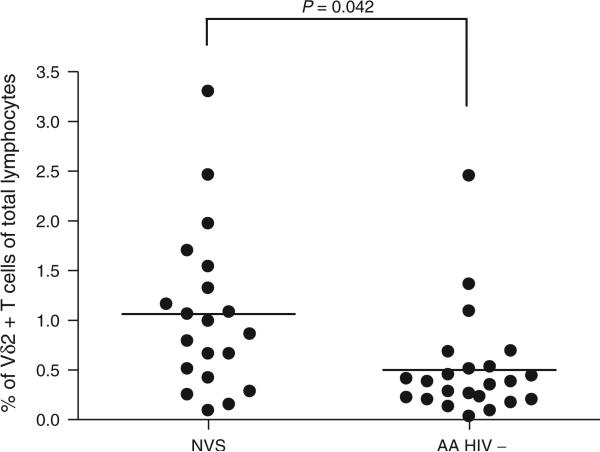
The baseline (unstimulated) frequency of CD3+Vδ2+ cells in total lymphocytes from 21 NVS patients is displayed in Table 1. The mean absolute number of CD3+Vδ2+ cells at baseline was 26 cells/μl (range 3.2–87 cells/μl). The mean frequency of CD3+Vδ2+ cells for NVS patients (1.06 ± 0.82%) was significantly higher than for HIV-infected patients from the HIV-P group (0.34 ± 0.37%; P = 0.002). NVS values were also significantly greater than a matched group of HIV-negative African–American volunteers (0.50 ± 0.53; P = 0.042). Although NVS appeared to have fewer Vγ2Vδ2 cells compared with previously used Caucasian control groups (3.99 ± 1.7%), the use of a racially matched group showed that NVSs have Vγ2Vδ2 cells significantly above control values (Fig. 1). Due to depletion of Vδ2 T cells in HIV-infected patients, the typical ratio of Vδ2:Vδ1 is characteristically inverted [21], though this ratio is also altered in some healthy populations, particularly adults in Ghana [27]. This reversal was found in most (71%) NVS patients; only two NVS patients (9.5%) had a Vδ2:Vδ1 ratio of more than 2 (Table 1).
Fig. 1. The percentage of Vδ2 T cells in natural viral suppressors is higher than for African–American HIV-negative patients.
Statistical comparisons were made using the unpaired Student's t-test. Horizontal line represents the mean. AA, African–American.
One measure of Vδ2+ cell reactivity to IPP expansion is the stimulation index. We calculated stimulation index for each NVS and compared the mean stimulation index to HIV-P patients and to African–American controls. Stimulation index values were higher for NVSs (17.6 ± 14.8) than for 21 HIV-P patients (2.8 ± 4.8; P < 0.001), indicating that the increased cell numbers were also associated with higher function. The NVS stimulation index values (17.6 ± 14.8) were lower than the stimulation index values for nine African–American controls (40.9 ± 49.9), though the differences did not reach statistical significance (P = 0.057).
HLA-B*57 genotype is not associated with preserved Vδ2 T cells
Several studies have associated specific HLA genotypes, especially the presence of HLA-B*57, with the NVS or elite controller phenotype [3,8]. Just over one-third of our NVS cohort are HLA-B*57 positive, and we tested whether HLA-B*57 was correlated with preservation of the Vδ2 subset. The mean Vδ2 frequency for seven HLA-B*57 positive NVS patients was 1.02 ± 1.04% compared with 1.08 ± 0.72% for 13 HLA-B*57 negative NVS patients (P = 0.9) (Fig. 2). There were no significant differences in Vδ2 frequency or Vδ2:Vδ1 ratios between HLA-B*57 positive and negative NVS patients.
Fig. 2. There were no significant differences in the percentage of Vδ2 T cells for HLA-B*57-positive and negative patients.
We measured Vδ2 T cells among total lymphocytes in HLA-B*57-positive patients (N = 8) and HLA-B*57 negative (N = 13) patients, and the values were not significantly different at baseline or after IPP expansion (data not shown). Statistical comparisons were made using the unpaired Student's t-test. Horizontal line represents the mean.
