Abstract
Biologic materials from various species and tissues are commonly used as surgical meshes or scaffolds for tissue reconstruction. Extracellular matrix (ECM) represents the secreted product of the cells comprising each tissue and organ, and therefore provides a unique biologic material for selected regenerative medicine applications. Minimal disruption of ECM ultrastructure and content during tissue processing is typically desirable. The objective of this study was to systematically evaluate effects of commonly used tissue processing steps upon porcine dermal ECM scaffold composition, mechanical properties, and cytocompatibility. Processing steps evaluated included liming and hot water sanitation, trypsin/SDS/TritonX-100 decellularization, and trypsin/TritonX-100 decellularization. Liming decreased the growth factor and glycosaminoglycan content, the mechanical strength, and the ability of the ECM to support in vitro cell growth (p ≤ 0.05 for all). Hot water sanitation treatment decreased only the growth factor content of the ECM (p ≤ 0.05). Trypsin/SDS/TritonX-100 decellularization decreased the growth factor content and the ability of the ECM to support in vitro cell growth (p ≤ 0.05 for both). Trypsin/TritonX-100 decellularization also decreased the growth factor content of the ECM but increased the ability of the ECM to support in vitro cell growth (p ≤ 0.05 for both). We conclude that processing steps evaluated in the present study affect content, mechanical strength, and/or cytocompatibility of the resultant porcine dermal ECM, and therefore care must be taken in choosing appropriate processing steps to maintain the beneficial effects of ECM in biologic scaffolds.
1. Introduction
The use of mammalian extracellular matrix (ECM) as surgical mesh materials and as scaffolds for regenerative medicine applications is commonplace. The tissues from which such biologic scaffolds are prepared include dermis [1, 2], pericardium [3–5], small intestine [6–9], and urinary bladder [10–12], among others. These tissues are harvested from several different species including pig, cow, horse and human. Commercially available products can be found from each source (Supplemental Table 1). Dermis is a commonly used source tissue for biologic scaffolds. Unlike porcine small intestinal submucosa and urinary bladder matrix that can be effectively decellularized by mechanical delamination and a brief exposure to peracetic acid and deionized water rinses, the thickness, density, and complexity of the dermis require the use of a variety of mechanical and chemical methods during tissue harvest and decellularization. Hot water sanitation and liming are common practices in the pre-decellularization processing of dermis: hot water treatment sanitizes the skin and aids in hair removal; liming serves the dual purpose of disinfection and hair removal. Decellularization is a necessary process in the preparation of medical devices composed of ECM, and decellularization protocols typically include combinations of detergents, organic solvents, and enzymatic solutions. Each of the methods used during tissue harvest and decellularization can affect the content, ultrastructure and mechanical properties of the material, and consequently the host response to the material [13–15].
The present study investigates the effect of commonly used processing steps upon growth factor and glycosaminoglycan content, mechanical properties, and in vitro cell growth properties of ECM derived from porcine dermis. It has long been known that growth factors are present in ECM scaffolds [16–18], but the role of growth factors in the host remodeling response has not been identified [19–21]. Glycosaminoglycans are also present in ECM scaffolds [22] and facilitate the maintenance of hydration and the binding of growth factors in extracellular matrices [19, 23–26]. Although the ability of an ECM scaffold to support cell growth in vitro cannot mimic the in vivo environment, the decreased ability of an ECM to support cell growth in vitro indicates that cell compatibility with the scaffold material may not be optimal. Finally, the strength of the material used as a biologic scaffold is a critical parameter for many clinical applications, such as hernia repair and orthopedic surgery [27–29]; therefore, the effect of processing methods upon ECM strength and mechanical properties is important.
The objectives of this study were to determine, quantify and compare effects of selected harvesting and processing steps on the growth factor and glycosaminoglycan content, the ability to support cell growth in vitro, and the mechanical strength of a biologic scaffold material composed of ECM harvested from porcine dermis.
2. Materials and Methods
2.1. Overview of experimental design
Dermis was harvested from market weight (approximately 110–130 kg) pigs. Four different preliminary tissue processing methods were evaluated, including treatment with lime, hot water sanitation, both lime and hot water sanitation, and neither lime nor hot water sanitation. After the preliminary processing, dermis samples that were neither limed nor hot water sanitized were processed for decellularization by two different methods. Effects of the various processing steps upon growth factor content, glycosaminoglycan content, mechanical properties, and the ability to support in vitro cell growth were assessed.
2.2. Preparation of porcine dermis samples
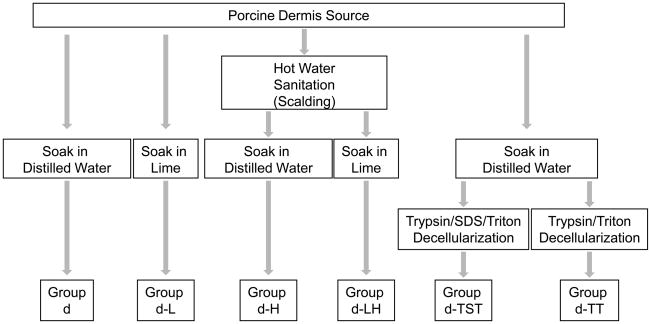
Porcine full thickness skin from the dorsolateral flank of market weight pigs immediately after euthanasia was harvested and processed by one of four methods (Figure 1). Specimens treated with hot water sanitation were subjected to water at approximately 60°C before removal of the skin from the carcass. All full thickness skin sheets were cut into 35-cm × 50-cm rectangles and the subcutaneous fat was mechanically removed. Limed samples were treated with a mixture of calcium hydroxide, soda ash and sodium sulfate for 20 to 24 hours with 15 minutes per hour of tumbling to remove hair, while non-limed samples were soaked in distilled water for 1 to 3 hours. All samples were then delaminated to remove subcutaneous fat, connective tissue and the epidermis. The harvested sheets of porcine dermis were immediately frozen at −80°C. Dermis samples not treated with lime or hot water sanitation were referred to as d; samples treated with lime, with hot water sanitation, and with both lime and hot water sanitation were referred to as d-L, d-H, and d-LH, respectively.
