Abstract
Aim
To investigate the usefulness of some clinical and laboratory parameters in assessing the prognosis and survival of CLL in a resource-limited setting.
Methods
Between September 1986 and March 2007, 79 consecutive patients were retrospectively studied. Diagnosis was based on clinical and haematological findings.
Results
A total of 79 patients, aged 30 to 81 (median = 60) years were managed. There were 34 males and 45 females (ratio = 0.8:1). About 86.1% were aged above 50 years. Massive splenomegaly and hepatomegaly were recorded in 70.9% and 29.1% of patients, respectively. More than 63% presented in stage C. Anaemia was recorded in 74.7%. Haematocrit correlated negatively with WBC but positively with platelet count. The spleen correlated positively with liver. The overall survival at 2 years was 70.2%. Logistic regression showed that younger age, male sex, higher haematocrit, and lower platelet count improved survival, while lower WBC, moderate hepatomegaly and splenomegaly conferred survival advantage.
Conclusion
It could be concluded that massive splenomegaly is a common finding in the majority of our patients. Non availability of immunophenotyping facility is a major constraint.
Keywords: CLL, diagnostic problems, resource-limited setting
Introduction
Chronic lymphocytic leukaemia (CLL) is an indolent malignancy which is common in the Western World1 and characterised by gradual accumulation in the blood, bone marrow and lymphoid organs, of immunologically incompetent B lymphocytes. The clinical features in CLL at presentation are related to these accumulated leukaemic cells. The course of CLL is very variable, with some patients having a long survival and requiring only supportive treatment and, sometimes “dying with their disease”; others have a progressive disease and dying from the disease or its complications. In view of the variable nature of the disease, many studies have investigated factors identifying poor prognosis and those predicting long survival. The newer prognostic factors, such as genetic markers, mutational status of the immunoglobulin heavy chain variable region (IgV [H] genes and surrogate markers such as CD38 and zeta-associated protein (ZAP) -70 gene expression, lipoprotein lipase expression, telomerase length and telomerase activity2, cannot be determined in most Nigerian tertiary hospitals, hence the need to investigate the usefulness of some simple biologic and laboratory parameters in assessing the prognosis and survival of CLL patients in a resource-limited setting like ours.
Methods
Between September 1986 and March 2007, seventy-nine consecutively previously untreated patients with full clinical and laboratory data were retrospectively studied. Data extracted included demographic parameters, clinical features at presentation, haematological parameters, including bone marrow cytology, management instituted and outcome of such therapy. Results of viral screenings (HBsAg, HCV, and HIV) were also extracted. Diagnosis of chronic lymphocytic malignancy was made on the basis of a clinical examination (peripheral lymphadenopathy, splenomegaly and hepatomegaly), peripheral blood and bone marrow aspiration cytological findings (absolute lymphocytosis). No immunophenotypic characterisation was carried out on any of the patients. Patients with chronic lymphocytic leukaemia were staged at the time of diagnosis according to the International (Binet's) system. They were classified into CLL, CLL/PL and PLL based on the percentage of prolymphocytes (less than 10%, more than 10% but less 50%, and more than 50%, respectively) in the peripheral circulation3. All subjects were censored on the 31st of March, 2007. The length of management in months and status at the cut-off date was recorded.
Data analysis
Data are presented as means and standard deviations (means ± SD). Survival was computed at presentation based on age (< or > 50 years), sex (male or female), stage (stage A, B, C), spleen size (less or eual to 9cm or greater or equal to 10cm), liver size (less or eual to 9cm or greater or equal to 10cm), haematocrit (les or equal to 30 and greater or equal to 31), total leukocyte count (less or equal to 50,000/µL or greater or equal to 51,000), absolute lymphocytosis (less than or equal to 30,000/µL or greater or equal to 31,000/µL), platelet count (less or equal to 100,000/µL or greater or equal to 101,000/µL). Logistic regressions of survival by age, sex, stage, spleen and liver sizes, haematocrit, total leukocyte count, platelet count and absolute lymphocytosis at diagnosis was computed. Data were analysed using SPSS 11 (SPSS Inc, Chicago, USA, SPSS Inc 1989 – 2001) statistical software.
Results
Presentation
Between September 1986 and March 2007, a total of 79 patients with chronic lymphoid leukaemias, aged 30 to 81 years (median = 60) were managed. The majority (88.1%) of the cases were chronic lymphocytic leukaemia (CLL), 10.1% were CLL/PL and only 1.3% being hairy cell leukaemia (HCL). More than 50% of our patients had no peripheral lymphadenopathy, but massive splenomegaly and hepatomegaly were recorded in 79% and 29.1%, respectively. More than 86% of them were 50 years or above at the time of diagnosis with more than 63% of them in advanced stage C disease. More than 74% of them also presented with anaemia (PCV less than 30%) at diagnosis.