Vγ2 T-cell receptor length profile is altered in natural viral suppressor patients
Spectratyping was used to determine the size distribution of Vγ2 T-cell receptor (γTCR) chains in the NVS population and to analyze changes in the TCR repertoire after infection with HIV. The Vγ2 chain length profiles for NVSs, HIV-P, African–American controls, and Caucasian control patients are displayed (Fig. 3). Cumulative frequency plots from spectratype data were used to compare groups. In these plots, each point represents the sum of the proportion of all chains of a particular length for all values less than or equal to that value. The normal bias observed for healthy controls results in a curve shifted to the right for Caucasian and African–American donors, but the curve is generally shifted to the left in HIV-infected individuals. In these data, the HIV-P curve is shifted farthest to the left, consistent with a profound repertoire defect even after lengthy and successful antiretroviral therapy. Despite demonstrating increased frequencies and responsiveness of their Vγ2Vδ2+ cells, the NVS curve was more similar to the HIV-P curve than to either of the HIV-uninfected groups. These data reveal an impact of HIV on the Vγ2 chain repertoire in NVS patients.
Fig. 3. Cumulative frequency plot of spectratype data comparing natural viral suppressors to other populations.
The coding region length (in nucleotides) for the Vγ2 chain is plotted against the proportion of the population at each interval. Cumulative frequency is described in the text. Boxes indicate Caucasian controls, diamonds are for African–American controls, triangles represent the NVS group and crosses identify HIV-positive individuals with viremia suppressed by HAART. AA, African–American; NVS, natural viral suppressor.
We also used quantitative methods to compare Vγ2 repertoire differences between groups. The repertoire bias in healthy controls reflects stable accumulation of cells expressing the Vγ2-Jγ1.2 rearrangement; most of these chains are found at coding region lengths of 990, 993, or 996 nucleotides. By combining the proportions of Vγ2 chains at these three lengths, we obtain a metric related to the degree of repertoire bias [26]. For the HIV-negative African–American control group, the proportion of Vγ2 chains between 990 and 996 was significantly higher than that for the NVS group (0.57 ± 0.06 vs. 0.32 ± 0.04; P = 0.016 by unpaired t test). We also observed significantly higher 990–996 values among NVS donors when compared with the HIV-P group (0.32 ± 0.04 vs. 0.22 ± 0.03; P = 0.03). Overall, we observed that the Vγ2 repertoire for NVSs was intermediate between uninfected and HIV-P control groups.
CD27/CD45RA expression is altered in natural viral suppressor patients
The distribution of Vδ2 cells among naive/memory/effector subsets was evaluated in PBMC from NVS patients (Table 2). NVS had distinctive CD27 and CD45RA expression patterns when compared with HIV-P or control patients. NVS had a lower frequency of naive (CD27+/CD45RA+) Vδ2 cells (6.1 ± 5.0%) than either HIV-P (28.8 ± 13.6%) or African–American control patients (8.4 ± 7.0%) and a higher frequency of central memory (CD27+/CD45RA–) Vδ2 cells [70.6 ± 16.5% vs. 28.5 ± 18.8% (HIV-P) vs. 65.7 ± 14.2% (African–American controls); Table 2]. In the effector compartment (CD27–/CD45RA– ), NVS had a similar frequency of these cells compared with control patients (19.6 ± 14.2% vs. 20.8 ± 12.9%; P = 0.35) but a significantly higher proportion compared with HIV-P patients (19.6 ± 14.2% vs. 5.7 ± 5.9%; P < 0.001).
Table 2.
