Abstract
An epidermis surrounds all vertebrates, forming a water barrier between the external environment and the internal space of the organism. In the zebrafish, the embryonic epidermis consists of an outer enveloping layer (EVL) and an inner basal layer that have distinct embryonic origins. Differentiation of the EVL requires the maternal effect gene poky/ikk1 in EVL cells prior to establishment of the basal layer. This requirement is transient and maternal Ikk1 is sufficient to allow establishment of the EVL and formation of normal skin in adults. Similar to the requirement for Ikk1 in mouse epidermis, EVL cells in poky mutants fail to exit the cell cycle or express specific markers of differentiation. In spite of the similarity in phenotype, the molecular requirement for Ikk1 is different between mouse and zebrafish. Unlike the mouse, EVL differentiation requires functioning Poky/Ikk1 kinase activity but does not require the HLH domain. Previous work suggested that the EVL was a transient embryonic structure, and that maturation of the epidermis required replacement of the EVL with cells from the basal layer. We show here that the EVL is not lost during embryogenesis but persists to larval stages. Our results show that while the requirement for poky/ikk1 is conserved, the differences in molecular activity indicate that diversification of an epithelial differentiation program has allowed at least two developmental modes of establishing a multilayered epidermis in vertebrates.
Keywords: zebrafish, epidermis, enveloping layer (EVL), chuk/ikk1/ikka, maternal effect
Introduction
Superficial epithelia mediate the interaction of organisms with their environment. These epithelia provide barriers to solute passage, allowing survival in external environments that are different from their internal environments. As freshwater fish, zebrafish (Danio rerio) must establish a permeability barrier early in embryogenesis to survive in a hypotonic external environment. During early embryogenesis, the blastula forms a squamous epithelial monolayer called the Enveloping Layer (EVL). During early cleavages, the EVL arises from external blastomeres and covers the blastoderm, contacting the yolk at the blastoderm margin (Kimmel et al., 1990, 1995). The EVL becomes lineage-restricted by the mid-blastula stage (sphere stage), with rare cell divisions contributing daughters only to the EVL and not the underlying Deep Layer (DEL) (Kane and Kimmel, 1993; Kimmel et al., 1990; Ho, 1992). At this stage, the EVL cells slow their cell cycle and begin to express specific keratins indicative of differentiation (Imboden et al., 1997; Sagerström et al., 2005; Thisse et al., 2001). During epiboly, the EVL and the underlying DEL spread over the surface of the large yolk cell. Following epiboly, the EVL forms the external layer of the epidermis surrounding the embryo. This layer has been described as a transient periderm that is shed during differentiation of the embryonic epidermis. By 1 day post fertilization (dpf) the embryonic epidermis consists of the EVL and a p63 positive basal layer that arises independently from ventral ectoderm and migrates under the EVL (Bakkers et al., 2002). Together, the EVL and the basal layer form the embryonic epidermis.
Like the zebrafish, the mouse embryo also forms a periderm surrounding the developing embryo in a single-layered squamous epithelium. Unlike the zebrafish EVL, the mouse periderm forms directly from the p63 positive basal layer by embryonic day 12 (E12) (M’Boneko and Merker, 1988). A third layer appears between the periderm and basal layer at E14.5. This intermediate layer originates from the basal layer and maintains a high mitotic index, but is distinct from the basal layer by its position and by its expression of Keratins 1 and 10 (Koster and Roop, 2007; Weiss and Zelickson, 1975; Smart, 1970). The high mitotic index is a transient property of the intermediate layer. The intermediate layer gives rise to the granular layer, which in turn differentiates into the stratum cuteum. Upon differentiation of the stratum cuteum, the periderm is sloughed off. By E18.5, mouse skin is a stratified epithelium consisting of 4 cell layers of progressively more differentiated cells: the basal layer, the spinous layer (similar to the intermediate layer but with a lower mitotic index), the granular layer and the stratum cuteum.
Genetic analysis of skin differentiation has identified key genes required for discrete steps in the formation of mature skin. Mutants in mouse Conserved Helix-loop-helix Ubiquitious Kinase (chuk)/Inhibitor of NfkB Kinase 1 (ikk1) display defective skin differentiation (Hu et al., 1999; Sil et al., 2004; Takeda et al., 1999). Mutant skin displays a highly proliferative epidermis that fails to differentiate into granular layer and stratum cuteum cells. This function of IKK1 does not require the kinase activity, nor does it result in defective NfkB signaling (Hu et al., 2001). This is surprising since IKK1 was originally identified as a central mediator of NfkB activation through phosphorylation of IkB proteins, triggering their processing and/or degradation. Recent work has identified the related protein IKK2 as the principal mediator of NFkB signaling, phosphorylating multiple IkB proteins, while IKK1 prefers a single IkB substrate, NFkB2 (Senftleben et al., 2001).
Two interpretations of the skin differentiation phenotype are prominent in the literature. One is that IKK1 is required for differentiation of spinous layer cells to a granular layer identity (Hu et al., 1999; Sil et al., 2004; Takeda et al., 1999). The other is that the embryonic epidermis forms the proliferative embryonic intermediate layer but cannot transition to more mature skin cell types such as spinous layer cells (Koster et al., 2007).
Squamous cell carcinoma is frequently associated with decreased IKK1 activity in mouse and human (Liu et al., 2006, 2008; Park et al., 2007). IKK1 is also required for proliferation of mouse mammary gland epithelium during pregnancy (Cao et al., 2001). The role of IKK1 in keratinocyte differentiation and in suppression of squamous cell carcinoma may involve interaction with TGFBeta-Smad2/3 signaling (Descargues et al., 2008; Marinari et al., 2008). Loss of the IKK1 kinase activity inhibits the proliferation of breast and prostate tumors (Luo et al., 2007; Cao et al., 2001, 2007). In breast cancer proliferation, IKK1 regulates cyclin D1 expression (Cao et al., 2001, 2007) and in prostate cancer progression, IKK1 regulates maspin expression (Luo et al., 2007).
We have identified poky as a mutation in the zebrafish homolog of ikk1. poky mutants display a failure in EVL differentiation. The results presented here extend earlier evidence of molecular homology between differentiation of the mouse skin and zebrafish EVL. In the mouse, mutation of irf6 results in an almost identical skin differentiation phenotype as loss of ikk1 (Ingraham et al., 2006; Richardson et al., 2006). Likewise, expression of dominant negative Irf6 in the zebrafish results in a failure of EVL differentiation and a phenotype similar to that of poky (Sabel et al., 2009) indicating there may be extensive homology between the pathways required for EVL and mammalian skin differentiation.
Additional zebrafish genes have been identified that affect EVL morphogenesis and differentiation. All of these have a substantial maternal contribution, indicating that formation of the EVL occurs early. Loss of maternal and zygotic pou5f1 (oct4) increases protrusive activity of EVL cells (Lachnit et al., 2008). Loss of maternal and zygotic epcam results in decreased protrusive activity of EVL cells and decreased adherens junction formation (Slanchev et al., 2009). Knock-down of foxh1 results in a loss of keratin gene expression in the EVL (Pei et al., 2007). Interestingly, all of these also result in an epiboly delay. In addition to these early phenotypes affecting the EVL, several mutants affecting differentiation and maintenance of the basal layer have been identified. These include penner/lgl2, which is required for hemidesmosome formation, hai1 which is required for matrix maturation and psoriasis which is required to suppress basal cell proliferation (Webb et al., 2008; Carney et al., 2007). Together these mutants indicate that the zebrafish epidermis requires complex regulation for its establishment and maintenance.
We show here that poky/ikk1 mutants are defective in differentiation of the EVL and epithelial barrier formation but that basal cells are specified normally. In the absence of Ikk1 activity, the cells that form the outer layer of the embryo form a rudimentary epithelium, but fail to exit the cell cycle or express markers typical of differentiated EVL. This mutant phenotype depends on zygotic transcription. While it is required for EVL differentiation, Ikk1 is not required for maintenance of the adult skin. We hypothesize that maternal poky is required for formation of the early EVL, but that once established, the epidermis does not require IKK1 kinase activity because the EVL persists as a periderm that lasts into larval stages. The molecular homology between the zebrafish EVL and mouse embryonic skin demonstrates that continued investigation of the zebrafish EVL will reveal mechanisms of epithelial cell cycle regulation and differentiation, yielding insight into human diseases including squamous cell carcinoma and cancers of the breast and prostate.
Materials and Methods
Fish husbandry
Zebrafish (Danio rerio) were maintained at 28.5 °C as described (Westerfield, 2000). Embryos were collected and staged according to established methods (Kimmel et al., 1995). All work involving the use of animals was performed with approval from the Rice University Institutional Animal Care and Use Committee. The poky mutation was maintained on a mixed AB, TU, DZ genetic mapping background. Wild type controls were primarily from the DZ mapping reference strain (McCollum et al., 2007). Heterozygous females were used for mitotic index count controls.
Molecular methods and microinjection
The IKK1 NM_200317 and NF-kB2 NM_001001840 coding regions were amplified from full-length cDNA and cloned into TOPO vector pCR4.1 (Invitrogen), followed by gel extraction using the Wizard SV Gel and PCR Cleanup System (Promega) and ligation into pCS2+ and pCS2+ MT vectors. IKK1 primers:
Fwd: 5′-gaattcatggagaaaccccct-3′
Rev: 5′-ctcgagaatgcagacaccgacaaagtt-3′
NF-kB2 primers:
Fwd: 5′-gaattcatggctggagcactaaggat-3′
Rev: 5′-ctcgagtcagtgattgcccactgcagg-3′
Mutated versions of IKK1 were created using the QuikChange XL Site-Directed Mutagenesis Kit (Stratagene). The following primer sequences were used:
K-dead IKK1 (K44M):
Fwd 5′-gaaacatcagaaaaaatagcagtgatgctctgtcgccttgag-3′
Rev 5′-ctcaaggcgacagagcatcactgctattttttctgatgtttc-3′
T-loop dead (S177A, S181A):
Fwd 5′-cctggaccagggggccttatgcactgcatttgtggggac-3′
Rev 5′-gtccccacaaatgcagtgcataaggccccctggtccagg - 3.
