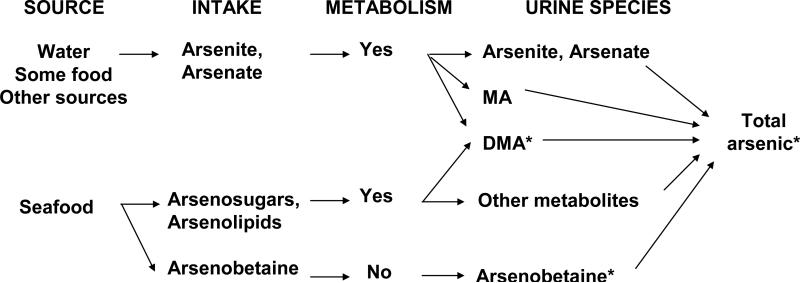
The role of inorganic arsenic exposure in chronic diseases, including type 2 diabetes, is a major public health research question. This has been underscored by recent epidemiologic1-4 and experimental5-8 evidence supporting increased risks at low exposure levels. In this context, it is critical to understand the biology and the technical limitations of biomarkers of inorganic arsenic exposure, usually measured in urine.9-11 Total urine arsenic integrates exposure from multiple sources including inorganic (arsenite, arsenate) and organic (mainly arsenobetaine, arsenosugars and arsenolipids) arsenic compounds and their metabolites (Figure 1). In population-based studies, arsenic speciation in urine is important to differentiate inorganic from organic exposure because organic arsenicals, mostly found in seafood, have little toxicity relative to inorganic arsenic and its metabolites. Despite analytic advances in the measurement of arsenosugars, arsenolipids and their metabolites,9 their determination remains technically challenging in epidemiologic studies. For example, those compounds were not measured in the 2003-2004 National Health Nutrition Examination Survey (NHANES). Moreover, because arsenite, arsenate and methylarsonate—species that directly reflect inorganic arsenic exposure and metabolism (Figure 1)—were measured in NHANES 2003-2004 with high limits of detection,9,12-14 only total arsenic, dimethylarsinate, arsenobetaine and arsenocholine (a minor seafood arsenical) were available for analyses of arsenic and health endpoints.
Figure 1.
Inorganic and organic arsenic exposure: relevant arsenic compounds in the general population, from source to urine. In NHANES 2003-2004, arsenite, arsenate, methylarsonate (MA) and arsenocholine were below the limit of detection in 96%, 94%, 65% and 98% of study participants, respectively. Other metabolites of arsenosugars and arsenolipids (thio-arsenic and oxo-arsenic compounds, etc.) were not measured. As a result, only total arsenic, dimethylarsinate (DMA) and arsenobetaine were available for arsenic analyses in NHANES 2003-2004.
To evaluate the association of inorganic arsenic exposure with the prevalence of type 2 diabetes in NHANES 2003-2004,2 we reported two main strategies to remove the contribution of organic arsenicals of marine origin to total urine arsenic. First, we conducted analyses of the association between total urine arsenic and the prevalence of type 2 diabetes adjusted for sociodemographic factors, diabetes risk factors, and two markers of seafood intake (urine arsenobetaine and blood mercury). More importantly, we reported analyses of the association between total urine arsenic and the prevalence of type 2 diabetes adjusted only for sociodemographic and diabetes risk factors but restricted to participants with low arsenobetaine levels (ie participants with unlikely seafood intake, in whom urine arsenic would be derived mainly from inorganic arsenic). The magnitude of the association in this subgroup was similar to the analysis adjusting for urine arsenobetaine and blood mercury, demonstrating that the association between arsenic and type 2 diabetes identified in the whole sample was driven by inorganic arsenic exposure and was not dependent on the statistical method used to control for organic arsenicals of marine origin.