CD27/CD45RA expression in natural viral suppressors, HIV-P and African-American HIV-negative patients ex vivo (day 0) and after 14-day expansion with isopentenyl pyrophosphate.
| CD27+/CD45RA+ (Mean % ±SD) | CD27+/CD45RA– (Mean % ± SD) | CD27–/CD45RA+ (Mean % ± SD) | CD27–/CD45RA– (Mean % ± SD) | |
|---|---|---|---|---|
| Day 0 | ||||
| NVS | 6.1 ± 5.0 | 70.6 ± 16.5 | 3.73 ± 5.2 | 19.6 ± 14.2 |
| AA HIV– | 8.4 ± 7.0 | 65.7 ± 14.2 | 5.1 ± 5.0% | 20.8 ± 12.9 |
| HIV-P | 28.8 ± 13.6 | 28.5 ± 18.8 | 37.1 ± 21.7 | 5.7 ± 5.9 |
| IPP | ||||
| NVS | 0.8 ± 0.9 | 25.6 ± 17.4 | 2.0 ± 2.4 | 72.0 ± 17.7 |
| AA HIV– | 8.9 ± 7.2 | 65.8 ± 13.9 | 20.1 ± 13.1 | 5.3 ± 5.0 |
| HIV-P | 15.0 ± 14.8 | 17.2 ± 9.8 | 20.0 ± 19.4 | 47.4 ± 25.2 |
AA, African–American; IPP, isopentenyl pyrophosphate; NVS, natural viral suppressor.
Isopentenyl pyrophosphate stimulation expands the CD27–/CD45RA– effector subset in natural viral suppressor patients
PBMCs were stimulated with IPP and stained for Vδ2, CD27, and CD45RA after 14 days in culture in order to determine responsiveness of the effector memory (CD27–/CD45RA–) compartment. With NVS patients, we observed significant increases in the fraction of Vδ2+ CD27–/CD45RA– cells: from 19.6 ± 14.2% to 72.0 ± 17.7%. In contrast, HIV-P patients had a blunted response (baseline 5.7 ± 5.9% to 47.4 ± 25.2%), and the difference between NVS and HIV-P patients was highly significant at 14 days (P = <0.001). The increased size of the effector compartment (CD27–/CD45RA–) in NVS was similar to that shown previously for Caucasian HIV-negative control patients [24].
CD56 expression on Vδ2+ cells from natural viral suppressor donors
Recent work showed the CD56+ subset of Vγ2Vδ2 T cells was most potent in tumor cytotoxic assays [28]. We evaluated CD56 expression on Vγ2Vδ2 T cells of NVS patients (n = 20) as a marker for functional effector cells. Vγ2Vδ2 T cells from NVS patients had a mean CD56+ frequency of 43.2 ± 4.0%, which was significantly greater than uninfected controls (29.2 ± 3.4; P = 0.01) (Fig. 4) and above the values for HIV-P patients (30.5 ± 5.6%). The differences between NVS and HIV-P patients were not statistically significant (P = 0.07).
Fig. 4. The proportion of Vγ2+ cells that express CD56 is higher for natural viral suppressors both ex vivo (a) and after IPP stimulation (b), compared with African–American or HIV-P control groups.
Statistical comparisons were made using the unpaired Student's t-test. Horizontal line represents the mean. AA, African–American; IPP, isopentenyl pyrophosphate; NVS, natural viral suppressor.
Because CD56 expression is inducible on Vγ2Vδ2 T cells, we next evaluated whether there were changes in CD56 expression among NVS patients after IPP stimulation. Vγ2Vδ2 T cells (n = 21) from NVS patients responded to IPP stimulation with increased CD56 expression (61.2 ± 4.6% from 43.2 ± 4.0% at baseline). CD56 expression after IPP expansion among NVS was significantly greater than that of HIV-P patients (n = 10) (61.2 ± 4.6% vs. 39.0 ± 6.4%; P = 0.01) and was similar to CD56 expression after IPP stimulation with uninfected control samples (n = 11) (61.2 ± 4.6% vs. 50.2 ± 6.2%; P = 0.17).