T-loop active (S177E, S181E):
Fwd 5′-atgccaaagacctggaccagggggaattatgcactgaattcgtggggactttacaatatctggc-3′
Rev 5′-gccagatattgtaaagtccccacgaattcagtgcataattccccctggtccaggtctttggcat-3′
-LZ IKK1 (M469S, M470S):
LZ2 fwd: 5′-atacaaatttgacaagatacaagaactcgaggttctccttttcacaacagctcaaag-3′
LZ2 rev: 5′-ctttgagctgttgtgaaaaggagaacctcgagttcttgtatcttgtcaaatttgtat-3′
LZ3 fwd: 5′-atacaaatagtactagatacaagaactcgagcttctccttttcacaacagctcaaag-3′
LZ3 rev: 5′-ctttgagctgttgtgaaaaggagaagctcgagttcttgtatctagtactatttgtat-3′
-HLH IKK1 (L609R, Y610P)
HLH fwd: 5′ ccagaatcaagaccgggtgctaagagataggcctgcacacctcagtaaaatccttctg 3′
HLH rev: 5′ cagaaggattttactgaggtgtgcaggcctatctcttagcacccggtcttgattctgg 3′
-NDB IKK1 (introduce a stop codon deleting last 19 amino acids)
NBD fwd gagaaatcggagagcataatgtaactcgaggctcaggactggag
NBD rev ctccagtcctgagcctcgagttacattatgctctccgatttctc
RNA was synthesized using the mMESSAGE mMACHINE kit (Ambion). Microinjections were carried out using the Harvard Apparatus PLI-90 microinjector. For double injections, embryos were injected separately with each solution.
Western blotting
For protein expression, each embryo was injected with 550 pg of NF-kB–myc mRNA. Protein samples from animal caps were obtained as previously described (McCollum et al., 2007) and separated by electrophoresis on a 10% SDS-PAGE gel. The presence of myc-tagged NF-kB protein was detected with monoclonal mouse c-myc antibody (1:10,000) (Santa Cruz) and peroxidase-conjugated goat-α-mouse antibody (1:20,000) (Rockland) using chemiluminescence (Amersham Biosciences).
Scatter label expression of Ikk1
For 64 cell injections, 100 pL of myc-tagged CHUK was injected into 1 blastomere. Embryos were fixed at 50% epiboly and processed for a Fast Red (Roche) keratin 4 in situ, followed immediately by antibody staining to detect the myc epitope.
In situ hybridization
All procedures for whole-mount in situ hybridization were carried out as described (Sagerström et al., 1996). The following probes were used: ntl (Schulte-Merker et al., 1994), gsc (Schulte-Merker et al., 1994), chordin (Miller-Bertoglio et al., 1997), eve1 (Joly et al., 1993), fbp1b (Thisse et al., 2001), keratin 4 (Thisse et al., 2001), keratin 8 (Imboden et al., 1997), cyt1 (Sagerström et al., 2005). Scatter-labeled embryos were processed identically except Fast Red (Roche) was used as a development reagent instead of NBT/BCIP.
Mitotic indices and EVL cell counting
Heterozygous and mutant embryos were fixed and stained for filamentous actin as described (Topczewski and Solnica-Krezel, 1999) and counterstained 1:1000 with DAPI. EVLs were peeled off and flat mounted for 10 embryos per group, and the deep cells of 10 other embryos were imaged. Images were imported to ImageJ, and nuclear morphologies were assessed. An average of 75 EVL and 148 DEL nuclei were examined per embryo. For EVL cell counting, embryos at 50% epiboly were stained with phalloidin and imaged using a Leica MZFLIII stereoscope. The number of EVL cells in a known area was counted on ImageJ, and the surface area of the blastoderm was calculated by using the formula for the surface area of a spherical cap (A= 2πrh).
Histology and antibody staining
Zebrafish embryos and steaks were embedded in gelatin or Tissue-Tek O.C.T. compound (Sakura Finetek) and cryosectioned at 10um, followed by staining in hematoxylin and eosin or processing for antibody staining or biotin-streptavidin assays. The following antibodies were used: mouse anti-ZO1 (1:25, Invitrogen), anti-cadherin 1:50, anti-beta-catenin (1:200, Sigma), anti-aPKC (1:50, Santa Cruz), rabbit anti-myc (1:50, Santa Cruz), Alexa-Fluor 488 goat anti-rabbit (1:500, Invitrogen) and Alexa-Fluor 488 goat anti-mouse (1:500, Invitrogen). For whole-mount antibody staining, embryos were treated with 1% SDS in PBS for 5 minutes after permeabilization in 1% Triton-PBS, and then washed 4×15′ in PBS. Biotin labeling was performed as described, with some modifications (Kiener et al., 2008). Live embryos were dechorionated and incubated for 1 hr in 1 mg/ml EZ-Link Sulfo-NHS Biotin (Thermo Scientific), washed 3×5′ in 1×E3, fixed 1 hr. at RT in 4% PFA, then washed 3×10′PBS. Sections were stained with 1:1000 streptavidin (Invitrogen) at RT, then washed 4×15′ in PBST.
Electron Microsocopy
For transmission electron microscopy, control heterozygote and poky embryos were fixed with 2.5% glutaraldehyde in PBS for 1 hr. at room temperature, washed 3 times in ice cold PBS, and post-fixed with 1% osmium tetroxide in PBS for 1 hr. at room temperature followed by 3 washes in PBS. Samples were washed 3 times for 5 min. each with 30% then 50% ethanol, and en bloc stained with saturated uranyl acetate in 50% ethanol for 1 hr. at room temperature. Further dehydration was then done with 70, 90, 95, and 100% ethanol, and samples were embedded using the Spurr’s low viscosity resin kit (Electron Microscopy Sciences). Ultrathin 75 nm sections were stained with Reynold’s lead citrate, and viewed at 80kV accelerating voltage in a Hitachi H7500 electron microscope equipped with a Gatan US1000 digital camera and DigitalMicrograph imaging software (Gatan, Inc: v.1.82.366.0).
Cell death and cell membrane viability assays
Apoptotic nuclei were identified using the ApopTag Tdt assay kit (Chemicon International) as previously described, with some modifications (Wagner et al., 2004). Fixed embryos were incubated at 37°C in terminal deoxynucleotidyl transferase and digoxigenin-labeled dUTP for 2 hrs, and washed 2×10′ in Stop/Wash buffer. Embryos were incubated overnight in alkalkine-phosphatase coupled anti-dioxygenin Fab fragments, washed, and developed as for in situs. To assay for cell membrane viability, embryos were dechorionated and incubated in 0.25 ng/ul propidium iodide (Invitrogen). Pictures were acquired using a Leica MZFLII stereoscope.
Transcription inhibition
Zygotic transcription was inhibited as described (Dalle Nogare et al., 2009). Embryos were injected with 2 nL of 0.2 mg/ml alpha-amanitin (Fluka BioChemika) into the yolk at 4–8 cells. in situ hybridization for keratin 4 revealed no expression in alpha-amanitin injected embryos.
Photography
All images were acquired using a Carl Zeiss Axiovert 200M, unless otherwise specified.
For time lapses, embryos were dechorionated and mounted in 0.5% low-melt agarose. For EVL time lapses, embryos were injected at 1 cell with 375 pg of membrane-localized GFP RNA. Interstitial space in the DEL was labeled by injecting 4 nL of 0.1% Alexa-488 10k dextran (Invitrogen) into the blastoderm at late blastula stage. For EVL lineage tracing, embryos were injected with 250 pL of 1% Alexa-488 10k dextran (Invitrogen) into 1 blastomere at the 32 or 64 cell stage; EVL cells were identified at Day 1 by morphology and size.
Results
poky mutant embryos are delayed in epiboly
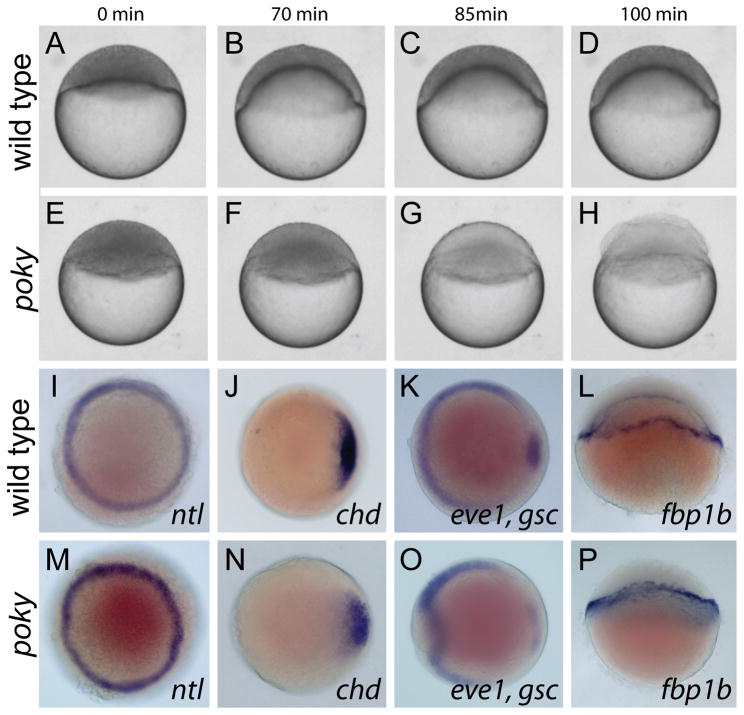
poky is a zebrafish maternal effect mutant that displays defective epiboly progression (Dosch et al., 2004; Wagner et al., 2004). While the pokyp20ad mutation was initially characterized by a delay in epiboly of the DEL, YSL and EVL (Wagner et al., 2004), subsequent breeding of poky mutants in a mixed AB/TU background led to an exacerbated phenotype. The strongly affected embryos described here usually failed to initiate epiboly, arresting at sphere stage. When age-matched control embryos reached approximately 50% epiboly, the blastoderm of poky mutant embryos cleared and lysed, exposing a bare yolk (Fig. 1A–H, Movie S1). Typically 90–100% of embryos from strong line females display the strong phenotype (Table 1). To determine if strongly delayed embryos were patterned normally, we examined the expression of several genes at 50% epiboly. no tail, goosecoid, even skipped 1, chordin and fructose-1,6-bisphosphatase 1b were all expressed in similar patterns in control and mutant embryos (Fig. 1I–P), indicating that in spite of the epiboly delay, specification of the YSL and patterning of the DEL occurred normally.
Figure 1. Poky mutants are delayed in epiboly.