Survival
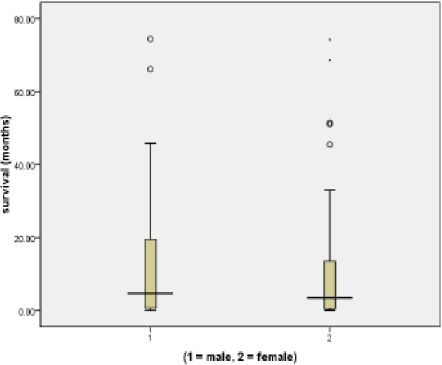
Out of which 34 were males and 45 were females (ratio = 0.8:1). More males (41.2%) survived beyond 6 months compared to 40% found in females (fig. 1). About 86.1% were aged 50 years or more.
Figure 1.
Differential suvival between sexes in Nigerians with CLL
More patients below 50 years (54.5%) survived longer than 6 months compared to those above 50 years (38.2%).
Massive (> 10cm below the costal margin) splenomegaly and hepatomegaly were recorded in 70.9% and 29.1% of patients, respectively. More patients (42.9%) with massive splenomegaly survived beyond 6 months compared to 34.8% found in those with mild to moderate splenomegaly.
Similarly, more survivors (43.5% compared to 39.3%) beyond 6 months were recorded in those with massive hepatomegaly
Minimal (one or no lymph node region) lymphadenopathy was found in 62% of patients. More patients (40.8%) with minimal lymphadenopathy survived beyond 6 months compared to 40% found in those with 2 or more lymph node regions. More than 63% of the patients presented in stage C.
However, 50% of those that presented in stage C survived for more than 6 months after diagnosis compared to 27.8% for stage B and 18.2% for stage A. More than 70% of patients had absolute lymphocytosis of greater than 30,000/µL at diagnosis. There was no difference in the length of survival between those with lymphocytosis below (40.5%) or above (40.5%) 30,000/µL at diagnosis. Symptomatic anaemia (PCV < 30%) were recorded in 74.7% of cases, with mean PCV (±SD) of 25.3 ± 13.9%. High haematocrit level improves survival.
The mean ± SD of WBC and platelet counts were 111851 ± 122795/µL and 110189 ± 74974µ/L, respectively. Leukocytosis appeared to favour longer survival as more patients (45.0%) with total WBC above 51,000/µL survived for more than 6 months compared to 36.8% found in those with total WBC of 50,000/µL and below. On the contrary, thrombocytosis appeared not to favour long survival as only 33.3% of those with platelet count above 90,000/µL survived beyond 6 months compared to 48.6% found in those with platelet count of less than 90,000/µL. The overall survival (OS) in our cohort of patients was 70.2% at 2 years.
Only 2 of the 18 patients that had HBsAg screening done were found positive, while only 1 of the 30 patient that had Coombs. test done was to be positive. None of the patients screened for HIV and HCV was positive.
There were no significant difference in mean survival based on differences in age, spleen or liver sizes, haematocrit level, WBC or platelet counts, and extent of lymphadenopathy. However, logistic regression of survival with these parameters showed that younger age, male sex, higher haematocrit, and lower platelet count improved survival, while lower WBC count, moderate hepatosplenomegaly and moderate lymphocytosis appeared not to confer better survival advantage in our cohort of patients. Correlation analysis revealed negative correlation between haematocrit and white cell count (r = −0.357, p = 0.001) and but a positive correlation between haematocrit and platelet count (r = 0.281, p = 0.012). A positive correlation was also noted between spleen and liver (r = 0.371, p = 0.001).
Thirteen (16.5%) were confirmed dead from causes such as liver failure, renal failure, congestive heart failure and intracranial bleeding. A significant proportion (45/79 or 56.9%) of our patients was lost to follow-up with only 21 (26.6%) still being followed-up as at the time of analysis.
Twenty-two did not receive chemotherapy due to a stable disease, 42 received chlorambucil or cyclophosphamide with or without prednisolone, thirteen with progressive disease had a combination of cyclophosphamide, vincristine and prednisolone, while 2 with acute lymphoblastic transformation received methylprednisolone. Ten (12.6%) had splenectomy due to symptomatic hypersplenism
Discussion
Chronic lymphocytic leukaemia (CLL) is an indolent disease with outcome relating to some factors at presentation. Assessment of some of these prognostic factors are not available or affordable in resource-limited environment, hence the need to assess the usefulness of some simple clinical and laboratory parameters that are available and affordable.