Discussion
We characterized the Vγ2Vδ2 T-cell population in NVS. Changes in this subset of T cells have been well characterized for other HIV-infected groups [20,23,24, 29–31], leading to the general conclusions that depletion of Vγ2Vδ2 T cells occurs early in HIV disease, HIV infection preferentially affects a subset of γδ cells that express the Vγ2-Jγ1.2 rearrangement, and the repertoire defect was not corrected by successful antiretroviral therapy [23]. Thus, changes among Vγ2Vδ2 T cells document an impact of HIV infection on T-cell immunity that is quantitative and can be used to compare individual patients or groups. Our studies of NVS individuals were directed toward understanding whether Vγ2Vδ2 T cells were different in this group compared with HIV-infected patients with low viremia during HAART. The NVS cohort is unique because of their naturally suppressed viremia, which allowed us to investigate potential ways that they differ from HIV-infected patients responding to treatment.
Overall, we noted that NVS individuals not only preserved but also actually had increased levels of Vγ2Vδ2 T cells compared with demographically similar controls. For these comparisons, we assembled a control group of healthy African–American donors and showed that Vγ2Vδ2 T cells were approximately eight-fold lower in this population as compared with Caucasians [Cairo et al., unpublished observations]. It was important to use a racially matched control group because all individuals in our NVS cohort are African–American. Vγ2Vδ2 T cells were actually increased among NVS individuals to levels approximately twice what was observed in matched controls (P = 0.042).
Spectratype analysis showed a difference in Vγ2 chain repertoire among NVS patients compared with African–American controls consistent with an impact of HIV infection on this cell population. However, phosphoantigen responsiveness, a functional response that is decreased sharply among HIV-infected patients, was largely preserved in the NVS cohort. We also noted changes in the distribution among naive and memory subsets for Vγ2Vδ2 T cells in NVS individuals. The CD27–/CD45RA– effector compartment was expanded among NVS patients to a much greater degree than in HIV-infected patients with viremia suppressed by HAART, consistent with their better responses to phosphoantigen and preservation of the cell subset. Additionally, we showed that CD56 expression on Vγ2Vδ2 T cells from NVS patients was similar to that from HIV-infected patients at baseline but was increased with IPP stimulation more than what was observed for HIV-infected individuals on HAART. We thus found clear evidence of HIV-induced immunologic damage to the γδ T-cell population of lymphocytes in the NVS cohort, but the Vγ2 chain repertoire and in-vitro functional responses were intermediate between the HIV-P group and healthy African–American controls.
We did not find any relationship between HLA-B*57 genotype and γδ T cells in our NVS group. Similar to other examples of elite controllers/elite suppressors [3,32], our NVS cohort was enriched for the HLA-B*57 genotype. However, there was no correlation between the HLA-B*57 genotype and either the Vδ2 frequency or the Vδ2:Vδ1 ratios. This finding may have been expected as γδ T cells are major histocompatibility complex (MHC)-unrestricted, which suggests that mechanisms controlling γδ T-cell levels are independent of MHC genotype.
γδ T cells with the effector phenotype CD27–/CD45RA– are depleted in active tuberculosis [33], tuberculosis/HIV coinfection [33], and in progressing HIV infection [24,33]. Interestingly, this subset of effector cells was preserved in our NVS cohort, and the effector phenotype was increased by IPP stimulation in NVS, similar to what we observed for HIV-negative African–American controls [25]. Our previous study of T-effector memory cells in HIV-infected individuals on HAART (in which the duration of viral suppression was similar to that observed for this NVS cohort) showed that IPP stimulation did not reconstitute the effector memory subset but instead produced CD27–/CD45RA+ cells [24], similar to what had been observed for other T-cell subsets in HIV-infected individuals [34].
NVS patients demonstrated an increased number of Vγ2Vδ2 T cells even when compared with uninfected controls, and their Vγ2Vδ2 T cells were more functional than cells from HIV-infected individuals with viremia suppressed by HAART. The increased expression of CD56, increased proliferation response to phosphoantigen, and the capacity to generate CD27–/CD45RA– effector memory cells all point to a positive selection for Vγ2Vδ2 T cells among NVS donors that leads to higher cell levels in blood. This type of positive selection does not occur in HIV-infected individuals in whom viremia is suppressed by HAART and is a key difference between these groups that may reflect the mechanisms for achieving this favored status.