(A–H) Stills from time lapse analysis of epiboly in wild type (A–D) and poky mutant embryos (E–H) timed from sphere stage (0 min). (I–P) Whole mount in situ expression in 50% epiboly wild type (I–L) and age matched poky mutant (M–P) embryos, (I,M) ntl, (J,N) chd, (K,O) eve1, gsc, (L, P) fbp1b. (A–H, L and P) lateral views. (I–K, M–O) animal pole views.
Table 1.
Rescue of poky mutant phenotype by ikk1 mRNA constucts
| Construct | amount | total | poky phenotype | wild type phenotype | % Wild Type |
|---|---|---|---|---|---|
| Control | 515 | 479 | 36 | 7 | |
| Wt ikk1 | 500 pg | 76 | 32 | 44 | 58 |
| 200 pg | 54 | 13 | 41 | 76 | |
| 100 pg | 143 | 47 | 96 | 67 | |
| 10 pg | 114 | 32 | 82 | 72 | |
| 1 pg | 209 | 168 | 41 | 20 | |
| Control | 995 | 969 | 26 | 3 | |
| pokyp20ad ikk1 | 2 ng | 100 | 100 | 0 | 0 |
| k-dead | 1 ng | 97 | 97 | 0 | 0 |
| t-loop dead | 1 ng | 50 | 50 | 0 | 0 |
| -LZ | 1 ng | 41 | 41 | 0 | 0 |
| -HLH | 1 ng | 48 | 7 | 41 | 85 |
| -NBD | 1 ng | 98 | 5 | 93 | 95 |
poky encodes the zebrafish chuk/ikk1
We determined the map position of poky to lie between the markers g13250 and z14593 on chromosome 13 using meiotic mapping. This corresponded to an interval of 2.5 Mb in build Zv7 of the Ensembl zebrafish genome build. We examined this interval and prioritized the 48 annotated genes within it on the basis of described function. RT-PCR of ovary and oocyte mRNA samples revealed maternal expression in 20 of 23 genes tested. We sequenced 17 genes within the interval and found a single base change in ikk1 not found in the original TU strain. Sequence of the corresponding genomic region of ikk1 in the original mutagenized male lacked this nucleotide change, indicating that it was induced by ENU mutagenesis (data not shown). Zebrafish Ikk1 has extensive conservation with human IKK1 with overall 63% amino acid identity (Correa et al., 2005 and data not shown). Amino acid conservation extends beyond the kinase domain and includes the leucine zipper, helix-loop-helix and NEMO binding domains. Zebrafish Ikk1 has only 51% identity with the closely related human IKK2 (data not shown).
Ikk1 is a kinase component of the IKK complex, a central mediator of NFkB signaling in a variety of contexts (Häcker and Karin, 2006; Karin and Ben-Neriah, 2000; Karin, 2008; Solt and May, 2008). The nucleotide missense mutation in pokyp20ad results in the substitution F182S (Fig. 2A). This position lies immediately adjacent to the T-loop that contains two serines that are phosphorylated by upstream kinases to activate IKK1 (Ling et al., 1998). This phenylalanine is conserved in human and mouse IKK1 and IKK2 genes. Furthermore, the Drosophila ikk homolog contains a valine at this position, indicating that a hydrophobic residue is important and that substitution by the hydrophilic serine affects structure or recognition by upstream kinases.
Figure 2. poky encodes chuk/ikk1/ikkα.
(A) schematic of domains of Ikk1. Amino acid sequence around the T-loop activation domain in wild type; poky mutant and human sequence is aligned below. The site of the missense mutation in pokyp20ad is indicated by the asterisk (*). T-loop activating serines are indicated in red. Missense mutation in pokyp20ad substituting the conserved phenylalanine for serine is indicated in green. Nonconserved amino acids in the human sequence are indicated in blue. (B) percent rescue to 24 hpf by ikk1 mRNA. (C,D,E,H,I,J) Whole mount in situ hybridization for cyt1 (C,H), krt8 (D,I) and krt4 (E,J) at 50% epiboly. (F,G,K,L, M–V) whole mount in situ hybridization for krt4 at 50% epiboly following microinjection of the indicated mRNA. (W–Y) Cell autonomy of Ikk1. (W) Anti myc staining of myc-Ikk1 scatter labeled embryo. (X) krt4 expression of the same embryo. (Y) Merged image of W and X. Rescued EVL cells (arrowhead) and isolated myc positive DEL cells (arrow) can be observed.
Zebrafish ikk1 is maternally expressed and widely distributed throughout the early embryo (Correa et al., 2005). We rescued the poky epiboly delay and blastoderm lysis mutant phenotype by injecting in vitro transcribed mRNA encoding wild type Ikk1. As little as 1 pg of wild type ikk1 mRNA can rescue poky mutants to viability 1 dpf (Table 1 Fig. 2B), yet microinjection of 2 ng of poky mutant mRNA cannot rescue the mutant phenotype, indicating that the mutation results in a null or strong hypomorph (Table 1). Microinjection of up to 2 ng of wild type mRNA did not cause developmental defects in wild type embryos.
poky mutant embryos have a defect in EVL differentiation
Epiboly delay and blastoderm rupture have been observed in embryos with EVL differentiation defects. Knock-down of FoxH1 with high doses of morpholino results in epiboly delay and embryo lysis (Pei et al., 2007). This knock-down results in loss of expression of several keratin genes expressed in the EVL. Knock-down of these keratin genes also disrupts epiboly (Pei et al., 2007). However, in both cases the epiboly delay is less pronounced than we observe in poky mutants. Expression of dominant negative Irf6 in zebrafish embryos leads to a failure of EVL differentiation, epiboly delay and blastoderm rupture that closely resembles the phenotype we observe in poky mutant embryos (Sabel et al., 2009). A hallmark of the Irf6 dominant negative phenotype is the loss of keratin gene expression in the EVL. To determine if EVL differentiation was defective in the poky mutants, we examined the expression of robust markers of EVL differentiation cytokeratin 1 (cyt1), keratin 8 (krt8) and keratin 4 (krt4) in poky mutant embryos and controls. In wild type embryos, these genes initiate expression in the EVL around mid-blastula stage (sphere stage) and display robust expression by the time embryos have reached late blastula (50% epiboly). In strongly affected poky mutant embryos, keratin expression was limited to a few scattered cells or none at all (Fig. 2C–E,H–J). Thus poky mutant embryos do not have a fully differentiated EVL.
The kinase activity of ikk1 is required for EVL differentiation
We examined the requirement for identified domains in ikk1 for EVL differentiation. Microinjection of wild type ikk1 mRNA can rescue krt4 expression in the EVL (Fig. 2K). Based on data from in vitro differentiation models and knock-in mice, the kinase activity of Ikk1 is not required for epidermal differentiation (Cao et al., 2001; Hu et al., 2001). We tested the ability of kinase dead and T-loop dead mutant forms of ikk1 to rescue poky mutants. The pokyp20ad allele is a missense mutation immediately adjacent to the second activating serine in the T-loop, indicating that the T-loop is important for activity. As expected, mRNA in which the activating serines had been substituted for alanines failed to rescue the poky mutant phenotype (Table 1, Fig. 2N,S). The kinase dead ikk1 K44M also failed to rescue the mutant phenotype (Fig. 2M,R). We tested the requirement for the leucine zipper, HLH and NEMO binding domains of Ikk1. The leucine zipper and HLH domains are necessary for differentiation in cultured mouse keratinocytes while NEMO binding domain (-NBD) deletions are not (Hu et al., 2001). As observed in mouse keratinocyte differentiation, the leucine zipper mutant mRNA failed to rescue poky mutant embryos (Fig. 2O,T) while the NDB (May et al., 2002) was not required to rescue the mutant phenotype (Fig 2Q,V). Surprisingly, the HLH mutant was able to rescue the poky mutant phenotype, indicating that the HLH domain is not required (Fig. 2P,U); a direct contrast to work in mammalian keratinocyte differentiation (Hu et al., 2001; Zhu et al., 2007).
Loss of poky/ikk1 activity does not affect NfkB2 processing in the early embryo
The mouse IKK1 mutant does not display a defect in NfkB signaling (Hu et al., 2001). Zebrafish Ikk1 was previously described as an NfkB antagonist competing with Ikk2 for the IKK complex component NEMO (Correa et al., 2005). Given this difference in requirement for the kinase activity of IKK1 in zebrafish and mouse, we determined the effect of the poky mutation on NfkB signaling in zebrafish. Phosphorylation of NFkB2 by Ikk1 leads to the proteolytic processing of the IkB p100 into the active NFkB, p52 (Senftleben et al., 2001). We tested the ability of zebrafish IKK1 to promote p100 processing in the early zebrafish embryo. mRNA encoding myc-tagged zebrafish p100 was microinjected into poky mutant embryos, wild type embryos, wild type embryos coinjected with ikk1, and wild type embryos co-injected with a putative activated ikk1 with phosphomimetic substitutions at the activating serines in the catalytic domain. Western blot analysis of protein extracts revealed the presence of both p100 and p52 isoforms in equal relative proportions in all conditions (Fig. S1). This result indicates that the level of functional Ikk1 in the cell did not affect processing of p100.
