Summary
Objectives
The laboratory is considered the cornerstone of tuberculosis (TB) control programme. International review of Ghana's programme in the late nineties identified the laboratory services as the weakest component. Sputum smear microscopy (SSM) being the main method of diagnosing pulmonary TB in Ghana, the training objectives were to: (i) strengthen the knowledge and skills of laboratory personnel on SSM (ii) impart necessary techniques in biosafety and (iii) introduce a Quality Assurance (QA) system in order to strengthen SSM services.
Methods
Personnel were selected for training during a nationwide situation analysis of SSM centres in 2000/2001. Four training sessions on SSM/QA were held between 2001/2004.
Results
A total of 80 personnel were trained: 10 regional TB coordinators and 70 laboratory personnel. The participants upon return to their respective regions also organized training within their districts. This approach resulted in another 100 district TB coordinators and 200 laboratory personnel being trained. Improvement in smear preparation, staining and reading ability of the participants were observed during the post-test and subsequent visit to their respective laboratories. The training has led to strengthening of TB laboratory services in the country and has contributed to increase in case detection from 10,745 in 2000 to 11,827 in 2004 and 14,022 in 2008. It was observed during the post-training follow-up and quarterly supervision visits that morale of the personnel was high.
Conclusion
Continuous training and re-training of laboratory personnel on SSM and QA at regular intervals do play an important role for effective and efficient TB control programme.
Keywords: tuberculosis, laboratory training, sputum smear microscopy, quality assurance, Ghana
Introduction
Tuberculosis (TB), an ancient disease that has caused more suffering and death than any other infectious disease continues to be a major public health problem worldwide. One-third of the world's population is currently infected with the TB bacilli with nearly 2 million deaths each year. Of those infected, over 1.5 million cases occur annually in sub-Saharan Africa.1
Over 46,000 new cases of TB annually are estimated by the World Health Organization (WHO) in Ghana.2 However, in reality, less than a third of the estimated number of cases is officially reported each year by health facilities in the country. For instance, in 2008, only 14,022 TB cases of all forms were reported, the highest cases ever recorded officially in the country.3
It is believed that many people suffering from the disease do not report to health facilities due to the stigma attached to the disease. Furthermore, some of those who report are misdiagnosed by laboratory personnel due to inaccurate sputum smear examinations. This may lead to a false negative or false positive result which has adverse implications in the community and on the patient.
A false negative result means that a TB patient would not be diagnosed and would continue to be a source of infection in the community whereas a false positive result means that a TB suspect who does not have TB would be put on unnecessary treatment with accompanying social stigma, drug wastage and side effects of the anti-TB drugs.
Therefore, to ensure and maintain a reliable and efficient laboratory service that will facilitate accurate, reliable and timely diagnosis of TB, effective training of laboratory personnel and TB coordinators is of paramount importance.
A study in 2000/2001 that evaluated public laboratories performing sputum smear microscopy (SSM) in Ghana highly recommended the training of laboratory personnel in SSM and the establishment of a quality assurance (QA) system to improve TB diagnostic services in the country. This became necessary as non-standardised smear preparations and staining techniques and poor documentation were observed at regional and peripheral laboratories, and regular supporting or monitoring visits to all levels of the laboratory were non-existent4. To impart appropriate knowledge to laboratory personnel and TB coordinators in SSM and QA, four series of training sessions were held between 2001 and 2004.
The main method of pulmonary TB diagnosis in Ghana is SSM as the control programme's priority is the identification and treatment of infectious pulmonary TB cases. The objectives of the training were therefore to strengthen the skills of laboratory personnel nationwide on SSM, impart necessary techniques in biosafety and introduce a QA system in order to strengthen TB microscopy services in the country. We report here the role played by laboratory training in the diagnosis of TB in Ghana.
Methods
The training programmes were organized between September 2001 and August 2004 at the National Tuberculosis Reference Laboratory (NTBRL) at the Noguchi Memorial Institute for Medical Research (NMIMR), University of Ghana, which has enough suitable lecture rooms and laboratories for both lectures and practical sessions. Relevant materials used by each participant are shown in (Table 1).
Table 1.
Materials used by each participant for smear preparation, staining and microscopy
| Materials | |
| 1. 50 glass slides | 9. 5ml of immersion oil |
| 2. 20 pieces of wooden applicators | 10. 200ml of xylene |
| 3. 1 jar with disinfectant | 11. 1 microscope |
| 4. 1 staining bridge | 12. 1 slide box for 100 slides |
| 5. 3–5ml of fresh sputum specimen | 13. 1 set of panel slides |
| 6. 200ml of carbolfuchsin | 14. Form sheets for recording |
| 7. 600ml of 20% sulphuric acid | 15. Training evaluation sheet |
| 8. 200ml of 0.3% methylene blue |
Five facilitators were used for each training session: two experienced bacteriologists, one public health physician and two medical laboratory technologists. National TB laboratory manual and training guidelines developed to suit local conditions from information gathered from the nationwide evaluation of public laboratories performing SSM were used for the training4.