Despite the elevated cell levels and functional responses, the Vγ2Vδ2 subset in NVS patients shows clear evidence of an HIV-mediated defect. The substantial change in repertoire bias, with a Vγ2 repertoire more similar to the HIV-P group than to any of the uninfected controls, is likely a consequence of HIV infection. Again, differences between NVS and HIV-P patients include a capacity to limit and/or overcome the targeted depletion of Vγ2Vδ2 T cells. One other possibility to explain the elevated levels of Vγ2Vδ2 T cells in NVS is that members of this cohort had significantly elevated Vγ2Vδ2 T cells prior to HIV infection and that somehow the Vγ2Vδ2 T cells played a role in limiting the early damage from HIV infection and contributed to their eventual NVS status (i.e. chronic, natural suppression of viremia). We cannot test this hypothesis directly, but it is important to note that among Caucasian populations, in whom we have found an eightfold higher level of Vδ2+ cells before HIV infection, we do not have higher proportions of NVS (or elite controller/elite suppressor) compared with what we have observed [7]. These data argue that NVS individuals experienced a virulent HIV infection with early damage to Vγ2Vδ2 T cells and likely other subsets, but host responses stopped and reversed the damage. Baseline CD56 expression is low for NVS, but precursor cytotoxic T lymphocytes (CTLs) are abundant as indicated by the increased CD56+ subset after antigen stimulation. Importantly, HIV-P individuals have few γδ CTL precursors and express only low levels of CD56 after stimulation in vitro. Studies to discern why Vγ2Vδ2 cells have regained functionality among NVS or elite controller/elite suppressor patients may help to define therapeutic targets in the quest to mimic NVS status through treatment.
We do not yet know what stimulus promotes expansion of Vγ2Vδ2 T cells in NVS to levels above those seen for healthy controls. This may be a response to HIVor other infectious agents. Alternately, early HIV infection may have changed the normal regulation of Vγ2Vδ2 T cells by removing a suppressing mechanism. Future studies will address the mechanisms for Vγ2Vδ2 T-cell regulation in NVS.
Acknowledgements
This article was supported by public health service grants AI077394 and CA113261 (C.D.P.). M.M.S. was supported by K12RRO23250 (Alan R. Shuldiner was principal investigator).
References
- 1.Walker BD. Elite control of HIV infection: implications for vaccines and treatment. Top HIV Med. 2007;15:134–136. [PubMed] [Google Scholar]
- 2.Bailey JR, Lassen KG, Yang HC, Quinn TC, Ray SC, Blankson JN, Siliciano RF. Neutralizing antibodies do not mediate suppression of human immunodeficiency virus type 1 in elite suppressors or selection of plasma virus variants in patients on highly active antiretroviral therapy. J Virol. 2006;80:4758–4770. doi: 10.1128/JVI.80.10.4758-4770.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey JR, Williams TM, Siliciano RF, Blankson JN. Maintenance of viral suppression in HIV-1-infected HLA-B*57+ elite suppressors despite CTL escape mutations. J Exp Med. 2006;203:1357–1369. doi: 10.1084/jem.20052319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blankson JN, Bailey JR, Thayil S, Yang HC, Lassen K, Lai J, et al. Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J Virol. 2007;81:2508–2518. doi: 10.1128/JVI.02165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambotte O, Boufassa F, Madec Y, Nguyen A, Goujard C, Meyer L, et al. HIV controllers: a homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin Infect Dis. 2005;41:1053–1056. doi: 10.1086/433188. [DOI] [PubMed] [Google Scholar]
- 6.Sajadi MM, Heredia A, Le N, Constantine NT, Redfield RR. HIV-1 natural viral suppressors: control of viral replication in the absence of therapy. AIDS. 2007;21:517–519. doi: 10.1097/QAD.0b013e328013d9eb. [DOI] [PubMed] [Google Scholar]