To test if p100 activity was required for early development, we expressed p100 mRNA, which is predicted to act as a dominant negative through its IkB function. We also tested a p100 with mutations in the Ikk1 target sites, which should not be processed and should act as a more potent dominant negative (zebrafish S883A/ S887A, mouse S866/S870). However, neither mRNA phenocopied the poky mutant (data not shown). We also attempted to rescue the poky mutant phenotype by expression of the predicted activated form of p52 (aa 1–416), but it did not rescue (data not shown).
poky/Ikk1 acts in the blastoderm cell autonomously
Mouse Ikk1 has been described as having non-cell autonomous functions in skin differentiation (Gareus et al., 2007; Hu et al., 2001). Recent results have caused some of these experiments to be reinterpreted (Liu et al., 2008). However, Ikk1 has been shown to regulate growth factor gene expression, supporting these claims (Gareus et al., 2007; Sil et al., 2004; Descargues et al., 2008; Liu et al., 2008). In the zebrafish embryo, Ikk1 is expressed ubiquitously in all cell layers of the early embryo (Correa et al., 2005 and data not shown). In order to discriminate between location-specific requirements for Ikk1, we injected Ikk1 mRNA into the yolk syncytial layer (YSL) at the 1000 cell stage. We observed no rescue of either the epiboly phenotype or the EVL differentiation defect (data not shown), and concluded that Ikk1 is required in the blastoderm for both epiboly and EVL differentiation. In order to determine the specific cells that require Ikk1, we injected myc-tagged ikk1 mRNA into single blastomeres of poky embryos at the 64-cell stage, and assayed krt4 expression at 50% epiboly (Fig 2W–Y). krt4 expression coincided with EVL cell expression of myc-Ikk1 but not with expression of myc-Ikk1in underlying DEL cells. In 9/14 embryos all krt4+ cells were also myc+. A minority of embryos (5/14) had small numbers of myc−/ krt4+ cells comparable to the number of uninjected poky embryos with small numbers of krt4+ cells (5/16). Thus, we concluded that Ikk1 functions in a cell autonomous manner to promote EVL differentiation.
poky mutant EVL cells are polarized and localize tight junction proteins
The EVL cells in poky mutant embryos failed to express specific keratin genes typical of differentiated EVL. To determine if they had other features of epithelial organization, we examined the formation of tight and adherens junctions characteristic of this simple squamous epithelium. Components of tight junctions are localized to sites of cell-cell contact in external blastomeres by the 64-cell stage, prior to keratin gene expression (Kiener et al., 2008). By 50% epiboly, tight and adherens junction components are robustly localized to the sites of cell-cell contact in the EVL. The tight junction associated protein ZO1 was localized to the interface between EVL cells of wild type and poky mutants (Fig. 3I–P). It also showed variable nuclear localization in both wild type and poky mutants, but variation in this signal did not coincide with the phenotype. Sections through the EVL showed ZO1 localized to a small domain at the interface between cells, consistent with its localization to tight junctions in wild type and poky mutants (Fig. 3Q,R). Tight junctions are required to separate apical and basolateral domains in polarized epithelia. We examined the apical-basal polarity of the EVL cells of poky mutant embryos and found it to be normal. aPKC was localized to the apical surface (Fig. 3Q–T), while E-cadherin and B-catenin were localized to the basolateral domain (Fig. 3S–V). To confirm the presence of tight junctions we examined them by transmission electron microscopy at 30% epiboly and observed tight junctions at apical sites of cell-cell contact (Fig. 3W–Z). In sections the junctions appear superficially normal; however, whole mount staining allowed us a wider survey of EVL cells and revealed differences in junction marker localization. Whereas in wild type embryos, ZO1 and microfilaments were juxtaposed in adjacent cells (Fig. 3E,G,M,O), in poky mutant embryos, localization of junction components in less well defined, with ZO1 and microfilaments appearing as wide bands at cell boundaries (Fig. 3F,H,N,P). This defect was observable in the microfilament organization at cell-cell boundaries in early embryos (sphere stage) (Fig. 3A–D) and became more pronounced as embryos aged (Fig. 3E–H). One aspect of microfilament and ZO1 distribution in wild type EVL cells was the enrichment at many vertices of the polygonal EVL cells (Fig 3G,O). This localization was absent in poky mutants (Fig. 3H,P) Thus, poky mutants do form a tight-junctioned enveloping epithelium with apical-basal polarity. However, the irregularities observed in the poky mutants’ EVL suggest that it lacks the robust junctions of mature wild type EVL.
Figure 3. poky mutant embryos display junction protein localization and apical basal polarity.
Wild type embryos displayed tight localization of actin microfilaments at cell-cell boundaries (arrows) at sphere (A, magnified in C) and robust labeling of cell vertices at 50% epiboly (E magnified in G, arrowheads). poky mutant embryos displayed actin localization to cell cell boundaries (arrows) at sphere stage (B magnified in D). At 50% epiboly (F magnified in H) the localization to cell-cell boundaries was less robust. Gaps were observed between neighboring cells (arrows) and little labeling was observed at cell vertices (arrowheads). Wild type embryos displayed localization of ZO1 to the tight junction at EVL cell borders at sphere (I magnified in K) and 50% epiboly (M magnified in O) with strong localization to cell-cell boundaries (arrows) and cell vertices (arrowheads). poky mutants also displayed localization at sphere stage (J, magnified in L). By 50% epiboly poky mutant cells were smaller but still displayed localized ZO1 (N magnified in P, arrow), although they lacked cell vertex labeling (arrowhead, N,P). Wild type (Q,S,U) and poky mutant (R,T,V) embryos display apical localization of aPKC (green, Q–T), localization of ZO1 to sites of EVL cell-cell contact (red, Q,R), basolateral localization of β-catenin (red, S,T) and cadherin (U,V). (W–Z) TEM of wild type (W,X) and poky mutant (Y,Z) EVL cells at 30% epiboly (tight junctions, black arrows).
poky mutants fail to maintain an epithelial barrier
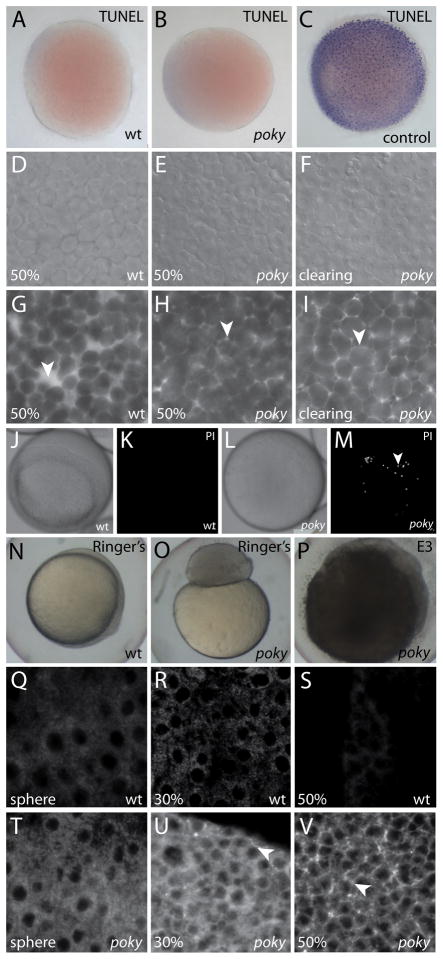
We suspected that the cells of the blastoderm cleared and lysed due to a hydrostatic pressure imbalance in DEL cells due to failure of the EVL barrier function. To test if the clearing of the blastoderm was due to widespread cell death at 50% epiboly in poky mutants, we performed TUNEL assays on embryos as they were clearing, but found no evidence for increased apoptosis (Fig. 4A–C). We examined the arrangement of the DEL cells by DIC microscopy and we labeled the interstitial space of sphere stage wild type and poky mutant embryos with Alexa 488 fluorescent dextran and examined them at 50% epiboly. Wild type embryos had round, loosely packed cells and easily observable large interstitial spaces in the DEL (Fig. 4D,G). Prior to clearing, poky mutant embryos had round, tightly packed cells with only small pockets of interstitial space between them (Fig. 4E,H). During clearing, poky mutant embryos were even more tightly packed and had less contrast in DIC, indicating close association between the DEL cells and little interstitial space due to swelling(Fig. 4F,I).
Figure 4. poky mutants fail to form an EVL barrier.
Wild type (A), poky mutant (B) and wild type positive control (C) processed for TUNEL. DIC (D–F) and interstitial space labeled with fluorescent dextran (G–I) of wild type (D,G), intact poky mutants (E,H) and clearing poky mutants (F,I) reveals large spaces in the wild type embryo at 50% epiboly (arrowhead, G) but little interstitial space in poky mutants (arrowheads, H,I). White light (J,L) and fluorescent (K,M) of propidium iodide stained live wild type (J,K) and clearing poky mutants (L,M) shows labeling of multiple nuclei in poky mutants (arrowhead, M). Wild type control embryos at 100% epiboly develop normally in 1 × Ringer’s saline (N). poky mutant embryo cultures in Ringer’s saline did not lyse but failed to initiate epiboly (O). poky mutant embryos cultured in E3 displayed blastoderm lysis when controls reached 50% epiboly (P). Wild type embryos (Q–S) displayed little interstitial biotin labeling. poky mutant embryos showed little interstitial label at sphere stage (T). 30% epiboly poky mutant embryos had some interstitial signal (arrowhead, U). At 50% epiboly poky mutants had strong interstitial label throughout the blastoderm (arrowhead, V).
To determine if individual cells of the blastoderm lysed prior to embryo clearing, we assayed for the ability of propidium iodide (PI) to stain live embryos, which would indicate that the cell membrane was permeable and the nucleus accessible (Macklis and Madison, 1990). We observed an average of 1.4 PI-positive nuclei in wild type (n=5) and 18.8 PI-positive nuclei in clearing but intact poky mutants (n=5) (Fig. 4J–M).
To further investigate whether the clearing and lysis of the blastoderm is due to hypotonic stress on the DEL, we cultured embryos in a more isotonic environment of 1X Ringer’s Saline and observed rescue of the blastoderm disintegration phenotype, but not rescue of the epiboly defects (Fig. 4N–P). We conclude that the swelling and rupture of the blastoderm in poky mutants is due to a failure to maintain an isotonic environment in the DEL.