Fig. 1.
Dermis was processed at the time of harvest with either no liming or hot water sanitation (sample d), liming but no hot water sanitation (sample d-L), hot water sanitation but no liming (sample d-H) or both liming and hot water sanitation (sample d-LH). Dermis sample d was treated with either the 0.25% trypsin/0.1% SDS/1% Triton X-100 decellularization protocol (d-TST) or the 0.25% trypsin/1% TritonX-100 decellularization protocol (d-TT).
Porcine dermis sheets of sample d (not limed or hot water sanitized) to be treated with decellularization protocols were removed from the freezer and cut into sections measuring 3–7 cm × 3–7 cm.
Dermis sections treated with the 0.25% Trypsin/0.1% SDS/1% Triton X-100 decellularization protocol were incubated on a vortex shaker (Thermo Scientific MaxQ3000, Thermo Fisher Scientific, Waltham, MA) at 300 RPM at room temperature in the following solutions: 0.25% trypsin for 6 hours one time (1x); deionized water, 15 minutes, 3x; 70% ethanol, 10 to 12 hours, 1x; 3% H2O2, 15 minutes, 1x; deionized water, 15 minutes, 2x; 0.1% SDS in 0.26% ethylenediaminetetraacetic acid-tetrasodium salt (EDTA)/0.69% Tris, 6 hours, 1x and then overnight, 1x; 1% Triton X-100 in 0.26% EDTA/0.69% Tris, 1 hour, 1x; deionized water, 15 minutes, 3x; 0.1% peracetic acid/4% ethanol, 2 hours, 1x; PBS, 15 minutes, 2x; and finally deionized water, 15 minutes, 2x. These samples were referred to as d-TST.
Dermis sections treated with the 0.25% Trypsin/1% Triton X-100 decellularization protocol were incubated on a vortex shaker at 300 RPM at room temperature in the following solutions: 0.25% trypsin for 6 hours, 1x; deionized water, 15 minutes, 3x; 70% ethanol, 10 to 12 hours, 1x; 3% H2O2, 15 minutes, 1x, deionized water, 15 minutes, 2x; 1% Triton X-100 in 0.26% EDTA/0.69% Tris, 6 hours, 1x and then overnight, 1x; deionized water, 15 minutes, 3x; 0.1% peracetic acid/4% ethanol, 2 hours, 1x; PBS, 15 minutes, 2x; and finally deionized water, 15 minutes, 2x. These samples were referred to as d-TT.
Dermis sheets to be assayed for growth factors or glycosaminoglycans were lyophilized and subsequently reduced to particulate form using a Waring blender and a Wiley Mill with a #20 mesh screen [30]. Dermis sheets to be used for in vitro cell growth assays were lyophilized, cut into 2 cm diameter circles, and sterilized by ethylene oxide (16 hour cycle at 50°C in a Series 3plus EOGas Sterilizer, Anderson Sterilizers, Inc., Haw River, NC). Dermis sheets to be evaluated for mechanical strength were tested as non-lyophilized sheets.
2.3. Assessment of Cellular Content
Cellular content of dermis samples was assessed by three criteria: (1) the absence of visible nuclear material on hematoxylin and eosin (H&E) stained and 4′,6-diamidino-2-phenylindole (DAPI) stained sections; (2) a Quant-iT PicoGreen assay (Invitrogen, Carlsbad, CA) for quantification of double-stranded DNA; (3) evaluation of a 2% agarose gel to determine the size of remaining DNA fragments [31].
2.4 Preparation of urea-heparin extracts for growth factor assays
Four hundred (400) mg of dermis powder was suspended in 6 ml of urea-heparin extraction buffer consisting of 2 M urea and 5 mg/ml heparin in 50 mM Tris with protease inhibitors [1mM Phenylmethylsulfonyl Fluoride (PMSF), 5 mM Benzamidine, and 10 mM N-Ethylmaleimide (NEM)] at pH 7.4. The extraction mixture was rocked at 4°C for 24 hours and then centrifuged at 12,000 g for 30 minutes at 4°C. Supernatants were collected and 6 ml of freshly prepared urea-heparin extraction buffer was added to each pellet. Pellets with extraction buffer were again rocked at 4°C for 24 hours, centrifuged at 12,000 g for 30 minutes at 4°C, and supernatants were collected. Supernatants from first and second extractions were dialyzed against Barnstead filtered water (three changes, 80 to 100 volumes per change) in Slide-A-Lyzer Dialysis Cassettes, 3500 MWCO (Pierce, Rockford, IL). The concentration of total protein in each dialyzed extract was determined by the bicinchoninic acid (BCA) Protein Assay (Pierce, Rockford, IL) following the manufacturer’s protocol.
2.5. Growth factor assays
Concentrations of basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), and transforming growth factor beta 1 (TGF-β1) in urea-heparin extracts of dermis samples were determined with the Quantikine Human FGF basic Immunoassay, Human VEGF Immunoassay, and Mouse/Rat/Porcine/Canine TGF-β1 Immunoassay (all R&D Systems, Minneapolis, MN). Manufacturer’s instructions were followed for all three growth factor assays. Each assay for bFGF and VEGF was performed in duplicate; each assay for TGF-β1 was performed in triplicate. Each growth factor assay was performed four times for sample d and two times for samples d-L, d-H, d-LH, d-TST and d-TT. Growth factor assays measured the concentration of each growth factor and did not measure growth factor activity.
2.6. Glycosaminoglycan (GAG) assay
Glycosaminoglycan concentrations of porcine dermis samples were determined using the Blyscan Sulfated Glycosaminoglycan Assay Kit (Biocolor Ltd., Carrickfergus, Co Antrim, United Kingdom). Samples were prepared by digestion of 50 mg/ml dry weight of each sample with 0.1 mg/ml proteinase K in buffer (10 mM Tris-HCl, pH 8, 100 mM NaCl, 25 mM EDTA) for 48 hours at 50°C. Digested samples were assayed following the manufacturer’s protocol, and the assay was performed in duplicate three times.