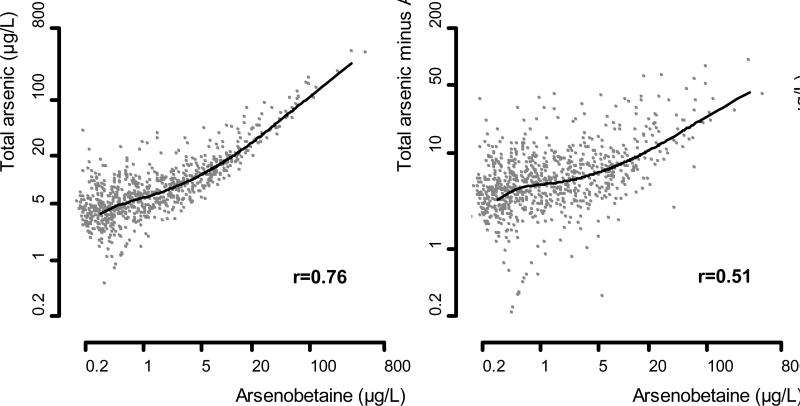
In a reanalysis of the association between arsenic exposure and diabetes in NHANES 2003-2004 published in this issue of EPIDEMIOLOGY, Steinmaus et al.15 used total urine arsenic minus urine arsenobetaine and urine arsenocholine (in 13 individuals in whom it was detected) as an “estimate of inorganic arsenic exposure,” in an attempt to remove the contribution of organic arsenicals of marine origin to total urine arsenic. Total urine arsenic minus arsenobetaine and arsenocholine, however, is an inadequate estimate of inorganic arsenic exposure because it does not remove other unmeasured organic arsenicals and their metabolites derived from seafood. Indeed, the correlation between arsenobetaine and total urine arsenic minus arsenobetaine and arsenocholine in NHANES 2003-2004 remained moderately strong (r=0.51, Figure 2). In their Discussion, the authors indicated that after subtracting arsenobetaine and arsenocholine from total arsenic, 24% of the variance in the remaining arsenic was still explained by arsenobetaine “probably due to some dimethylarsinate and some other arsenosugars that are present in the same seafoods that contain arsenobetaine”.15 The magnitude of the misclassification induced when estimating inorganic arsenic by total urine arsenic minus arsenobetaine and arsenocholine in the NHANES sample can be further appreciated by comparing the distribution of total arsenic minus arsenobetaine and arsenocholine (20th, 50th 80th, 90th and 99th percentiles of 2.7, 6.0, 11.9, 18.4 and 73.6 μg/L, respectively) with the distribution of total urine arsenic among participants with undetectable (<0.4 μg/L) urine arsenobetaine (20th, 50th 80th, 90th and 99th percentiles of 1.9, 3.9, 7.7, 11.8 and 30.4 μg/L, respectively). As a consequence, any analysis that uses total urine arsenic minus arsenobetaine and arsenocholine as an estimate of inorganic arsenic exposure still needs to adjust for markers of seafood intake, such as arsenobetaine itself. Futhermore, the misclassification induced by using total urine arsenic minus arsenobetaine and arsenocholine as an index of inorganic arsenic exposure is aggravated in categorical analyses, e.g. quintiles. Many participants in the highest quintile (≥80th percentile) of total urine arsenic minus arsenobetaine and arsenocholine in NHANES 2003-2004 were exposed to substantial amounts of organic arsenic compounds from seafood, and categorization in this situation could reduce the efficacy of statistical adjustment for seafood intake in multivariable models.
Figure 2.
Relationship of arsenobetaine with total arsenic and total arsenic minus arsenobetaine (AB) and arsenocholine (AC) in NHANES 2003-2004. Arsenocholine was subtracted only in 13 individuals with detectable levels. Lines represent dose-response relationships based on restricted quadratic spline models. The Pearson's correlation coefficients were estimated on log-transformed arsenic levels. Statistical analyses were weighted using specific sample weights for arsenic analysis in NHANES 2003-2004.