The EVL acts as a barrier between the external environment and the internal space of the blastoderm, providing resistance to the passage of solutes (Bennett and Trinkaus, 1970; Hagedorn et al., 2002; Kiener et al., 2008). We tested the permeability of poky mutant EVL by treating live embryos with sulfo-NHS-biotin at sphere, 30% and 50% epiboly and assaying for penetration of this small hydrophilic molecule into the interstitial space of the blastoderm (Kiener et al., 2008). Background signal from nonspecific streptavidin labeling of cytoplasm was visible in all samples. Penetration of biotin into the blastoderm was visible as strong interstitial labeling. The biotin was excluded from the interstitial space of wild type embryos (Fig. 4Q–S). At 30% epiboly the poky mutant embryos had elevated penetration of biotin into the interstitial space (Fig. 4U). When wild type controls had reached 50% epiboly, poky mutant embryos had widespread biotin distribution throughout the interstitial space (Fig. 4V), demonstrating failure of the EVL barrier at the time of clearing of the blastoderm and lysis of DEL cells. Embryos were treated for 1 hour at the indicated time point, so the increased signal reflects increased permeability, not increased accumulation over time. We concluded that poky mutant embryos have a progressive loss of barrier function culminating in failure when controls reach 50% epiboly and mutant blastoderms clear and lyse.
poky mutant EVL cells do not slow the cell cycle or show cell layer restriction
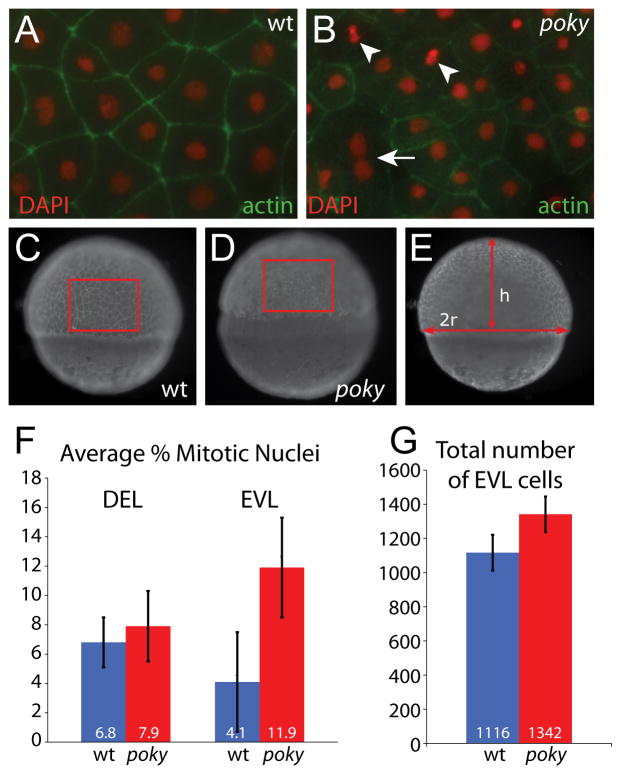
In addition to a loss of epidermal barrier function, IKK1 mutant mice have an expanded intermediate or spinous layer with a high mitotic index. These cells do not exit the cell cycle or form the post-mitotic granular layer (Takeda et al., 1999; Hu et al., 1999). To determine if a similar cell cycle defect was present in zebrafish ikk1 mutants, we examined cell cycle regulation in poky mutants. In wild type embryos the cell cycle slows in all cells at the mid-blastula transition (MBT, cell cycle 10) (Kane et al., 1993; Kane et al., 1992; Kane, 1999; Dalle Nogare et al., 2009). At sphere stage (cell cycle 11), the DEL and EVL have a cell cycle length of approximately 20 minutes. The EVL cell cycle length increases over the next two cycles to 65 minutes, whereas the DEL cell cycle increases to approximately 45 minutes (Kane et al., 1992). To determine if the cell cycle regulation of the EVL was affected in poky mutants, we examined the EVL cells in membrane GFP-labeled embryos at 50% epiboly by time lapse microscopy. We observed frequent cell divisions within the most external cell layer of poky mutant embryos (Movie S2). During epiboly, EVL cell divisions in wild type embryos result in two daughter cells that remain in the EVL (Kane et al., 1993). In DIC time lapse analysis of wild type and poky mutant embryos, we observed more cells in the outer layer of poky mutants and cells leaving this layer (Movie S3), indicating that the EVL cells are not lineage restricted. We quantified the mitotic index of EVL and DEL cells in poky mutants and wild type controls at 50% epiboly by counting mitotic figures in DAPI-stained embryos counter stained with phalloidin. The EVL was removed and flat mounted, and mitotic nuclei were identified based on their morphology (Fig. 5A,B). The DEL mitotic index was obtained by imaging deep into the blastoderm of DAPI/phalloidin stained embryos. The EVL cells of poky mutant embryos had a higher mitotic index than the EVL cells of the sibling controls (Fig. 5F, n= 10 each class, p<0.001, t-test). However, the DEL cells of wild type and mutants had a similar index, lower than the average observed for the poky EVL (Fig. 5F, n=10, p=0.26, t-test). This higher EVL mitotic index is reflected in the higher number of cells in the poky mutant EVL. We measured the number of EVL cells per unit area and then calculated the area of the EVL in wild type and poky mutant embryos when control embryos were at 50% epiboly (n= 8 each class) (Fig. 5C–E). In spite of the smaller surface area, poky mutants had more EVL cells (p=0.001, t-test) (Fig. 5M). One unexpected feature of EVL cells was the presence of occasional multinucleate EVL cells in both wild type and poky mutants (Fig. 5B, Movie S3).
Figure 5. poky mutants fail to down regulate cell division in the EVL.
Embryos were stained with phalloidin (green) to reveal actin localization at cell boundaries and DAPI (red) to reveal nuclear morphology. Dividing cells were identified by their nuclear morphology (arrowheads, A&B). Multinucleate EVL cells were observed in wild type and mutant embryos (arrow, B). Total number of EVL cells was calculated by determining the number of cells per unit area as C and D and then calculating the total EVL surface area by calculating the radius and height as in E. (F) Percent mitotic nuclei. (G) Total number of EVL cells.
Gene transcription is required for the EVL phenotype in poky mutants
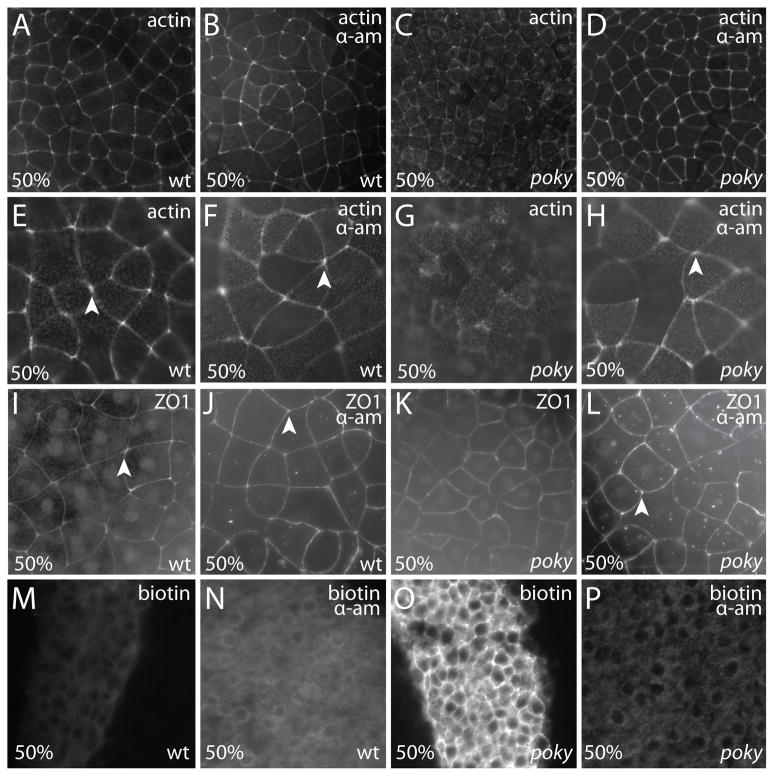
Although a difference between wild type and poky mutant EVL can be detected as early as sphere stage, in the slight disruption of cell-cell boundaries and slightly stronger biotin labeling, the severity of these phenotypes appears to increase with time. The progressive loss of EVL function indicates a transition in the composition or requirement for the EVL in the developing embryo. To test when poky is required for EVL differentiation, we treated wild type and mutant embryos with alpha-amanitin to block the onset of zygotic transcription. Alpha-amanitin treated wild type embryos continue to proliferate but do not initiate epiboly (Kane et al., 1996). Since we could not use the expression of keratin genes to determine if the EVL was differentiating, we stained fixed embryos with phalloidin and ZO1 to reveal the size and shape of the EVL cells. The EVL cells of alpha-amanitin treated wild type embryos displayed tightly juxtaposed cells with polygonal morphology and robust labeling at the cell vertices that resembled age matched wild type EVL cells at 50% epiboly (Fig. 6A,B,E,F,I,J). To our surprise, mutant embryos treated with alpha-amanitin also displayed EVL cells with a wild type morphology (Fig. 6D,H,L). In contrast, the untreated poky EVL cells were smaller and lacked robust localization of actin and ZO1 to the cell borders and vertices (Fig. 6C,G,K). In accordance with the rescue of the EVL cell morphology, alpha-amanitin treated poky mutant embryos had a functional EVL that was able to exclude biotin (Fig. 6M–P). Thus, blockage of zygotic transcription by alpha-amanitin rescued the EVL cell morphology and barrier function defects in poky mutants. This indicates that maternally supplied poky/ikk1 is required not for early EVL differentiation, but after the onset of zygotic transcription.
Figure 6. poky mutant phenotype requires zygotic transcription.
(A–H) EVL of phalloidin stained embryos (A–D) low magnification (E–H) high magnification. Untreated (A,E) and alpha-amanitin treated (B,F) wild type embryos display similar organization of actin microfilaments with robust localization to cell borders and cell vertices (arrowheads). Untreated poky mutants (C,G) display smaller EVL cells with less robust localization of actin to the EVL cell borders. Alpha-amanitin treated poky mutants (D,H) display a wild type EVL cell morphology, tight actin localization to the cell boundaries, and robust vertex labeling (arrowhead). (I–L) ZO1 localization at the same magnification as E–H. Untreated (I) and alpha-amanitin treated (J) wild type embryos display similar localization of ZO1 to tight junctions. Untreated poky mutants (K) display more EVL cells with less robust localization of ZO1 to cell borders and vertices. Alpha-amanitin treated poky mutants (L) display a wild type EVL cell morphology and tight ZO1 localization to the cell boundaries and robust localization to the vertices (arrowhead). (M,N) Wild type untreated and treated embryos exclude biotin from the DEL. (O) poky mutants do not exclude biotin and show strong interstitial labeling with fluorescent streptavidin. (P) alpha aminitin treated poky embryos exclude biotin from the DEL.