2.7. Ball burst testing
Porcine dermis samples from each test group were cut to a size of at least 6 cm × 6 cm and frozen at −80°C until time for testing (not more than 72 hours). The specimens were thawed in 0.9% sodium chloride solution for approximately 15 minutes. Each specimen was mounted into a ball burst fixture with a polypropylene mesh on either side of the specimen within the grips to prevent slippage. The ball-burst test, a measure of strength in response to multiaxial loading, has been previously described [10] and was conducted in compliance with the Standard Test Method for Bursting Strength of Knitted Goods, Constant-Rate-of-Traverse (CRT) Ball-burst Test (ASTM D 3787-89). A uniaxial tensile testing machine (MTS Insight; 2kN capacity, MTS Systems Corp., Eden Prairie, MN) was equipped with a ball-burst compression cage in which a 25.4-mm (1-inch) polished stainless steel hemisphere rod was pushed against the material at a rate of 25.4 mm/minute until failure. A total of 5 to 8 specimens were tested within each group.
2.8. Cell growth assays
The dermis sheets were lyophilized, cut to 2 cm diameter circles and sterilized by ethylene oxide (16 hour cycle at 50°C in a Series 3plus EOGas Sterilizer, Anderson Sterilizers, Inc., Haw River, NC) for use in cell growth studies. NIH-3T3 mouse fibroblast cells (American Type Culture # CRL-1658, ATCC, Manassas, Virginia) were grown in Dulbecco’s Modified Eagle Medium (DMEM; Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin in 95% air / 5% CO2. Lyophilized, sterile dermis discs were placed in 6-well plates and hydrated with sterile HBSS prior to cell seeding. NIH-3T3 mouse fibroblasts were seeded on each type of dermis in triplicate, at 1 ×106 cells per 2 cm diameter dermis disc. Media was changed after 24 hours. After seven days of growth, cell culture media was removed and the dermis discs with attached cells were immediately fixed in 10% neutral buffered formalin for 18 hours. Following fixation, the discs were paraffin embedded, sectioned and mounted on glass slides. Slides were stained with Hematoxylin and Eosin (H&E), representative microscopic images captured, and images scored for cellular confluence, infiltration into the dermis substrate, and phenotype using a standardized quantitative scaling system (Supplemental Figure 1). Images were scored by five individuals who were blinded with respect to the processing protocol of the dermis samples, and an average score for each image was determined.
2.9 Statistical Analysis
Growth factor and GAG assay results are reported as mean ± standard error. A one-way analysis of variance (ANOVA) with Dunnett’s comparison test was used to determine whether the growth factor or GAG content of the dermis that had been processed with a treatment was different than that of untreated dermis. Tukey’s comparison test was used to compare growth factor contents of dermis that had been processed with different treatments.
Ball burst test results are reported as mean ± standard deviation. A one-way ANOVA was performed to determine differences in the ball-burst strength of the devices using the SPSS package (version 16.0; SPSS, Inc, Chicago, Ill). Statistical significance was set at p < 0.05.
Cell growth results are shown as average scores ± standard error. A one-way analysis of variance was performed with Dunnett’s comparison test used to determine whether the ability to support in vitro cell growth of the dermis that had been processed with a treatment was different than the ability to support in vitro cell growth of untreated dermis. A one-way ANOVA with Tukey’s comparison was used to compare dermis processed with different treatments.
3. Results
3.1. Cellular content of porcine dermis
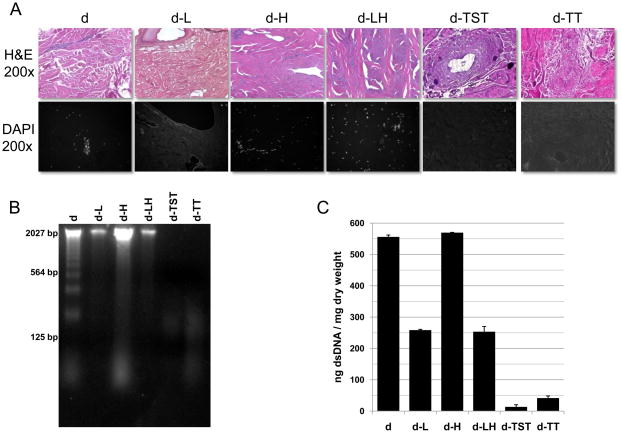
The pre-decellularization steps of liming and hot water sanitation do not decellularize the dermis, although liming does decrease the DNA content (Figure 2).
Fig. 2.
Decellularization of porcine dermis was assessed by imaging and analysis of DAPI and Hematoxylin and Eosin (H&E) stained sections (A), agarose gel analysis (B), and dsDNA per mg dry weight as measured with the Quant-iT PicoGreen dsDNA Assay Kit (C).
Both decellularization methods were effective at decellularization of the dermis. Dermis treated with both the trypsin/SDS/TritonX-100 protocol (D-TST) and the trypsin/TritonX-100 protocol (d-TT) was determined to be effectively decellularized based upon the established criteria described earlier; specifically, (1) no nuclei observed by imaging and analysis of both DAPI and Hematoxylin and Eosin stained sections, (2) no DNA ≥200 base pairs observed by agarose gel analysis, and (3) samples had a content of < 50 ng dsDNA per mg initial dry weight as measured with the Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen Corporation, Carlsbad, CA) (Figure 2).