- 7.Sajadi MM, Constantine NT, Mann DL, Charurat M, Dadzan E, Kadlecik P, et al. Epidemiologic characteristics and natural history of HIV-1 natural viral suppressors. J Acquir Immune Defic Syndr. 2009;50:403–408. doi: 10.1097/QAI.0b013e3181945f1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Migueles SA, Sabbaghian MS, Shupert WL, Bettinotti MP, Marincola FM, Martino L, et al. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci U S A. 2000;97:2709–2714. doi: 10.1073/pnas.050567397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emu B, Sinclair E, Hatano H, Ferre A, Shacklett B, Martin JN, et al. HLA class I-restricted T-cell responses may contribute to the control of human immunodeficiency virus infection, but such responses are not always necessary for long-term virus control. J Virol. 2008;82:5398–5407. doi: 10.1128/JVI.02176-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emu B, Sinclair E, Favre D, Moretto WJ, Hsue P, Hoh R, et al. Phenotypic, functional, and kinetic parameters associated with apparent T-cell control of human immunodeficiency virus replication in individuals with and without antiretroviral treatment. J Virol. 2005;79:14169–14178. doi: 10.1128/JVI.79.22.14169-14178.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunt PW, Brenchley J, Sinclair E, McCune JM, Roland M, Page-Shafer K, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197:126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saez-Cirion A, Lacabaratz C, Lambotte O, Versmisse P, Urrutia A, Boufassa F, et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci U S A. 2007;104:6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan Y, Sajadi MM, Kamin-Lewis R, Fouts TR, Dimitrov A, Zhang Z, et al. Discordant memory B cell and circulating anti-Env antibody responses in HIV-1 infection. Proc Natl Acad Sci U S A. 2009;106:3952–3957. doi: 10.1073/pnas.0813392106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahalanabis M, Jayaraman P, Miura T, Pereyra F, Chester EM, Richardson B, et al. Continuous viral escape and selection by autologous neutralizing antibodies in drug-naive human immunodeficiency virus controllers. J Virol. 2009;83:662–672. doi: 10.1128/JVI.01328-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, Pietzsch J, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458:636–640. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 16.Pereyra F, Addo MM, Kaufmann DE, Liu Y, Miura T, Rathod A, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis. 2008;197:563–571. doi: 10.1086/526786. [DOI] [PubMed] [Google Scholar]
- 17.Chase AJ, Yang HC, Zhang H, Blankson JN, Siliciano RF. Preservation of FoxP3+ regulatory T cells in the peripheral blood of human immunodeficiency virus type 1-infected elite suppressors correlates with low CD4+ T-cell activation. J Virol. 2008;82:8307–8315. doi: 10.1128/JVI.00520-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poccia F, Boullier S, Lecoeur H, Cochet M, Poquet Y, Colizzi V, et al. Peripheral V gamma 9/V delta 2 T cell deletion and anergy to nonpeptidic mycobacterial antigens in asymptomatic HIV-1-infected persons. J Immunol. 1996;157:449–461. [PubMed] [Google Scholar]
- 19.Parker CM, Groh V, Band H, Porcelli SA, Morita C, Fabbi M, et al. Evidence for extrathymic changes in the T cell receptor gamma/delta repertoire. J Exp Med. 1990;171:1597–1612. doi: 10.1084/jem.171.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enders PJ, Yin C, Martini F, Evans PS, Propp N, Poccia F, Pauza CD. HIV-mediated gammadelta T cell depletion is specific for Vgamma2+ cells expressing the Jgamma1.2 segment. AIDS Res Hum Retroviruses. 2003;19:21–29. doi: 10.1089/08892220360473934. [DOI] [PubMed] [Google Scholar]