Ikk1 is required transiently for the establishment of the larval epidermis
In the mouse, the epidermis derives from the p63 positive basal layer of the epidermis. In the zebrafish, the EVL arises independently from the outer blastomeres, with the basal layer forming later from ventral ectoderm. Loss of maternal poky results in defects in EVL differentiation. Following neurulation, the EVL forms the external layer of the embryonic skin with the p63 basal cells underlying them (Bakkers et al., 2002; Webb et al., 2008). To test if differentiation of the p63 positive non-neural ectoderm also requires Ikk1, we cultured embryos in Ringer’s saline to promote survival until 24 hpf and assayed p63 expression by whole mount antibody staining. p63 positive nuclei were present in poky mutants and in wild type controls (Fig. 7A,B) indicating that specification of the basal layer does not require Ikk1. To determine if poky/ikk1 continues to be required for terminal differentiation of cells arising from the basal layer in the larval or adult skin, we examined skin of zygotic mutant poky larvae and adults derived from heterozygous mothers by gross observation of live fish and histology (Fig. 7C–H). We did not observe any differences between mutants and wild type controls. Genetic redundancy may compensate for the absence of zygotic Ikk1. However, we were unable to find additional ikk1 orthologs in the latest assembly of the zebrafish genome, (Zv8, data not shown); but found that, as in mammals, no duplicates exist of either IKK gene that might compensate for loss of IKK1 function. Work in the mouse has shown that IKK1 and IKK2 are not redundant in their molecular roles (Luedde et al., 2008). Therefore we hypothesize that poky/ikk1 is not required for differentiation of the superficial epithelium during turn over in adult skin.
Figure 7. poky mutant adults have normal skin and wild type EVL cells persist into larval stages.
(A,B) Optical section through wild type (A) and poky mutant (B) epidermis expressing nuclear p63 protein (green) at 24 hpf. (C,D) 2 year-old wild type and poky mutants have no obvious skin lesions. (E,F) Pigment, scales and fins are normal in wild type (E) and poky mutants (F). Histology of adult skin reveals the typical bilayered epidermis (bracket) in wild type (G) and poky mutants (H). Lineage tracing of embryonic and larval EVL (I–N). EVL from embryos scatter labeled with fluorescent dextran were observed up to 9 dpf. (I) 2 dpf low magnification image of the tail shows multiple cells labeled; location at higher magnification in J. (J) A clone of EVL cells is easily observable at 2 dpf (yellow arrowhead marks a single EVL cell). The location of these cells was traced relative to underlying muscle cells (red asterisk) at 2 dpf (J), 3 dpf (K), 5 dpf (L), 7 dpf (M) and 9 dpf (N).
The EVL is conventionally considered an extra embryonic structure that is lost during differentiation of the embryonic skin (Kimmel et al., 1990; Bouvet, 1976). Another hypothesis is that the EVL, once formed, persists to form the external layer of the larval epidermis (Slanchev et al., 2009). To address this question we used fluorescent dextran to label one-cell at the 32 cell stage in wild type embryos and then identified clones of EVL cells 1 dpf on the basis of their position, size and morphology (Slanchev et al., 2009; Sonawane et al., 2009). For clarity we chose clones without underlying labeled epidermal basal cells. The EVL cell clones in the tail were clearly identifiable through the 5 days of embryogenesis, and up to 9 dpf during larval development, after which time the label was difficult to follow (Fig. 7I–N). The EVL cells were not replaced by unlabeled epidermal cells derived from the basal layer, demonstrating that EVL cells persist into the larval period and form the external layer of the bilayered epidermis. These results suggest that maternal poky/ikk1 is required to initiate the differentiation of the EVL and that once it is established, this structure persists at least until larval stages.
Discussion
poky/ikk1 is required for EVL differentiation
The external cell layer of poky mutants has some of the epithelial features of EVL cells, such as localization of tight junction components and apical basal polarity. However, the cells do not slow their cell cycle, express specific markers of differentiation or maintain a permeability barrier. This phenotype is similar to that observed in IKK1 mutant mice which have excessive proliferation of epidermal cells, fail to express the markers of differentiated skin and fail to form a permeability barrier.
Despite the similarities of the ikk1 mutant phenotypes in the zebrafish EVL and mouse epidermis, the molecular function of Ikk1 in these animals is different. In the mouse, kinase activity of IKK1 is not required for skin differentiation: T-loop dead mutant knock-in mice have normal epidermal differentiation, although they display decreased mammary epithelium cell proliferation (Cao et al., 2001). However, the leucine zipper domain and the HLH domain (Hu et al., 2001) are required. In contrast to the results in mouse epidermis, we have found that the kinase activity and the leucine zipper domain are essential for poky function in EVL differentiation. However, the HLH domain is not. These results indicate that the molecular mechanisms driving cell cycle suppression and differentiation may differ between the mouse skin and zebrafish EVL, although the requirement for IKK1 function is shared.
The molecular role of poky in EVL differentiation
Ikk1 was originally identified as a component of the IKK complex with NFkB2 as its primary IkB kinase target (Senftleben et al., 2001). Our results indicate that Ikk1 is not acting through activation of the NFkB pathway. Correa et al identified Ikk1 as an NFkB pathway antagonist and proposed that Ikk1 functions in mesoderm patterning by competing for NEMO in IKK complexes (Correa et al., 2005). This is unlikely to be the role for Ikk1 in the EVL due to the fact that Ikk1 missing the NEMO binding domain rescues poky mutant embryos. Additionally, treatment of poky mutant embryos with the NfKB pathway inhibitor (NAI IV Calbiochem #481412) does not rescue the mutant phenotype (data not shown).
The kinase domain is required for EVL differentiation implicating specific targets of Ikk1 in this process. Independently of Nfkb signaling, diverse other Ikk1 targets have been identified in several systems (reviewed in Chariot, 2009). The identity of the targets required for EVL differentiation is currently under investigation.
EVL differentiation has transcription dependent and independent phases
The EVL begins to form before the onset of zygotic transcription. Tight junctions form between external blastomeres as early the 64-cell stage (Kiener et al., 2008). However, subsequent changes in proliferation rate, lineage restriction and gene expression that define the EVL require Ikk1. We observed that an initial barrier forms in poky mutants but is lost, suggesting that Ikk1 is required not for EVL formation per se but for later differentiation. Suppressing zygotic transcription in poky mutant embryos rescues EVL permeability, morphology and organization, apparently by preventing a disruptive transcriptional program. The mechanism of alpha-amanatin rescue may be indirect. Ikk1 may be required in the EVL to resist changes in the embryo associated with other transcription dependent processes such as epiboly and gastrulation. However, poky mutant embryos that fail to undergo epiboly still display defective EVL structure and lysis, indicating that the EVL differentiation phenotype we observe in poky mutants is not secondary to epiboly progression.
A more likely hypothesis is that Ikk1 may directly suppress a transcriptional program that promotes proliferation of the EVL. Prior to epiboly, the mid-blastula EVL proliferates rapidly. The cell cycles first slow during epiboly, then speed up again after epiboly is complete (Kane et al., 1992). Ikk1 may be required to suppress proliferation during a critical period to allow differentiation to occur. Current work in the mouse indicates that Ikk1 directly regulates gene transcription of specific genes required for suppressing proliferation and promoting differentiation (Liu et al., 2008; Zhu et al., 2007; Descargues et al., 2008), consistent with our observation that the poky mutant phenotype requires transcription. Myc-tagged Ikk1 expressed in the EVL is robustly localized to the nucleus (Fig. 2W), supporting a direct role for Ikk1 in transcriptional regulation.
The relationship among the processes of gene transcription, cell cycle regulation, and tight junction formation is currently unclear, but premiliminary data suggest that Ikk1 may regulate one process directly and the others indirectly. Knock-down of claudin E (cldnE), an EVL tight junction component, leads to the loss of keratin expression (Siddiqui et al., 2010, and unpublished data) suggesting that specific cell-cell contacts are required for EVL differentiation. However, cldnE knock-down embryos do not show elevated EVL cell division (CS and DSW unpublished data) indicating that loss of gene expression does not necessarily imply EVL hyperproliferation. Therefore, we currently hypothesize that, as with mouse Ikk1 (Liu et al., 2008), the critical role of Ikk1 is to slow the cell cycle and allow the transition of epidermal cells to a more differentiated state and that loss of keratin expression and tight junction integrity are secondary.
The role of IKK1 in epiboly
In the original description of the mutant phenotype, embryos were delayed in epiboly but did not display obvious EVL differentiation defects. The stronger mutant phenotype described here has an even more pronounced delay in epiboly, with mutants often failing to initiate epiboly before clearing and lysing. These different phenotypes may reflect independent requirements for IKK1 in these processes. Several genes have been identified that are exclusively required in the yolk cell for epiboly progression such as mk2a/betty boop (Holloway et al., 2009) and mtx2 (Bruce et al., 2005). Therefore, we hypothesized that if a similar requirement existed for Ikk1, microinjection of ikk1 mRNA into the yolk cell would rescue the epiboly delay. However, this did not rescue epiboly progression or blastoderm lysis (data not shown).
Differentiation of the EVL may be required for epiboly progression. Loss of cldnE results in epiboly defects, highlighting a requirement during epiboly for tight junctions between the EVL and the yolk or between EVL cells themselves (Siddiqui et al., 2010). Contact between the EVL and the surface of the yolk cell at the margin of the blastoderm may provide cues to the underlying yolk cell required to organize the cytoskeleton into the distinct domains associated with epiboly progression. Dramatic disorganization of the yolk cell cytoskeleton was observed in poky mutants that do initiate epiboly but have strongly delayed progression (data not shown). Taken together, these data suggest that the failure to undergo epiboly in poky mutants is most likely secondary to a defect in EVL differentiation.
pokyp20ad does not display an adult skin defect
Maternal message and protein are likely lost within the first few days of embryogenesis, but zygotic poky mutants born from heterozygous mothers have no apparent epidermal defect. In current models, the differentiated cells of the epidermis in older embryos are produced from cycling stem cells in the basal layer. This lower level production of epidermis may not require Ikk1 due to the lower proliferation rate of the differentiating cells. Results in the mouse support this hypothesis: Deletion of IKK1 in more mature keratinocytes results in a less dramatic overproliferation phenotype than the germ line deletion (Gareus et al., 2007; Liu et al., 2008). In these experiments, the skin differentiates but it is thickened with compromised barrier function and has a high rate of squamous cell carcinoma. Thus, once a critical early requirement for Ikk1 is past, Ikk1 is required only to slow cell division and maintain skin homeostasis. The simple architecture and regenerative capacity of the zebrafish may mask a similar role in larvae and adults. If ikk1 is analogously required in adult fish skin to maintain homeostasis it might be necessary to severely challenge the skin through wounding or chemical insult in order to see a phenotype.