Given the longstanding experience of Steinmaus et al. in investigating the health effects of arsenic, it is difficult to understand why the authors used total urine arsenic minus arsenobetaine and arsenocholine as an “estimate of inorganic arsenic exposure” without controlling for seafood intake in their main analyses and conclusions. Indeed, when controlling for seafood intake, Steinmaus et al. reported findings consistent with out 2008 paper.2 (Table 3 of their manuscript).
Updated Analysis in NHANES 2003-2006
Since the publication of our study using NHANES 2003-2004 data,2 new urine arsenic data has become available for NHANES 2005-2006. Sampling and laboratory methods (including limits of detection for arsenic species) are consistent with NHANES 2003-2004, so that 4 years of data can be combined to evaluate the association between arsenic exposure and the prevalence of type 2 diabetes in US adults. Using NHANES 2003-2006, we have thus updated our original analysis, adding total arsenic minus arsenobetaine and arsenocholine as an exposure variable. The exclusion criteria and endpoint definition were similar to those in our original paper,2 except for participants missing glycosylated hemoglobin (an endpoint not used here). A total of 1279 participants (160 with diabetes) aged 20 years or older were included. Statistical analyses were performed as described previously, taking into account the complex NHANES sampling design and specific weights for arsenic analyses, as recommended by the National Center for Health Statistics.
Median (interquartile range) urine concentrations for total arsenic, total arsenic minus arsenobetaine and arsenocholine (in 21 participants with detectable concentrations), and arsenobetaine were 7.4 (4.0, 14.6) μg/L, 5.4 (3.0, 9.7) μg/L and 1.0 (0.3, 4.0) μg/L, respectively. Among study participants with undetectable (<0.4 μg/L) arsenobetaine concentrations (n = 381), the median (interquartile range) total urine arsenic concentration was 3.6 (2.0, 6.6) μg/L. Total arsenic was highly correlated with arsenobetaine (r = 0.78). Total arsenic minus arsenobetaine and arsenocholine also showed a moderately strong correlation with arsenobetaine (r = 0.50), confirming again that subtracting arsenobetaine is not sufficient to eliminate the contribution of unmeasured organic arsenicals from total urine arsenic.
We conducted analyses of total arsenic (as in our original study2) and total arsenic minus arsenobetaine and arsenocholine (as in Steinmaus et al15), progressively adjusting for markers of seafood intake (urine arsenobetaine and blood mercury). The findings (Table 1) were similar to our original analyses. In the categorical analyses, the highest quintiles of total urine arsenic and total arsenic minus arsenobetaine and arsenocholine were not clearly associated with an increased risk of diabetes because, rather than high inorganic arsenic, these quintiles mostly included participants with high intakes of organic arsenic compounds from seafood that are difficult to control for in the analysis. (The percentage of subjects with undetectable urine arsenobetaine levels decreased markedly from 57% in the lowest quintile to 12% in the highest quintile of total arsenic minus arsenobetaine and arsenocholine).
Restricting the analysis to participants with undetectable arsenobetaine concentrations, and adjusting only for sociodemographic and diabetes risk factors, participants with diabetes had 28% (95% Confidence Interval 7% - 53%) higher total arsenic concentrations compared with participants without diabetes, and the odds ratio for diabetes comparing participants at the 80th vs. the 20th percentiles of total urine arsenic (7.4 vs. 1.6 μg/L) was 2.60 (95% CI = 1.12 - 6.03) (Table 1). These analyses were not adjusted for arsenobetaine, ruling out collinearity as an explanation of our findings. The corresponding odds ratio for diabetes comparing the highest vs. the lowest quintiles (≥ 80th vs. ≤ 20th percentile) of total urine arsenic in this subgroup was 4.26 (0.83 - 21.8) (Table). This odds ratio directly estimates the association between exposure to inorganic arsenic and the prevalence of diabetes without assuming a linear dose-response relationship.
Table.