3.2. bFGF content of porcine dermis
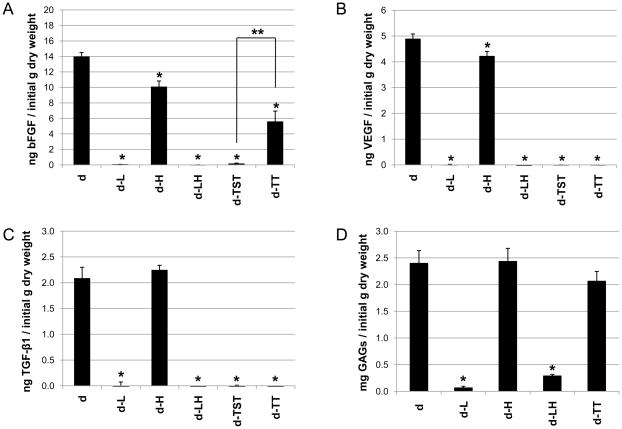
While hot water sanitation treatment resulted in a significant decrease in the bFGF content of porcine dermis, liming resulted in removal of all detectable bFGF from the porcine dermis (Figure 3A). Porcine dermis processed without liming or hot water sanitation (sample d) contained an average of 13.96 ± 0.54 ng of bFGF per g initial dry weight of dermis. bFGF in porcine dermis processed with hot water sanitation (sample d-H) was decreased by 28.0% from sample d (p ≤ 0.05).
Fig. 3.
Liming, hot water sanitation, and decellularization treatments affected the growth factor and glycosaminoglycan contents of the porcine dermis samples. The bFGF (A), VEGF (B), TGF-β1 (C) and glycosaminoglycan (D) contents of the dermis were measured. Results are plotted as mean ± standard error. * signifies a p value of ≤.05 as compared to d; ** signifies a p value of ≤.05.
Porcine dermis samples processed with either of the two decellularization protocols contained less bFGF compared to the preprocessed sample d. (Figure 3A). While sample d-TST showed a 99.0% loss in bFGF compared to sample d (p ≤ 0.05), sample d-TT showed a 60.1% loss compared to sample d (p ≤ 0.05). Thus, processing with the trypsin/TritonX-100 decellularization protocol resulted in a significantly higher retention of bFGF in the dermis samples compared to processing with the trypsin/SDS/TritonX-100 decellularization protocol (p ≤ 0.05).
3.3. VEGF content of porcine dermis
While hot water sanitation did have an effect on the VEGF content of porcine dermis, liming resulted in removal of all detectable VEGF from the porcine dermis (Figure 3B). Porcine dermis processed without liming or hot water sanitation (sample d) and porcine dermis processed with hot water sanitation (sample d-H) contained averages of 4.89 ± 0.1 ng of VEGF per g initial dry weight of dermis and 4.22 ± 0.19 ng of VEGF per g initial dry weight of dermis, respectively: sample d-H showed a 13.7% decrease in VEGF compared with sample d (p ≤ 0.05).
Porcine dermis samples processed with either of the two decellularization protocols (samples d-TST and d-TT) contained no detectable VEGF (Figure 3B).
3.4. TGF-β1 content of porcine dermis
While hot water sanitation did not have a noticeable effect on the TGF-β1 content of porcine dermis, liming resulted in removal of all detectable TGF-β1 from the porcine dermis (Figure 3C). Porcine dermis processed without hot water sanitation or liming (sample d) and porcine dermis processed with hot water sanitization (sample d-H) contained averages of 2.08 ± 0.22 ng of TGF-β1 per g initial dry weight of dermis and 2.24 ± 0.10 ng of TGF-β1 per g initial dry weight of dermis, respectively.
Porcine dermis samples processed with either of the two decellularization protocols (samples d-TST and d-TT) contained no detectable TGF-β1 (Figure 3C).
3.5. GAG content of porcine dermis
While samples processed with hot water sanitation showed a similar GAG content to samples processed without hot water sanitation, liming resulted in a marked decrease in GAG content of the porcine dermis (Figure 3D). Porcine dermis processed without hot water sanitation or liming (sample d) and porcine dermis processed with hot water sanitation (d-H) contained averages of 2.40 ± 0.24 mg and 2.44 ± 0.24 mg GAGs per g dry weight, respectively. Porcine dermis processed with liming but not with hot water sanitation (sample d-L) showed a 97.3% decrease in GAG content compared with sample d (p ≤ 0.05); porcine dermis that was both limed and treated with hot water sanitation (sample d-LH) showed an 88.0% decrease in GAG content compared with sample d (p ≤ 0.05).
The trypsin/TritonX-100 decellularization protocol did not have a noticeable effect on the GAG content of the porcine dermis samples: sample d-TT did not show a significant difference with regard to GAG content from sample d (Figure 3D). . Since SDS interferes with the assay for glycosaminoglycans, we were unable to measure the GAG content of the trypsin/SDS/TritonX-100 treated dermis.
3.6. Mechanical strength of porcine dermis
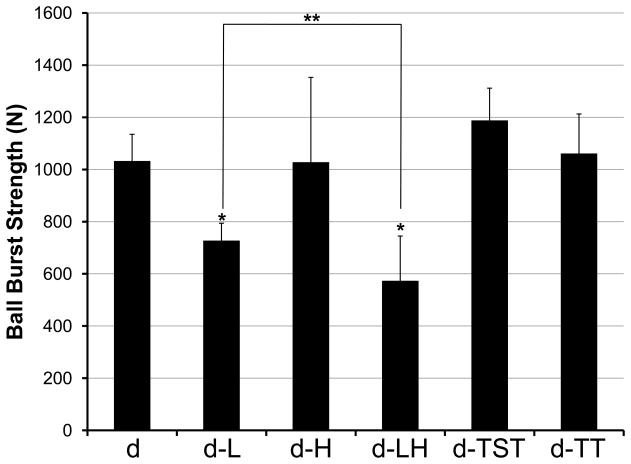
Liming treatment decreased the ball-burst strength of porcine dermis (p ≤ 0.05), while hot water sanitation treatment alone had no effect on strength (Figure 4). Porcine dermis processed without liming or hot water sanitation (sample d) and porcine dermis processed with hot water sanitation (sample d-H) showed ball-burst strengths of 1031 ± 104 N and 1027 ± 326 N, respectively (p = 0.97). In contrast, porcine dermis processed with liming (sample d-L) and with both liming and hot water sanitation (sample d-LH) showed ball-burst strengths of 726 ± 68 N and 572 ± 173 N, respectively, which correspond to 30% and 45% reductions in strength compared to porcine dermis processed without liming or hot water sanitation (p ≤ 0.05). The ball-burst strength of porcine dermis subjected to both liming and hot water sanitation treatments (sample d-LH) was markedly less than the that of porcine dermis subjected to hot water sanitation only (sample d-H) (p < 0.05), and also was significantly lower than the ball-burst strength of dermis subjected to liming only (sample d-L) (p ≤ 0.05). While sample d-H did not show a loss in mechanical strength compared with sample d, sample d-LH showed greater loss in mechanical strength than sample d-L, suggesting that hot water sanitation may alter the dermis, but that any effects upon mechanical strength are observed only when combined with the liming process.