- 21.Autran B, Triebel F, Katlama C, Rozenbaum W, Hercend T, Debre P. T cell receptor gamma/delta+ lymphocyte subsets during HIV infection. Clin Exp Immunol. 1989;75:206–210. [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Peng H, Ma P, Ruan Y, Su B, Ding X, et al. Association between Vgamma2Vdelta2 T cells and disease progression after infection with closely related strains of HIV in China. Clin Infect Dis. 2008;46:1466–1472. doi: 10.1086/587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hebbeler AM, Propp N, Cairo C, Li H, Cummings JS, Jacobson LP, et al. Failure to restore the Vgamma2-Jgamma1.2 repertoire in HIV-infected men receiving highly active antiretroviral therapy (HAART). Clin Immunol. 2008;128:349–357. doi: 10.1016/j.clim.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cummings JS, Cairo C, Armstrong C, Davis CE, Pauza CD. Impacts of HIV infection on Vgamma2Vdelta2 T cell phenotype and function: a mechanism for reduced tumor immunity in AIDS. J Leukoc Biol. 2008;84:371–379. doi: 10.1189/jlb.1207847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans PS, Enders PJ, Yin C, Ruckwardt TJ, Malkovsky M, Pauza CD. In vitro stimulation with a nonpeptidic alkylphosphate expands cells expressing Vgamma2-Jgamma1.2/Vdelta2 T-cell receptors. Immunology. 2001;104:19–27. doi: 10.1046/j.0019-2805.2001.01282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNeil DR, Tukey JW. Higher-order diagnosis of two-way tables, illustrated on two sets of demographic empirical distributions. Biometrics. 1975;31:487–510. [PubMed] [Google Scholar]
- 27.Hviid L, Akanmori BD, Loizon S, Kurtzhals JA, Ricke CH, Lim A, et al. High frequency of circulating gamma delta T cells with dominance of the v(delta)1 subset in a healthy population. Int Immunol. 2000;12:797–805. doi: 10.1093/intimm/12.6.797. [DOI] [PubMed] [Google Scholar]
- 28.Alexander AA, Maniar A, Cummings JS, Hebbeler AM, Schulze DH, Gastman BR, et al. Isopentenyl pyrophosphate-activated CD56+ {gamma}{delta} T lymphocytes display potent antitumor activity toward human squamous cell carcinoma. Clin Cancer Res. 2008;14:4232–4240. doi: 10.1158/1078-0432.CCR-07-4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gougeon ML, Poccia F, Boullier S. Human gamma delta T lymphocytes in HIV disease: effector functions and control by natural killer cell receptors. Springer Semin Immunopathol. 2000;22:251–263. doi: 10.1007/s002810000046. [DOI] [PubMed] [Google Scholar]
- 30.Wallace M, Scharko AM, Pauza CD, Fisch P, Imaoka K, Kawabata S, et al. Functional gamma delta T-lymphocyte defect associated with human immunodeficiency virus infections. Mol Med. 1997;3:60–71. [PMC free article] [PubMed] [Google Scholar]
- 31.Bordon J, Evans PS, Propp N, Davis CE, Jr, Redfield RR, Pauza CD. Association between longer duration of HIV-suppressive therapy and partial recovery of the V gamma 2 T cell receptor repertoire. J Infect Dis. 2004;189:1482–1486. doi: 10.1086/382961. [DOI] [PubMed] [Google Scholar]
- 32.Saez-Cirion A, Pancino G, Sinet M, Venet A, Lambotte O. HIV controllers: how do they tame the virus? Trends Immunol. 2007;28:532–540. doi: 10.1016/j.it.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Gioia C, Agrati C, Casetti R, Cairo C, Borsellino G, Battistini L, et al. Lack of CD27-CD45RA-V gamma 9V delta 2+ T cell effectors in immunocompromised hosts and during active pulmonary tuberculosis. J Immunol. 2002;168:1484–1489. doi: 10.4049/jimmunol.168.3.1484. [DOI] [PubMed] [Google Scholar]
- 34.Oswald-Richter K, Grill SM, Leelawong M, Tseng M, Kalams SA, Hulgan T, et al. Identification of a CCR5-expressing T cell subset that is resistant to R5-tropic HIV infection. PLoS Pathog. 2007;3:e58. doi: 10.1371/journal.ppat.0030058. [DOI] [PMC free article] [PubMed] [Google Scholar]