EVL is not a transient embryonic structure
The EVL has been described as a transient structure homologous to the mammalian periderm that is shed when the embryonic epidermis differentiates. We have shown that EVL cells persist through embryonic stages and into larval stages, supporting the hypothesis that a persistent EVL forms a superficial epithelial layer distinct in origin from the basal layer. The relationship of these layers in later larval and adult stages is currently unknown. Presumably, the superficial epidermis in adults arises from the basal layer, however, long term lineage tracing experiments are necessary to address this question.
Molecular conservation of epidermal differentiation
We have shown that poky/ikk1 is required for differentiation of the zebrafish EVL as shown previously for irf6 (Sabel et al., 2009). The ikk1 and irf6 connection further confirms molecular homology between zebrafish EVL and mouse embryonic skin. In mice, the Irf6 and Ikk1 mutants have essentially the same phenotype, suggesting that they may act within the same pathway. In zebrafish, expression of a dominant negative Irf6 results in a phenotype similar to the poky/ikk1 mutant, supporting a conserved relationship between Irf6 and Ikk1 in regulating the differentiation of the epidermis. Future work will establish the relationship among these and other genes required at the same step of skin differentiation, shedding light on conserved mechanisms of epidermal differentiation across species.
Supplementary Material
Wild type embryo (left) undergoes normal epiboly movements. Age matched poky mutant embryos (right) fail to initiate epiboly, the blastoderm clears and then disintegrates. Images were captured with 5 minute intervals.
Wild type EVL (left) expressing a membrane localized GFP spread during epiboly. Age matched poky mutant EVL (right) show a high rate of cell division with multiple mitoses visible during this interval. Images were captured with 1 minute intervals.
DIC timelapse of poky mutant EVL. Cell indicated by < leaves the EVL and enters the DEL. Multinucleated cells were observed within the EVL (asterisk). Images were captured with 5 minute intervals.
Expression of mRNA encoding myc-NfkB2 (p100) was detected by anti-myc western blot analysis in wild type (lane 3), poky mutant (lane 4), wild type expressing IKK1 (lane 1) and wild type expressing a T-loop phosphomimetic substitution of Ikk1 (lane 2). In all conditions p100 and processed p52 were observed.
Acknowledgments
We would like to thank Dr Mary Mullins and Dr Rob Cornell for thoughtful discussion and comments on the manuscript. We are grateful to the Integrated Microscopy Core Laboratory, Baylor College of Medicine, for the assistance with the electron microscopy work. This work was supported in part by NIH R01GM77429 to DSW
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bakkers J, Hild M, Kramer C, Furutani-Seiki M, Hammerschmidt M. Zebrafish DeltaNp63 is a direct target of Bmp signaling and encodes a transcriptional repressor blocking neural specification in the ventral ectoderm. Dev Cell. 2002;2:617–627. doi: 10.1016/s1534-5807(02)00163-6. [DOI] [PubMed] [Google Scholar]
- Bennett MV, Trinkaus JP. Electrical coupling between embryonic cells by way of extracellular space and specialized junctions. J Cell Biol. 1970;44:592–610. doi: 10.1083/jcb.44.3.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvet J. Enveloping layer and periderm of the trout embryo (Salmo trutta fario L.)20U. Cell Tissue Res. 1976;170:367–382. doi: 10.1007/BF00219418. [DOI] [PubMed] [Google Scholar]
- Bruce AEE, Howley C, Dixon Fox M, Ho RK. T-box gene eomesodermin and the homeobox-containing Mix/Bix gene mtx2 regulate epiboly movements in the zebrafish. Dev Dyn. 2005;233:105–114. doi: 10.1002/dvdy.20305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Bonizzi G, Seagroves TN, Greten FR, Johnson R, Schmidt EV, Karin M. IKKalpha provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell. 2001;107:763–775. doi: 10.1016/s0092-8674(01)00599-2. [DOI] [PubMed] [Google Scholar]
- Cao Y, Luo J, Karin M. IkappaB kinase alpha kinase activity is required for self-renewal of ErbB2/Her2-transformed mammary tumor-initiating cells. Proc Natl Acad Sci USA. 2007;104:15852–15857. doi: 10.1073/pnas.0706728104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney TJ, von der Hardt S, Sonntag C, Amsterdam A, Topczewski J, Hopkins N, Hammerschmidt M. Inactivation of serine protease Matriptase1a by its inhibitor Hai1 is required for epithelial integrity of the zebrafish epidermis. Development. 2007;134:3461–3471. doi: 10.1242/dev.004556. [DOI] [PubMed] [Google Scholar]
- Chariot A. The NF-kappaB-independent functions of IKK subunits in immunity and cancer. Trends Cell Biol. 2009;19:404–413. doi: 10.1016/j.tcb.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Correa RG, Matsui T, Tergaonkar V, Rodriguez-Esteban C, Izpisua-Belmonte JC, Verma IM. Zebrafish IkappaB kinase 1 negatively regulates NF-kappaB activity. Curr Biol. 2005;15:1291–1295. doi: 10.1016/j.cub.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Dalle Nogare DE, Pauerstein PT, Lane ME. G2 acquisition by transcription-independent mechanism at the zebrafish midblastula transition. Dev Biol. 2009;326:131–142. doi: 10.1016/j.ydbio.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Descargues P, Sil AK, Sano Y, Korchynskyi O, Han G, Owens P, Wang X, Karin M. IKKα is a critical coregulator of a Smad4-independent TGFβ-Smad2/3 signaling pathway that controls keratinocyte differentiation. Proceedings of the National Academy of Sciences. 2008;105:2487–2492. doi: 10.1073/pnas.0712044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosch R, Wagner DS, Mintzer KA, Runke G, Wiemelt AP, Mullins MC. Maternal control of vertebrate development before the midblastula transition: mutants from the zebrafish I. Dev Cell. 2004;6:771–780. doi: 10.1016/j.devcel.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Gareus R, Huth M, Breiden B, Nenci A, Rösch N, Haase I, Bloch W, Sandhoff K, Pasparakis M. Normal epidermal differentiation but impaired skin-barrier formation upon keratinocyte-restricted IKK1 ablation. Nat Cell Biol. 2007;9:461–469. doi: 10.1038/ncb1560. [DOI] [PubMed] [Google Scholar]
- Hagedorn M, Lance SL, Fonseca DM, Kleinhans FW, Artimov D, Fleischer R, Hoque ATMS, Hamilton MB, Pukazhenthi BS. Altering fish embryos with aquaporin-3: an essential step toward successful cryopreservation. Biol Reprod. 2002;67:961–966. doi: 10.1095/biolreprod.101.002915. [DOI] [PubMed] [Google Scholar]
- Häcker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006;2006:re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- Ho RK. Cell movements and cell fate during zebrafish gastrulation. Dev Suppl. 1992:65–73. [PubMed] [Google Scholar]
- Holloway BA, Gomez de la Torre Canny S, Ye Y, Slusarski DC, Freisinger CM, Dosch R, Chou MM, Wagner DS, Mullins MC. A novel role for MAPKAPK2 in morphogenesis during zebrafish development. PLoS Genet. 2009;5:e1000413. doi: 10.1371/journal.pgen.1000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Baud V, Delhase M, Zhang P, Deerinck T, Ellisman M, Johnson R, Karin M. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKalpha subunit of IkappaB kinase. Science. 1999;284:316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- Hu Y, Baud V, Oga T, Kim KI, Yoshida K, Karin M. IKKalpha controls formation of the epidermis independently of NF-kappaB. Nature. 2001;410:710–714. doi: 10.1038/35070605. [DOI] [PubMed] [Google Scholar]
- Imboden M, Goblet C, Korn H, Vriz S. Cytokeratin 8 is a suitable epidermal marker during zebrafish development. C R Acad Sci III, Sci Vie. 1997;320:689–700. doi: 10.1016/s0764-4469(97)84816-0. [DOI] [PubMed] [Google Scholar]
- Ingraham CR, Kinoshita A, Kondo S, Yang B, Sajan S, Trout KJ, Malik MI, Dunnwald M, Goudy SL, Lovett M, et al. Abnormal skin, limb and craniofacial morphogenesis in mice deficient for interferon regulatory factor 6 (Irf6) Nat Genet. 2006;38:1335–1340. doi: 10.1083/ng1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly JS, Joly C, Schulte-Merker S, Boulekbache H, Condamine H. The ventral and posterior expression of the zebrafish homeobox gene eve1 is perturbed in dorsalized and mutant embryos. Development. 1993;119:1261–1275. doi: 10.1242/dev.119.4.1261. [DOI] [PubMed] [Google Scholar]
- Kane DA. Cell cycles and development in the embryonic zebrafish. Methods Cell Biol. 1999;59:11–26. doi: 10.1016/s0091-679x(08)61817-8. [DOI] [PubMed] [Google Scholar]
- Kane DA, Hammerschmidt M, Mullins MC, Maischein HM, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Haffter P, Heisenberg CP, et al. The zebrafish epiboly mutants. Development. 1996;123:47–55. doi: 10.1242/dev.123.1.47. [DOI] [PubMed] [Google Scholar]
- Kane DA, Kimmel CB. The zebrafish midblastula transition. Development. 1993;119:447–456. doi: 10.1242/dev.119.2.447. [DOI] [PubMed] [Google Scholar]
- Kane DA, Warga RM, Kimmel CB. Mitotic domains in the early embryo of the zebrafish. Nature. 1992;360:735–737. doi: 10.1038/360735a0. [DOI] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- Karin M. The IkappaB kinase - a bridge between inflammation and cancer. Cell Res. 2008;18:334–342. doi: 10.1038/cr.2008.30. [DOI] [PubMed] [Google Scholar]
- Kiener TK, Selptsova-Friedrich I, Hunziker W. Tjp3/zo-3 is critical for epidermal barrier function in zebrafish embryos. Dev Biol. 2008;316:36–49. doi: 10.1016/j.ydbio.2007.12.047. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Warga RM, Schilling TF. Origin and organization of the zebrafish fate map. Development. 1990;108:581–594. doi: 10.1242/dev.108.4.581. [DOI] [PubMed] [Google Scholar]