Fig. 4.
The ball burst test was used to assess the mechanical strength of porcine dermis samples. Results are plotted as mean ± standard deviation. * signifies a p value of ≤.05 as compared to d; ** signifies a p value of ≤.05.
Neither decellularization treatment had a noticeable effect on the mechanical strength of the porcine dermis: samples d-TST and d-TT showed no change in ball burst strength from sample d.
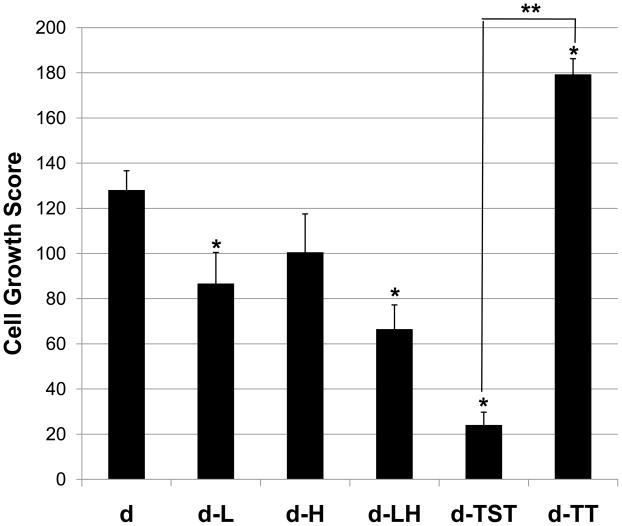
3.7. Ability of porcine dermis to support in vitro cell growth
Liming affected the ability of the porcine dermis to support cell growth, while hot water treatment alone had no significant effect on the ability of the dermis to support cell growth (Figure 5). Porcine dermis processed without liming or hot water sanitation (sample d) supported NIH-3T3 cell growth with a score of 128 ± 9. The cell growth score for dermis that had been limed (sample d-L) showed a 32% decrease from sample d (p ≤ 0.05). For dermis processed with both liming and hot water sanitation (sample d-LH) the cell growth score showed a 48% decrease from sample d (p≤ 0.05).
Fig. 5.
Processing treatments affected the ability of the porcine dermis samples to support the growth of NIH-3T3 mouse fibroblasts in vitro. Results are shown as average scores ± standard error. * signifies a p value of ≤.05 as compared to d; ** signifies a p value of ≤.05.
The two decellularization protocols were very different from one another in their effects on the ability of the porcine dermis to support cell growth in vitro (p≤ 0.05) (Figure 5). Porcine dermis decellularized with the trypsin/SDS/TritonX-100 protocol (sample d-TST) had a cell growth score of 24 ± 10, an 81% decrease from sample d (p≤ 0.05). Porcine dermis decellularized with the trypsin/TritonX-100 protocol (Sample d-TT) had a cell growth score of 179 ± 1.5, a 40% increase from sample d (p ≤ 0.05).
4. Discussion
The manufacture of a medical device from a biologic source presents unique challenges: contamination must be prevented, biologic variability must be controlled or at least understood, and a number of practical concerns such as sourcing, transportation of the raw material to a manufacturing plant, and preservation of tissue integrity must be addressed. For medical devices composed of ECM, minimal disruption of the ECM ultrastructure and content during tissue processing is typically desirable [32, 33]. A variety of methods are used to preserve tissue integrity while removing cellular components and reducing the bioburden during the manufacturing process. This study represents the first systematic evaluation of the effects of major processing steps upon ECM scaffold composition, mechanical properties, and cytocompatibility. The results of the present study confirm that certain processing steps clearly and unequivocally affect the composition, the mechanical properties, and the in vitro cytocompatibility of porcine dermal ECM.
The ECM represents the secreted product of the resident cells of each tissue and organ. These secreted products include both functional and structural molecules arranged in a unique three dimensional ultrastructure that supports the phenotype and function of the resident cells [34, 35]. Ideally, processing methods would be designed that could create a biologic scaffold composed of ECM with no changes in composition or structure. However, such preparation is not possible since decellularization and terminal sterilization of the native tissue is necessary in order to manufacture a medical device intended for surgical implantation. In reality, composition and ultrastructure are affected by each and every processing step.
Certain preliminary processing steps that have marked negative effects on the properties of dermis ECM were identified in this study. Both liming and hot water sanitation are common preliminary processing steps in the harvest of dermis ECM. While hot water sanitation had modest effects on the properties of the porcine dermis, liming had significant and dramatic negative effects on the composition, on the mechanical strength, and on the ability of the dermis samples to support cell growth in vitro.