Ratio of total urine arsenic concentrations in diabetes cases vs noncases and association of (OR[95%CI] of high vs low urine arsenic concentration with type 2 diabetes in NHANES 2003-2006.
| Geometric mean of urine arsenic concentrations Ratio (95% CI) |
Model 1 Odds ratio (95% CI)a |
Model 2 Odds ratio (95% CI)a |
|
|---|---|---|---|
| Total urine arsenic (n=1279, 160 with diabetes) | |||
| Model 1 | 0.99 (0.83 - 1.19) | 0.95 (0.65 - 1.40) | 0.90 (0.37 - 2.19) |
| Model 2 | 1.07 (0.91 - 1.26) | 1.07 (0.72 - 1.61) | 0.90 (0.37 - 2.20) |
| Model 2 + urine arsenobetaine | 1.19 (1.04 - 1.35) | 2.57 (1.10 - 6.00) | 1.62 (0.57 - 4.62) |
| Model 2 + blood mercury | 1.21 (1.02 - 1.44) | 1.41 (0.89 - 2.25) | 1.35 (0.49 - 3.73) |
| Model 2 + both | 1.21 (1.06 - 1.38) | 2.86 (1.23 - 6.63) | 1.78 (0.60 - 5.30) |
| Total urine arsenic minus arsenobetaine and arsenocholine (n=1268, 159 with diabetesb | |||
| Model 1 | 1.04 (0.89 - 1.21) | 1.06 (0.72 - 1.54) | 0.68 (0.32 - 1.44)c |
| Model 2 | 1.13 (0.97 - 1.31) | 1.25 (0.83 - 1.88) | 0.79 (0.33 - 1.92)c |
| Model 2 + urine arsenobetaine | 1.18 (0.98 - 1.43) | 1.58 (0.80 - 3.13) | 0.96 (0.28 - 3.33)c |
| Model 2 + blood mercury | 1.22 (1.02 - 1.46) | 1.53 (0.93 - 2.52) | 1.02 (0.40 - 2.56)c |
| Model 2 + both | 1.22 (1.00 - 1.48) | 1.72 (0.85 - 3.45) | 1.04 (0.30 - 3.59)c |
| Total urine arsenic in participants with undetectable (< 0.4 μg/L) arsenobetaine (n=381, 62 with diabetes) | |||
| Model 1 | 1.16 (0.96 - 1.41) | 1.78 (0.77 - 4.15) | 2.34 (0.52 - 10.6) |
| Model 2 | 1.28 (1.07 - 1.53) | 2.60 (1.12 - 6.03) | 4.26 (0.83 - 21.8) |
OR (95%CI) for diabetes in participants in the 80th vs 20th percentile of urine arsenic distribution For the whole study sample (n=1279), the 20th and 80th percentiles of total arsenic distribution were 3.4 and 17.2 μg/L, respectively. For total urine arsenic minus arsenobetaine and arsenocholine (n=1268), the 20th and 80th percentiles were 2.5 and 11.0 μg/L, respectively. For participants with urine arsenobetaine < 0.4 μg/L (n=381), the 20th and 80th percentiles of total arsenic distribution were 1.6 and 7.4 μg/L, respectively. Model 1: adjusted for age, sex, race/ethnicity, and urine creatinine. Model 2: Further adjusted for education, body mass index, serum cotinine, and hypertension medication.
A total of 11 participants with negative values for total urine arsenic minus arsenobetaine were excluded. Arsenocholine was subtracted in 21 participants with detectable levels.
For comparison with Steinmaus et al15, the odds ratios, (95% CIs) not corrected for the complex survey design, for model 1, model 2, model 2 + urine arsenobetaine, model 2 + blood mercury, and model 2 + urine arsenobetaine and blood mercury were 1.08 (0.50 - 2.34), 1.45 (0.65 - 2.34), 1.86 (0.78 - 4.42), 1.59 (0.70 - 3.62), and 1.89 (0.79 - 4.49), respectively.