This study found patients younger than 50 years at presentation to survive longer than those older, though the difference was not statistically significant. This finding has been corroborated by other studies. Applying period analysis to age-specific long-term analysis of survival in patients with CLL, Brenner et al4 found patients younger than 70 years to survive longer than those older. In another study by Thurmes et al5, investigating the prevalence and prognostic implications of co morbidities in patients with newly diagnosed CLL, they found age and stage at presentation to predict prognosis in survival in newly diagnosed patients with CLL. Age also had significant prognostic effect on the survival of patients in another study conducted in Turkey6.
Some studies have shown superior survival for female patients with CLL7,8 and which was attributed to a major biological difference between the sexes9. The present study showed, on the contrary, a higher but insignificant survival advantage in males. The poor socio-economic status of many women in the sub-Saharan Africa which could have hampered them from seeking and receiving prompt and adequate treatment might have contributed. Longer survival was noted be positively associated with splenomegaly, hepatomegaly, high leukocyte count, and higher stage of the disease at presentation. This is an unusual finding when compared with results from other parts of the world8,10. This is because patients with splenomegaly and hepatomegaly are prone to more anaemia and thrombocytopenia and which are expected to worsen the prognosis in them. Although the majority of our patients presented with massive splenomegaly, the unavailability of other diagnostic modalities such as flow Cytometry or immunocytochemistry to rule out other lymphoid malignancies such as splenic marginal zone lymphoma, mantle cell lymphoma or follicular lymphoma, create diagnostic problem in our setting. However, the possibility of extramedullary haematopoiesis in these organs could possibly contribute to the observed survival advantage in them. However, this has not been described in CLL patients. It is equally intriguing that more patients in stage C at diagnosis survived better than those in stages B and A; which is contrary to finding from other parts of the world11,12 More of our patients presented with splenomegaly rather than peripheral lymph node enlargement; but with 60% of those with significant lymphadenopathy surviving for less than six months post diagnosis. Survival was also found to be better without lymphadenopathy in a study conducted by de Faria et al, and Montserrat and Rozman8,11 Lymphadenopathy being part of tumour load could probably explain the poorer prognosis in those with significant lymph node enlargement.
Similar to reports elsewhere, this study showed that high leukocytes count is associated with poor prognosis with 55% of our patients surviving for less than 6 months. Complications of hyperleukocytosis resulting from the high leukocyte count (and neutropenia) that is common in them, could probably account for high mortality when compared with those with moderate leukocytosis. Platelet count of > 100,000µL was found not to be favourable to prolonged survival in our cohort as more than 66.7% of them with high platelet count could not survive beyond 6 month after diagnosis. This contrary to the findings of de Faria et al8 who found longer survival time in patients with platelet count of more than 100,000µL and those of de Rossi et al13 who found thrombocytopenia to significantly influence survival. The reason for the disparity on the effects of platelet count on survival between this present study in mainly Black population and the mainly Caucasian population studies are not clear. However, in a study conducted by Koller et al14, they found high serum thrombopoietin levels to be associated with shorter survival. In this present study, serum thrombopoietin levels were not assessed in our patients.
Survival is better in patients with haematocrit of less than 30% as haematocrit of more than 30% does not appear to improve survival as only 35% of those with haematocrit of 30% and above survived for more than six months post diagnosis. Reason(s) for the relatively insignificant effect of anaemia on our patients' survival is not clear. Adaptation to low haematocrit values as in sickle cell anaemia patients could probably be contributory. Anaemia in chronic lymphocytic leukaemia could result from several factors including bone marrow infiltration, immune haemolysis, and hypersplenism and pooling of blood in the massive spleen. In this cohort of patients, severe bone marrow infiltration and massive splenomegaly could have been responsible for the anaemia, as 99% of patients had lymphocytic infiltration in the bone marrow of more than 70% (range 70 to 100%) while more than 70% of patients had massive splenomegaly. Immune cytopenias (anaemia or thrombocytopenia) is not common in CLL15,16, and has been shown in this study, as only one of 30 patients who had Coombs' test done was positive. Studies have, however, shown that immune cytopenias does not adversely affect prognosis in CLL patients16,17 as steroid is an effective therapy. The negative correlation found between haematocrit and leukocytosis could be an indirect evidence of bone marrow failure resulting from bone marrow infiltration by the malignant leukocytes. The positive correlation recorded between platelet count and haematocrit level could be cytokine related, as erythropoietin has effect on both erythroid and platelet cell lines18. On the other hand, the positive correlation noted between the spleen and the liver could reflect the infiltration of leukocytes in the two organs.