Decellularization is a necessary process in the preparation of a medical device composed of ECM. The presence of cells and cellular debris has been shown to elicit an undesirable M1 phenotype in host macrophages following exposure to the ECM scaffold, with negative downstream effects upon remodeling [36] . The results of the present study confirm that certain decellularization processes have a marked negative effect on the properties of porcine dermal ECM, and that the cumulative amount of negative effects varies depending upon the method of decellularization. Dermis decellularized with the trypsin/SDS/TritonX-100 protocol retained significantly less bFGF than dermis decellularized with the trypsin/TritonX-100 protocol, a finding not surprising given the potentially harsh effects of SDS [37, 38] . Additionally, the dermis decellularized with the trypsin/TritonX-100 protocol retained GAG content similar to that of untreated dermis. Growth factors and GAGs have been correlated with in vivo constructive remodeling of biologic scaffolds [19–21, 23, 26]. The retention of growth factor and GAG content in the trypsin/TritonX-100 treated dermis is consistent with the greater biocompatibility found in the in vitro cell growth studies. While treatment with the trypsin/TritonX-100 decellularization protocol resulted in a 40% increase in the ability of the dermis to support cell growth compared with untreated dermis, treatment with the trypsin/SDS/TritonX-100 decellularization protocol resulted in an 81% decrease in the ability to support cell growth compared with untreated dermis. The difference in procedure between the two decellularization protocols is the inclusion of an SDS step in the trypsin/SDS/TritonX-100 method, which may in part explain the differential decrease in bFGF content and biocompatibility. It is evident from the marked differences in the effects of the two decellularization methods that the choice of a decellularization protocol is critical when preparing biologic scaffolds.
It is interesting that Sample d-TT showed an increased ability to support cell growth compared to sample d even though growth factor content in sample d-TT was markedly decreased compared to sample d. This finding emphasizes the fact that there are many facets contributing to the final effectiveness of an ECM scaffold; evaluation of an ECM scaffold should include the assessment of several factors including composition, mechanical strength, and cytocompatibility. Additionally, sample d is not decellularized: in vivo the presence of cellular content in scaffolds has been associated with an M1 type macrophage response and a reduction in constructive tissue remodeling [36]; it is possible that cellular remnants in sample d may play a role in the decreased ability of sample d to support cell growth in vitro compared with sample d-TT.
Numerous studies confirm the considerable effects that processing steps have upon on the properties of ECM scaffolds [15, 39, 40] For example, chemical crosslinking markedly changes the surface topology of the ECM [41], the chosen method of terminal sterilization (including particular conditions and dosage) affects the structural properties of the ECM [42–45], and lyophilization alone affects the mechanical properties of ECM and the host response to that ECM [46–49]. The effects of liming and of SDS on the composition and mechanical strength of porcine dermal ECM observed in the present study emphasize once again the potential adverse consequences that a single processing step may have on the properties of an ECM scaffold.
Although the present study was conducted upon extracellular matrix derived from porcine dermis, it is possible that these fundamental principles will be applicable to the preparation of biologic scaffolds from other tissues and organs. The processing of tissues for the purpose of creating an ECM biologic scaffold will vary depending upon the tissue source and intended clinical application. The optimal processing methods will likely vary between tissues, but the findings of the present study provide guidance for the intelligent selection of processing steps that minimize adverse effects upon structure and function to maximize clinical utility.
5. Conclusions
The present study represents the first systematic evaluation of the effects of major processing steps upon ECM scaffold composition, mechanical properties, and cytocompatibility. The results show that during the processing of porcine dermis (1) liming decreases the growth factor and glycosaminoglycan content, the mechanical strength, and the ability of the ECM to support in vitro cell growth (p ≤ 0.05 for all), (2) hot water sanitation treatment decreases the growth factor content of the ECM (p ≤ 0.05), (3) the trypsin/SDS/TritonX-100 decellularization method decreases the growth factor content of the ECM and the ability of the ECM to support in vitro cell growth (p ≤ 0.05 for both), and (4) the trypsin/TritonX-100 decellularization method decreases the growth factor content of the ECM and increases the ability of the ECM to support in vitro cell growth (p ≤ 0.05 for both). The two different decellularization methods in the present study differ from one another with regard to their effects on the ECM. Although the trypsin/SDS/TritonX-100 and the trypsin/ TritonX-100 protocols both significantly decrease the bFGF content of the ECM, the dermis treated with the trypsin/TritonX-100 protocol retains 40% of the bFGF content, retains GAGs, and shows a 40% increase in the support of cell growth in vitro compared with untreated dermis; the dermis treated with the trypsin/SDS/TritonX-100 protocol does not retain any detected bFGF, is not able to be assessed for GAG content likely due to the presence of residual SDS, and shows an 81% decrease in the support of cell growth in vitro compared with untreated dermis. The difference in procedure between the two decellularization protocols is the inclusion of an SDS step in the trypsin/SDS/TritonX-100 protocol, which may in part be responsible for the lower bFGF content and the decreased ability to support cell growth. It can be concluded that each processing step evaluated in the present study affects the content, mechanical strength, and/or cytocompatibility of the resultant porcine dermal ECM, and therefore care must be taken in choosing appropriate processing steps to maintain selected properties of ECM in biologic scaffolds.