In summary, when adequately controlling for markers of seafood intake, our original analysis2 and those of Steinmaus et al15 of NHANES 2003-2004 resulted in similar conclusions. Furthermore, updated findings in NHANES 2003-2006, presented here, confirm that inorganic arsenic is associated with the prevalence of diabetes in US adults and rule out collinearity as an explanation to our findings. As we indicated in our original paper, these findings are inherently limited by the cross-sectional nature of NHANES data. Prospective evidence in populations exposed to a wide range of arsenic exposure levels, including arsenic in drinking water < 50 μg/L and other sources, is clearly needed. We also recognized that the use of spot urine samples and the need to account for urine dilution are limitations of our data common to many epidemiologic studies, and that single urine arsenic measures may not reflect long-term arsenic exposure.
From the perspectives of both science and public health, there can be no question about the need to evaluate the impact of inorganic arsenic exposure on diabetes development at low to moderate exposure levels (and not only at arsenic levels in drinking water from 50 to 200 μg/L, as Steinmaus et al indicate). First, evidence linking arsenic with diabetes endpoints in populations exposed to low1 and moderate16 levels is already available. Second, increasing experimental evidence supports several diabetogenic mechanisms of arsenic.5,6 Third, low to moderate arsenic exposure levels are widespread, affecting most populations worldwide. Inorganic arsenic is an established carcinogen and a high priority contaminant for screening in drinking water sources.17 While 10 μg/L is the current safety standard for arsenic levels in drinking water in most countries, the World Health Organization indicated that 10 μg/L is a provisional standard because many uncertainties remain at low exposure levels, in particular for non-cancer endpoints.17,18 Evaluating these health effects of low to moderate arsenic exposure is a public health priority. Knowledgeable use of biomarkers of arsenic exposure,10,11 high quality laboratory methods with adequate limits of detection,19-21 high-quality prospective epidemiologic designs, and adequate statistical analysis techniques are needed to evaluate the impact of low to moderate inorganic arsenic exposure on cardiovascular disease and diabetes risk.
Acknowledgments
Funding: Supported by grants 1R01HL090863 from the National Heart Lung and Blood Institute
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ettinger AE, Zota AR, Amarasiriwardena CJ, et al. Maternal arsenic exposure and impaired glucose tolerance during pregnancy. Environ Health Perspect. 2009 doi: 10.1289/ehp0800533. Epub 6 March 2009. doi:10.1289/ehp.0800281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, Guallar E. Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA. 2008;300:814–822. doi: 10.1001/jama.300.7.814. [DOI] [PubMed] [Google Scholar]
- 3.Andrew AS, Jewell DA, Mason RA, Whitfield ML, Moore JH, Karagas MR. Drinking-water arsenic exposure modulates gene expression in human lymphocytes from a u.s. Population. Environ Health Perspect. 2008;116:524–531. doi: 10.1289/ehp.10861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y, Parvez F, Gamble M, et al. Arsenic Exposure at Low-to-Moderate Levels and Skin Lesions, Arsenic Metabolism, Neurological Functions, and Biomarkers for Respiratory and Cardiovascular Diseases: Review of Recent Findings from the Health Effects of Arsenic Longitudinal Study (HEALS) in Bangladesh. Toxicol Appl Pharmacol. 2009 doi: 10.1016/j.taap.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paul DS, Harmon AW, Devesa V, Thomas DJ, Styblo M. Molecular mechanisms of the diabetogenic effects of arsenic: inhibition of insulin signaling by arsenite and methylarsonous acid. Environ Health Perspect. 