- Koster MI, Roop DR. Mechanisms regulating epithelial stratification. Annu Rev Cell Dev Biol. 2007;23:93–113. doi: 10.1146/annurev.cellbio.23.090506.123357. [DOI] [PubMed] [Google Scholar]
- Lachnit M, Kur E, Driever W. Alterations of the cytoskeleton in all three embryonic lineages contribute to the epiboly defect of Pou5f1/Oct4 deficient MZspg zebrafish embryos. Dev Biol. 2008;315:1–17. doi: 10.1016/j.ydbio.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Ling L, Cao Z, Goeddel DV. NF-kappaB-inducing kinase activates IKK-alpha by phosphorylation of Ser-176. Proc Natl Acad Sci USA. 1998;95:3792–3797. doi: 10.1073/pnas.95.7.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Park E, Zhu F, Bustos T, Liu J, Shen J, Fischer SM, Hu Y. A critical role for I kappaB kinase alpha in the development of human and mouse squamous cell carcinomas. Proc Natl Acad Sci USA. 2006;103:17202–17207. doi: 10.1073/pnas.0604481103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Xia X, Zhu F, Park E, Carbajal S, Kiguchi K, DiGiovanni J, Fischer SM, Hu Y. IKKalpha is required to maintain skin homeostasis and prevent skin cancer. Cancer Cell. 2008;14:212–225. doi: 10.1016/j.ccr.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedde T, Heinrichsdorff J, de Lorenzi R, De Vos R, Roskams T, Pasparakis M. IKK1 and IKK2 cooperate to maintain bile duct integrity in the liver. Proceedings of the National Academy of Sciences. 2008;105:9733–9738. doi: 10.1073/pnas.0800198105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Tan W, Ricono JM, Korchynskyi O, Zhang M, Gonias SL, Cheresh DA, Karin M. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature. 2007;446:690–694. doi: 10.1038/nature05656. [DOI] [PubMed] [Google Scholar]
- M’Boneko V, Merker HJ. Development and morphology of the periderm of mouse embryos (days 9–12 of gestation) Acta Anat (Basel) 1988;133:325–336. doi: 10.1159/000146662. [DOI] [PubMed] [Google Scholar]
- Macklis JD, Madison RD. Progressive incorporation of propidium iodide in cultured mouse neurons correlates with declining electrophysiological status: a fluorescence scale of membrane integrity. J Neurosci Methods. 1990;31:43–46. doi: 10.1016/0165-0270(90)90007-3. [DOI] [PubMed] [Google Scholar]
- Marinari B, Moretti F, Botti E, Giustizieri ML, Descargues P, Giunta A, Stolfi C, Ballaro C, Papoutsaki M, Alemà S, et al. The tumor suppressor activity of IKKα in stratified epithelia is exerted in part via the TGF-β antiproliferative pathway. Proceedings of the National Academy of Sciences. 2008;105:17091–17096. doi: 10.1073/pnas.0809288105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May MJ, Marienfeld RB, Ghosh S. Characterization of the Ikappa B-kinase NEMO binding domain. J Biol Chem. 2002;277:45992–46000. doi: 10.1074/jbc.M206494200. [DOI] [PubMed] [Google Scholar]
- McCollum CW, Amin SR, Pauerstein P, Lane ME. A zebrafish LMO4 ortholog limits the size of the forebrain and eyes through negative regulation of six3b and rx3. Dev Biol. 2007;309:373–385. doi: 10.1016/j.ydbio.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller-Bertoglio VE, Fisher S, Alej, Sánchez R, Mullins MC, Halpern ME. Differential Regulation ofchordinExpression Domains in Mutant Zebrafish. Developmental Biology. 1997;192:537–550. doi: 10.1006/dbio.1997.8788. [DOI] [PubMed] [Google Scholar]
- Park E, Zhu F, Liu B, Xia X, Shen J, Bustos T, Fischer SM, Hu Y. Reduction in IkappaB kinase alpha expression promotes the development of skin papillomas and carcinomas. Cancer Res. 2007;67:9158–9168. doi: 10.1158/0008-5472.CAN-07-0590. [DOI] [PubMed] [Google Scholar]
- Pei W, Noushmehr H, Costa J, Ouspenskaia MV, Elkahloun AG, Feldman B. An early requirement for maternal FoxH1 during zebrafish gastrulation. Dev Biol. 2007;310:10–22. doi: 10.1016/j.ydbio.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RJ, Dixon J, Malhotra S, Hardman MJ, Knowles L, Boot-Handford RP, Shore P, Whitmarsh A, Dixon MJ. Irf6 is a key determinant of the keratinocyte proliferation-differentiation switch. Nat Genet. 2006;38:1329–1334. doi: 10.1038/ng1894. [DOI] [PubMed] [Google Scholar]
- Sabel JL, d’Alençon C, O’Brien EK, Van Otterloo E, Lutz K, Cuykendall TN, Schutte BC, Houston DW, Cornell RA. Maternal Interferon Regulatory Factor 6 is required for the differentiation of primary superficial epithelia in Danio and Xenopus embryos. Dev Biol. 2009;325:249–262. doi: 10.1016/j.ydbio.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagerström CG, Grinbalt Y, Sive H. Anteroposterior patterning in the zebrafish, Danio rerio: an explant assay reveals inductive and suppressive cell interactions. Development. 1996;122:1873–1883. doi: 10.1242/dev.122.6.1873. [DOI] [PubMed] [Google Scholar]
- Sagerström CG, Gammill LS, Veale R, Sive H. Specification of the enveloping layer and lack of autoneuralization in zebrafish embryonic explants. Dev Dyn. 2005;232:85–97. doi: 10.1002/dvdy.20198. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Hammerschmidt M, Beuchle D, Cho KW, De Robertis EM, Nüsslein-Volhard C. Expression of zebrafish goosecoid and no tail gene products in wild-type and mutant no tail embryos. Development. 1994;120:843–52. doi: 10.1242/dev.120.4.843. [DOI] [PubMed] [Google Scholar]
- Senftleben U, Cao Y, Xiao G, Greten FR, Krähn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, et al. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- Siddiqui M, Sheikh H, Tran C, Bruce AEE. The tight junction component Claudin E is required for zebrafish epiboly. Dev Dyn. 2010;239:715–722. doi: 10.1002/dvdy.22172. [DOI] [PubMed] [Google Scholar]
- Sil AK, Maeda S, Sano Y, Roop DR, Karin M. IkappaB kinase-alpha acts in the epidermis to control skeletal and craniofacial morphogenesis. Nature. 2004;428:660–664. doi: 10.1038/nature02421. [DOI] [PubMed] [Google Scholar]
- Slanchev K, Carney TJ, Stemmler MP, Koschorz B, Amsterdam A, Schwarz H, Hammerschmidt M. The epithelial cell adhesion molecule EpCAM is required for epithelial morphogenesis and integrity during zebrafish epiboly and skin development. PLoS Genet. 2009;5:e1000563. doi: 10.1371/journal.pgen.1000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart IH. Variation in the plane of cell cleavage during the process of stratification in the mouse epidermis. Br J Dermatol. 1970;82:276–282. doi: 10.1111/j.1365-2133.1970.tb12437.x. [DOI] [PubMed] [Google Scholar]
- Solt LA, May MJ. The IkappaB kinase complex: master regulator of NF-kappaB signaling. Immunol Res. 2008;42:3–18. doi: 10.1007/s12026-008-8025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonawane M, Martin-Maischein H, Schwarz H, Nüsslein-Volhard C. Lgl2 and E-cadherin act antagonistically to regulate hemidesmosome formation during epidermal development in zebrafish. Development. 2009;136:1231–1240. doi: 10.1242/dev.032508. [DOI] [PubMed] [Google Scholar]
- Takeda K, Takeuchi O, Tsujimura T, Itami S, Adachi O, Kawai T, Sanjo H, Yoshikawa K, Terada N, Akira S. Limb and skin abnormalities in mice lacking IKKalpha. Science. 1999;284:313–316. doi: 10.1126/science.284.5412.313. [DOI] [PubMed] [Google Scholar]
- Thisse B, Pflumio S, Fürthauer M, Loppin B, Heyer V, Degrave A, Woehl R, Lux A, Steffan T, Charbonnier XC, et al. Expression of the zebrafish genome during embryogenesis (NIH R01 RR15402) ZFIN Direct Data Submission. 2001 ( http://zfin.org)
- Topczewski J, Solnica-Krezel L. Cytoskeletal dynamics of the zebrafish embryo. Methods Cell Biol. 1999;59:205–226. doi: 10.1016/s0091-679x(08)61827-0. [DOI] [PubMed] [Google Scholar]
- Wagner DS, Dosch R, Mintzer KA, Wiemelt AP, Mullins MC. Maternal control of development at the midblastula transition and beyond: mutants from the zebrafish II. Dev Cell. 2004;6:781–790. doi: 10.1016/j.devcel.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Webb AE, Driever W, Kimelman D. psoriasis regulates epidermal development in zebrafish. Dev Dyn. 2008;237:1153–1164. doi: 10.1002/dvdy.21509. [DOI] [PubMed] [Google Scholar]
- Weiss LW, Zelickson AS. Embryology of the epidermis: ultrastructural aspects. II. Period of differentiation in the mouse with mammalian comparisons. Acta Derm Venereol. 1975;55:321–329. [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book: A guide for the laboratory use of zebrafish (Danio rerio) 4. University of Oregon Press; Eugene: 2000. [Google Scholar]
- Zhu F, Xia X, Liu B, Shen J, Hu Y, Person M, Hu Y. IKKalpha shields 14-3-3sigma, a G(2)/M cell cycle checkpoint gene, from hypermethylation, preventing its silencing. Mol Cell. 2007;27:214–227. doi: 10.1016/j.molcel.2007.05.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Wild type embryo (left) undergoes normal epiboly movements. Age matched poky mutant embryos (right) fail to initiate epiboly, the blastoderm clears and then disintegrates. Images were captured with 5 minute intervals.
Wild type EVL (left) expressing a membrane localized GFP spread during epiboly. Age matched poky mutant EVL (right) show a high rate of cell division with multiple mitoses visible during this interval. Images were captured with 1 minute intervals.
DIC timelapse of poky mutant EVL. Cell indicated by < leaves the EVL and enters the DEL. Multinucleated cells were observed within the EVL (asterisk). Images were captured with 5 minute intervals.
Expression of mRNA encoding myc-NfkB2 (p100) was detected by anti-myc western blot analysis in wild type (lane 3), poky mutant (lane 4), wild type expressing IKK1 (lane 1) and wild type expressing a T-loop phosphomimetic substitution of Ikk1 (lane 2). In all conditions p100 and processed p52 were observed.