Supplementary Material
Acknowledgments
Funding for this study was provided through a grant from the National Institutes of Health (NIH 5R01 AR054940-03) and by C. R. Bard, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Armour AD, Fish JS, Woodhouse KA, Semple JL. A comparison of human and porcine acellularized dermis: interactions with human fibroblasts in vitro. Plast Reconstr Surg. 2006;117(3):845–856. doi: 10.1097/01.prs.0000204567.28952.9d. [DOI] [PubMed] [Google Scholar]
- 2.Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84(1):209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 3.Walles T, Herden T, Haverich A, Mertsching H. Influence of scaffold thickness and scaffold composition on bioartificial graft survival. Biomaterials. 2003;24(7):1233–1239. doi: 10.1016/s0142-9612(02)00490-8. [DOI] [PubMed] [Google Scholar]
- 4.Cebotari S, Lichtenberg A, Tudorache I, Hilfiker A, Mertsching H, Leyh R, et al. Clinical application of tissue engineered human heart valves using autologous progenitor cells. Circulation. 2006;114(1 Suppl):I132–137. doi: 10.1161/CIRCULATIONAHA.105.001065. [DOI] [PubMed] [Google Scholar]
- 5.Lichtenberg A, Tudorache I, Cebotari S, Suprunov M, Tudorache G, Goerler H, et al. Preclinical testing of tissue-engineered heart valves re-endothelialized under simulated physiological conditions. Circulation. 2006;114(1 Suppl):I559–565. doi: 10.1161/CIRCULATIONAHA.105.001206. [DOI] [PubMed] [Google Scholar]
- 6.Sacks MS, Gloeckner DC. Quantification of the fiber architecture and biaxial mechanical behavior of porcine intestinal submucosa. J Biomed Mater Res. 1999;46(1):1–10. doi: 10.1002/(sici)1097-4636(199907)46:1<1::aid-jbm1>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Badylak SF, Tullius R, Kokini K, Shelbourne KD, Klootwyk T, Voytik SL, et al. The use of xenogeneic small intestinal submucosa as a biomaterial for Achilles tendon repair in a dog model. J Biomed Mater Res. 1995;29(8):977–985. doi: 10.1002/jbm.820290809. [DOI] [PubMed] [Google Scholar]
- 8.Badylak SF, Lantz GC, Coffey A, Geddes LA. Small intestinal submucosa as a large diameter vascular graft in the dog. J Surg Res. 1989;47(1):74–80. doi: 10.1016/0022-4804(89)90050-4. [DOI] [PubMed] [Google Scholar]
- 9.Kropp BP, Eppley BL, Prevel CD, Rippy MK, Harruff RC, Badylak SF, et al. Experimental assessment of small intestinal submucosa as a bladder wall substitute. Urology. 1995;46(3):396–400. doi: 10.1016/S0090-4295(99)80227-1. [DOI] [PubMed] [Google Scholar]
- 10.Freytes DO, Badylak SF, Webster TJ, Geddes LA, Rundell AE. Biaxial strength of multilaminated extracellular matrix scaffolds. Biomaterials. 2004;25(12):2353–2361. doi: 10.1016/j.biomaterials.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Ewalt DH, Howard PS, Blyth B, Snyder HM, 3rd, Duckett JW, Levin RM, et al. Is lamina propria matrix responsible for normal bladder compliance? J Urol. 1992;148(2 Pt 2):544–549. doi: 10.1016/s0022-5347(17)36650-8. [DOI] [PubMed] [Google Scholar]
- 12.Chen F, Yoo JJ, Atala A. Acellular collagen matrix as a possible "off the shelf" biomaterial for urethral repair. Urology. 1999;54(3):407–410. doi: 10.1016/s0090-4295(99)00179-x. [DOI] [PubMed] [Google Scholar]
- 13.Valentin JE, Badylak JS, McCabe GP, Badylak SF. Extracellular matrix bioscaffolds for orthopaedic applications. A comparative histologic study. J Bone Joint Surg Am. 2006;88(12):2673–2686. doi: 10.2106/JBJS.E.01008. [DOI] [PubMed] [Google Scholar]
- 14.Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A. 2008;14(11):1835–1842. doi: 10.1089/ten.tea.2007.0264. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27(19):3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Voytik-Harbin SL, Brightman AO, Kraine MR, Waisner B, Badylak SF. Identification of extractable growth factors from small intestinal submucosa. J Cell Biochem. 1997;67(4):478–491. [PubMed] [Google Scholar]
- 17.Hodde JP, Record RD, Liang HA, Badylak SF. Vascular endothelial growth factor in porcine-derived extracellular matrix. Endothelium. 2001;8(1):11–24. doi: 10.3109/10623320109063154. [DOI] [PubMed] [Google Scholar]
- 18.McDevitt CA, Wildey GM, Cutrone RM. Transforming growth factor-beta1 in a sterilized tissue derived from the pig small intestine submucosa. J Biomed Mater Res A. 2003;67(2):637–640. doi: 10.1002/jbm.a.10144. [DOI] [PubMed] [Google Scholar]
- 19.Rosso F, Giordano A, Barbarisi M, Barbarisi A. From cell-ECM interactions to tissue engineering. J Cell Physiol. 2004;199(2):174–180. doi: 10.1002/jcp.10471. [DOI] [PubMed] [Google Scholar]
- 20.Marra KG, Defail AJ, Clavijo-Alvarez JA, Badylak SF, Taieb A, Schipper B, et al. FGF-2 enhances vascularization for adipose tissue engineering. Plast Reconstr Surg. 2008;121(4):1153–1164. doi: 10.1097/01.prs.0000305517.93747.72. [DOI] [PubMed] [Google Scholar]
- 21.Ota T, Gilbert TW, Schwartzman D, McTiernan CF, Kitajima T, Ito Y, et al. A fusion protein of hepatocyte growth factor enhances reconstruction of myocardium in a cardiac patch derived from porcine urinary bladder matrix. J Thorac Cardiovasc Surg. 2008;136(5):1309–1317. doi: 10.1016/j.jtcvs.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodde JP, Badylak SF, Brightman AO, Voytik-Harbin SL. Glycosaminoglycan content of small intestinal submucosa: a bioscaffold for tissue replacement. Tissue Eng. 1996;2(3):209–217. doi: 10.1089/ten.1996.2.209. [DOI] [PubMed] [Google Scholar]
- 23.Folkman J, Shing Y. Control of angiogenesis by heparin and other sulfated polysaccharides. Adv Exp Med Biol. 1992;313:355–364. doi: 10.1007/978-1-4899-2444-5_34. [DOI] [PubMed] [Google Scholar]
- 24.Hardingham TE, Fosang AJ. Proteoglycans: many forms and many functions. FASEB J. 1992;6(3):861–870. [PubMed] [Google Scholar]