2007;115:734–742. doi: 10.1289/ehp.9867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pysher MD, Sollome JJ, Regan S, et al. Increased hexokinase II expression in the renal glomerulus of mice in response to arsenic. Toxicol Appl Pharmacol. 2007;224:39–48. doi: 10.1016/j.taap.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Straub AC, Stolz DB, Vin H, et al. Low level arsenic promotes progressive inflammatory angiogenesis and liver blood vessel remodeling in mice. Toxicol Appl Pharmacol. 2006 doi: 10.1016/j.taap.2006.10.011. In press. Epub Oct 24 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Straub AC, Clark KA, Ross MA, et al. Arsenic-stimulated liver sinusoidal capillarization in mice requires NADPH oxidase-generated superoxide. J Clin Invest. 2008;118:3980–3989. doi: 10.1172/JCI35092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francesconi KA, Kuehnelt D. Determination of arsenic species: a critical review of methods and applications, 2000-2003. Analyst. 2004;129:373–395. doi: 10.1039/b401321m. [DOI] [PubMed] [Google Scholar]
- 10.Hughes MF. Biomarkers of exposure: a case study with inorganic arsenic. Environ Health Perspect. 2006;114:1790–1796. doi: 10.1289/ehp.9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navas-Acien A, Guallar E. Measuring arsenic exposure, metabolism, and biological effects: the role of urine proteomics. Toxicol Sci. 2008;106:1–4. doi: 10.1093/toxsci/kfn172. [DOI] [PubMed] [Google Scholar]
- 12.National Center for Environmental Health [May 11, 2009];Laboratory procedure manual. Total arsenic. 2008 http://0-www.cdc.gov.pugwash.lib.warwick.ac.uk/nchs/data/nhanes/nhanes_03_04/l06uas_c_met _arsenic_total.pdf .
- 13.National Center for Environmental Health [May 11, 2009];Laboratory procedure manual. Urine arsenic species. 2008 http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/l06uas_c_met_arsenic_speciated.pdf.
- 14.Caldwell KL, Jones RL, Verdon CP, Jarrett JM, Caudill SP, Osterloh JD. Levels of urinary total and speciated arsenic in the US population: National Health and Nutrition Examination Survey 2003-2004. J Expo Sci Environ Epidemiol. 2008 doi: 10.1038/jes.2008.32. [DOI] [PubMed] [Google Scholar]
- 15.Steinmaus C, Yuan Y, Liaw J, Smith AH. Low Level population exposure to inorganic arsenic in the United States and diabetes mellitus. Epidemiology. 2009;20:xxx–xxx. doi: 10.1097/EDE.0b013e3181b0fd29. [DOI] [PubMed] [Google Scholar]
- 16.Coronado-Gonzalez JA, Del Razo LM, Garcia-Vargas G, Sanmiguel-Salazar F, Escobedo-de la PJ. Inorganic arsenic exposure and type 2 diabetes mellitus in Mexico. Environ Res. 2007;104:383–389. doi: 10.1016/j.envres.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization . Guidelines for Drinking-water quality. Third Edition. Geneva, WHO: 2003. Ref Type: Report. [Google Scholar]
- 18.National Research Council . Arsenic in drinking water. National Academy Press; Washington DC: 1999. [Google Scholar]
- 19.Navas-Acien A, Umans JG, Howard BV, et al. Urine arsenic concentrations and species excretion patterns in American Indian Communities over a 10-year period. The Strong Heart Study. Environ Health Perspect. 2009 doi: 10.1289/ehp.0800509. Epub May 7 2009. doi:10.1289/ehp.0800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen YC, Amarasiriwardena CJ, Hsueh YM, Christiani DC. Stability of arsenic species and insoluble arsenic in human urine. Cancer Epidemiol Biomarkers Prev. 2002;11:1427–1433. [PubMed] [Google Scholar]
- 21.Hall M, Gamble M, Slavkovich V, et al. Determinants of arsenic metabolism: blood arsenic metabolites, plasma folate, cobalamin, and homocysteine concentrations in maternal-newborn pairs. Environ Health Perspect. 2007;115:1503–1509. doi: 10.1289/ehp.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]