More than half of the patients were lost to follow-up as at the time of writing this report. This is a major problem in the management of malignancies in resource-limited environment19,20 as patients sometimes stop attending clinic due to inability to continue to fund their treatment and possibly died at home. All patients received only the older generation chemotherapeutic agents in the management of their CLL, such as cyclophosphamide or chlorambucil with or without prednisolone and vincristine. None could receive Fludarabine as this is neither avaialble nor affordable to most patients.
Conclusion
It could be concluded from this study that accurate diagnosis in a major problem in our setting as without some form of immunochemical analysis, diagnosis of lymphoid malignancies may be faulty. By and large, massive splenomegaly, younger age, male sex, moderate haematocrit level, moderate platelet count, and fewer lymph node regions are good prognostic factors in our environment. The study has also shown that unavailability of newer generation of chemotherapeutic agents and poverty could hinder effective management of patients in resource-limited environment and could significantly affect their survival which was 70.2% at only 2 years.
Table 1.
Mean ± SD Clinical and laboratory parameters at diagnosis
| Parameter | Mean ± SD |
| Age (yrs) | 59.00 ± 13.92 |
| PCV (%) | 25.00 ± 7.52 |
| WBC (/µL) | 111851.27 ± 122795.30 |
| ALC (/µL) | 103954.91 ± 116897.97 |
| BML (%) | 78.04 ± 16.99 |
| Spleen (cm) | 13.32 ± 8.14 |
| Liver (cm) | 6.38 ± 5.00 |
PCV = packed cell volume
WBC = white blood cell count
ALC = absolute lymphocyte count
BML = bone marrow lymphocyte count
cm = centimetre (below costal margin)
yrs = years
% = percentage, /µL = per microlitre
Table 2.
Survival advantage based on clinical and laboratory parameters
| Parameter | Percentage that survived beyond 6 months |
|
| Sex | Male = 41.2% | Female = 40.0% |
| Age | <50years = 54.5% | >50years = 38.2% |
| Stage | A = 18.2%, B = 27.8%, C = 50.0% | |
| Spleen size (cm) | <9cm = 34.8%, | >10cm = 42.9% |
| Liver size (cm) | <9cm = 39.3%, | >10cm = 43.5% |
| Lymph node regions | 1 = 40.8% | >2 = 40.0% |
| PCV (%)<20% = 41.7%, <30% = 42.9%, >30 =35% | ||
| WBC count (/µL) | <50000 = 36.8%, | >50000 = 45% |
| Platelet count (/µL) | <90000 = 48.6%, | >90000 = 33.3% |
PCV = packed cell volume (percentage)
WBC = white blood cell, (/µL) per microlitre
cm = centimetre (below the costal margin)
% = percentage
References
- 1.Cmunt E, Michalova K, Sindelarova L, Karban J, Zemanova Z, Kurkova S, et al. Importance of prognostic factors in patients with chronic B-lymphocytic leukaemia at the time of diagnosis . Sb Lek. 2002;103:359–370. [PubMed] [Google Scholar]
- 2.Gowda A, Byrd JC. Use of prognostic factors in risk stratification at diagnosis and time of treatment of patients with chronic lymphocytic leukaemia. Cur Opin Haematol. 2006;13:266–272. doi: 10.1097/01.moh.0000231425.46148.b0. [DOI] [PubMed] [Google Scholar]
- 3.Thomas JK. Chronic lymphocytic leukaemia and related disorders. In: Lichtman MA, Beutler E, Kipps TJ, Seligsohn U, Kaushansky K, Prchal JT, editors. Williams Haematology. 7th Edition. New York: McGraw-Hill; 2006. pp. 1343–1383. [Google Scholar]
- 4.Brenner H, Gondos A, Pulte D. Trends in longterm survival of patients with chronic lymphocytic leukaemia from the 1980s to the early 21st century. Blood. 2008;111:4916–4921. doi: 10.1182/blood-2007-12-129379. [DOI] [PubMed] [Google Scholar]
- 5.Thurmes P, Call T, Slager S, Zent C, Jenkins G, Schwager S, et al. Comorbid conditions and survival in unselected, newly diagnosed patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2008;49:49–56. doi: 10.1080/10428190701724785. [DOI] [PubMed] [Google Scholar]