- 25.Souza-Fernandes AB, Pelosi P, Rocco PR. Bench-to-bedside review: the role of glycosaminoglycans in respiratory disease. Crit Care. 2006;10(6):237. doi: 10.1186/cc5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lovekamp JJ, Simionescu DT, Mercuri JJ, Zubiate B, Sacks MS, Vyavahare NR. Stability and function of glycosaminoglycans in porcine bioprosthetic heart valves. Biomaterials. 2006;27(8):1507–1518. doi: 10.1016/j.biomaterials.2005.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grabow N, Schmohl K, Khosravi A, Philipp M, Scharfschwerdt M, Graf B, et al. Mechanical and structural properties of a novel hybrid heart valve scaffold for tissue engineering. Artif Organs. 2004;28(11):971–979. doi: 10.1111/j.1525-1594.2004.00007.x. [DOI] [PubMed] [Google Scholar]
- 28.Gloeckner DC, Sacks MS, Billiar KL, Bachrach N. Mechanical evaluation and design of a multilayered collagenous repair biomaterial. J Biomed Mater Res. 2000;52(2):365–373. doi: 10.1002/1097-4636(200011)52:2<365::aid-jbm17>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 29.Freytes DO, Rundell AE, Vande Geest J, Vorp DA, Webster TJ, Badylak SF. Analytically derived material properties of multilaminated extracellular matrix devices using the ball-burst test. Biomaterials. 2005;26(27):5518–5531. doi: 10.1016/j.biomaterials.2005.01.070. [DOI] [PubMed] [Google Scholar]
- 30.Gilbert TW, Stolz DB, Biancaniello F, Simmons-Byrd A, Badylak SF. Production and characterization of ECM powder: implications for tissue engineering applications. Biomaterials. 2005;26(12):1431–1435. doi: 10.1016/j.biomaterials.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 31.Daly KA, Stewart-Akers AM, Hara H, Ezzelarab M, Long C, Cordero K, et al. Effect of the alphaGal epitope on the response to small intestinal submucosa extracellular matrix in a nonhuman primate model. Tissue Eng Part A. 2009;15(12):3877–3888. doi: 10.1089/ten.TEA.2009.0089. [DOI] [PubMed] [Google Scholar]
- 32.Badylak SF. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl Immunol. 2004;12(3–4):367–377. doi: 10.1016/j.trim.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 33.Gilbert TW. Immune Response to Biologic Scaffold Materials. Semin Immunol. 2008;20:109–116. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nature Medicine. 2010 doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 35.Sellaro TL, Ravindra AK, Stolz DB, Badylak SF. Maintenance of hepatic sinusoidal endothelial cell phenotype in vitro using organ-specific extracellular matrix scaffolds. Tissue Eng. 2007;13(9):2301–2310. doi: 10.1089/ten.2006.0437. [DOI] [PubMed] [Google Scholar]
- 36.Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30(8):1482–1491. doi: 10.1016/j.biomaterials.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rieder E, Kasimir M, Silberhumer G, Seebacher G, Wolner E, Simon P, et al. Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. J Thorac Cardiovasc Surg. 2004;127(2):399–405. doi: 10.1016/j.jtcvs.2003.06.017. [DOI] [PubMed] [Google Scholar]
- 38.Flynn L, Semple JL, Woodhouse KA. Decellularized placental matrices for adipose tissue engineering. J Biomed Mater Res A. 2006;79(2):359–369. doi: 10.1002/jbm.a.30762. [DOI] [PubMed] [Google Scholar]
- 39.Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009;5(1):1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert TW, Wognum S, Joyce EM, Freytes DO, Sacks MS, Badylak SF. Collagen fiber alignment and biaxial mechanical behavior of porcine urinary bladder derived extracellular matrix. Biomaterials. 2008;29(36):4775–4782. doi: 10.1016/j.biomaterials.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown BN, Barnes CA, Kasick RT, Michel R, Gilbert TW, Beer-Stolz D, et al. Surface characterization of extracellular matrix scaffolds. Biomaterials. 2010;31(3):428–437. doi: 10.1016/j.biomaterials.2009.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freytes DO, Stoner RM, Badylak SF. Uniaxial and biaxial properties of terminally sterilized porcine urinary bladder matrix scaffolds. J Biomed Mater Res B Appl Biomater. 2008;84(2):408–414. doi: 10.1002/jbm.b.30885. [DOI] [PubMed] [Google Scholar]
- 43.Dziedzic-Goclawska A, Kaminski A, Uhrynowska-Tyszkiewicz I, Stachowicz W. Irradiation as a safety procedure in tissue banking. Cell Tissue Bank. 2005;6(3):201–219. doi: 10.1007/s10561-005-0338-x. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen H, Morgan DA, Forwood MR. Sterilization of allograft bone: is 25 kGy the gold standard for gamma irradiation? Cell Tissue Bank. 2007;8(2):81–91. doi: 10.1007/s10561-006-9019-7. [DOI] [PubMed] [Google Scholar]
- 45.Akkus O, Belaney RM, Das P. Free radical scavenging alleviates the biomechanical impairment of gamma radiation sterilized bone tissue. J Orthop Res. 2005;23(4):838–845. doi: 10.1016/j.orthres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Freytes DO, Tullius RS, Valentin JE, Stewart-Akers AM, Badylak SF. Hydrated versus lyophilized forms of porcine extracellular matrix derived from the urinary bladder. J Biomed Mater Res A. 2008;87(4):862–872. doi: 10.1002/jbm.a.31821. [DOI] [PubMed] [Google Scholar]
- 47.Hafeez YM, Zuki AB, Yusof N, Asnah H, Loqman MY, Noordin MM, et al. Effect of freeze-drying and gamma irradiation on biomechanical properties of bovine pericardium. Cell Tissue Bank. 2005;6(2):85–89. doi: 10.1007/s10561-004-1888-z. [DOI] [PubMed] [Google Scholar]
- 48.Curtil A, Pegg DE, Wilson A. Freeze drying of cardiac valves in preparation for cellular repopulation. Cryobiology. 1997;34(1):13–22. doi: 10.1006/cryo.1996.1982. [DOI] [PubMed] [Google Scholar]
- 49.Gilbert TW, Gilbert S, Madden M, Reynolds SD, Badylak SF. Morphologic assessment of extracellular matrix scaffolds for patch tracheoplasty in a canine model. Ann Thorac Surg. 2008;86(3):967–974. doi: 10.1016/j.athoracsur.2008.04.071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.