- 6.Pamuk ON, Pamuk GE, Soysal T, Ongören S, Ba°lar Z, Ferhanođlu B, et al. Chronic lymphocytic leukemia in Turkey: experience of a single center in Istanbul. South Med J. 2004;97:240–245. doi: 10.1097/01.SMJ.0000053674.03385.B7. [DOI] [PubMed] [Google Scholar]
- 7.Molica S. Sex differences in incidence and outcome of chronic lymphocytic leukaemia patients. Leuk Lymphoma. 2006;47:1477–1480. doi: 10.1080/10428190600555819. [DOI] [PubMed] [Google Scholar]
- 8.de Faria JR, de Oliveira JSR, de Faria RMD, Silva MRR, Goihman S, Yamamoto M, et al. Prognosis related to staging systems for chronic lymphocytic leukaemia. Sao Paulo Med J. 2000;118:83–88. doi: 10.1590/S1516-31802000000400002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catovsky D, Fooks J, Richards S. Prognostic factors in chronic lymphocytic leukaemia: the importance of age, sex and response to treatment in survival. A report from the MRC CCL 1 trial - MRC Working Party on Leukaemia in Adults. Cr J Haematolo. 1989;72:141–149. doi: 10.1111/j.1365-2141.1989.tb07674.x. [DOI] [PubMed] [Google Scholar]
- 10.Sriphatphiriyakun T, Auewarakul CU. Clinical presentation and outcome of Thai patients with chronic lymphocytic leukaemia: retrospective analysis of 184 cases. Asian Pac J Allergy immunol. 2005;23:197–203. [PubMed] [Google Scholar]
- 11.Montserrat E, Rozman C. Chronic lymphocytic leukaemia: prognostic factors and natural history. Baillieres Clin Haematol. 1993;6:849–866. doi: 10.1016/s0950-3536(05)80179-9. [DOI] [PubMed] [Google Scholar]
- 12.Chen PM, Lin SH, Fan SF, Chiou TJ, Hsieh RK, Yu IT, et al. Genotypic characterisation and multivariate survival analysis of chronic lymphocytic leukaemia in Taiwan. Acta Haematol. 1997;97:196–204. doi: 10.1159/000203683. [DOI] [PubMed] [Google Scholar]
- 13.De Rossi G, Granati L, Girelli G, Gandolo G, Perrone P, Martelli M, et al. Prognostic value of autoantibodies against erythrocytes and platelets in chronic lymphocytic leukaemia. Jumori. 1991;77:100–104. doi: 10.1177/030089169107700202. [DOI] [PubMed] [Google Scholar]
- 14.Koller C, Bekele BN, Zhou X, Park C, Estrov Z, O'Brien S, et al. Plasma thrombopoietin compared with immunoglobulin heavy-chain mutation status as a predictor of survival in chronic lymphocytic leukaemia. Blood. 2006;108:1001–1006. doi: 10.1182/blood-2005-05-2110. [DOI] [PubMed] [Google Scholar]
- 15.Catovsky D. Chronic lymphocytic leukaemia and other B cell disorders. In: Hoffbrand AV, Catovsky D, Tuddenham TGD, editors. Postgraduate Haematology. 5th ed. London: Blackwell Publishing; 2005. pp. 619–643. [Google Scholar]
- 16.Mauro FR, Fao R, Cerretti R, Giannarelli D, Coluzzi S, Mandelli F, et al. Anaemia in chronic lymphocytic leukaemia: clinical, therapeutic, and prognostic features. Blood. 2000;95:2786–2792. [PubMed] [Google Scholar]
- 17.Zent CS, Ding W, Schwager SM, Reinalder MS, Hoyer JD, Jelinek DF. The prognostic significance of cytopenia in chronic lymphocytic leukaemia/small lymphocytic lymphoma. Br J Haematol. 2008;141:615–621. doi: 10.1111/j.1365-2141.2008.07086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byrne JL, Russell NH. Haemopoietic growth factors. In: Hoffbrand AV, Catovsky D, Tuddenham TGD, editors. Postgraduate Haematology. 5th ed. London: Blackwell Publishing; 2005. pp. 303–317. [Google Scholar]
- 19.Salawu L, Durosinmi MA. Myelomatosis: Clinical and laboratory features in Nigerians. West African Journal of Medicine. 2005;24:54–57. doi: 10.4314/wajm.v24i1.28164. [DOI] [PubMed] [Google Scholar]
- 20.Durosinmi MA, Adediran IA. Cancer management under structural adjustment programme (SAP): Experience in Ile-Ife; Nigeria. Nigerian Medical Journal. 1993;25:92–96. [Google Scholar]