Laboratory personnel on SSM bench at regional and district laboratories and regional TB coordinators were selected during the nationwide evaluation to participate in the training. Two of laboratory personnel were from a private laboratory. The regional TB coordinators made up of medical officers, public health nurses and disease control officers were invited to participate so that they could appreciate SSM and also create a forum where some of the problems encountered in TB control in their respective regions could be addressed.
The training courses were in the form of TOT as the trained personnel went back to their respective regions and acted as facilitators during their regional SSM training. Based on field observation during the evaluation study,4 a training curriculum was developed by the facilitators with more time allotted to hands-on practical session i.e. practicals 43%, lectures 27%, assessment 16%, workshop 8% and others such as video presentation, demonstrations and breaks 6%. A summary of topics covered are shown in (Table 2). Each training session lasted 5 days and 20 personnel participated per session.
Table 2.
Lectures and practical topics covered in the laboratory training
| Lectures | Practical |
|
|
Participants were assessed before (pre-test) and after (post-test) training in the form of written and practical tests. The same questions were given to participants in both cases so that the impact of the training could be assessed. Successful participants were awarded certificates signed by National TB Programme (NTP) Manager and the Director of the National Health Laboratory Services (NHLS). The initial lectures were on the bacteriology and epidemiology of TB with emphasis on global and national TB burden; the role of the microscopist in TB control and biosafety in a TB laboratory. The presentations were interactive which allowed the participants to gain knowledge through exchange of information.
In order to evaluate the proficiency of participants before the practical training, each participant was provided with 3 sputum specimen of different consistency: mucopurulent, mucoid and salivary collected 24 hours prior to the practical. All the samples were known smear negative as some of the participants were inexperienced in performing SSM5. The participants were allowed to do smear preparation and staining without instructions in order to identify their weaknesses if any. The prepared slides were evaluated based on the six parameters of smear preparation 6,7 (i) sputum quality - this was evaluated microscopically. The presence of more than 25 leukocytes per field at x100 magnification or presence of macrophages indicated good quality sputum; (ii) smear size - this was evaluated macroscopically. A smear size of approximately 1x2 and 2x3 cm was considered acceptable; (iii) smear evenness - this was evaluated by macroscopic observation. A smear of acceptable evenness should not be too thick or not too thin on the slide; (iv) smear thickness - this was evaluated by both microscopic and macroscopic observations. A smear is considered to be of acceptable thickness if the entire depth of the smear layer can be focused sharply in each field; (v) staining technique - this was evaluated by microscopic observation. A good staining technique ensures that the smear is neither under-stained nor over-stained; (vi) smear cleanness - this was evaluated by microscopic observation. A smear was considered clean if stained smear was free from stain deposits, dirt, debris and crystals produced by overheating during staining. The scores obtained by each participant were used as baseline value for comparison with post training results.7
Smear preparation and staining
Lectures focused on technical aspects of smear preparation and staining by the Ziehl-Neelsen method; 0.3% carbolfuchsin for 5 minutes, 20% sulphuric acid as a decolourizer for 5 minutes and 0.3% methylene blue as a counterstain for 1 minute. Emphasis was laid on the participants weaknesses identified during the initial practice. Participants were instructed on how to rectify and correct their weaknesses. Participants were made to repeat the practice of smear preparation and staining and evaluated as in the initial practice. Each participant prepared 10 stained smears. A video “Acid-Fast Direct Smear Microscopy”8 was used to introduce participants to the procedural steps for sputum collection, smearing, staining and microscopy.
Practice in microscopy
A panel of 10 stained slides were prepared and numbered: 5 negative slides, 2 scanty positive slides, 1 moderate positive slide (1+), and 2 heavy positive slides (2+ and 3+). Each participant was given a panel of 10 slides and a form to enter the results to the corresponding slide number. The procedure was repeated in three different sessions with the slide set changing among participants at each session. Results obtained by the participants were compared with standard results by the use of correlation table.7
Manipulation and maintenance of microscope was identified during the evaluation study to be weak spot of most laboratory personnel hence more time was allotted for this session. The various components of the microscope (eyepiece lens, observation tube, objective lens, mechanical stage, condenser, filter, lamp and coarse/fine focus adjustment knobs) and its importance, handling and manipulation were explained to the participants. The importance of using x10 magnification without immersion oil to focus the image before changing to x100 immersion objective in order to avoid slide breakage was demonstrated to the participants. The need to protect lenses from fungus by keeping the glass surface as clean as possible and free from dirt and fingerprints were also emphasized. Removal of dust attached to microscopes with a blower and proper storage and replacement of bulbs were also demonstrated.
A workshop was organized to address constraints in laboratory diagnosis of TB in the country and was attended by the NTP Manager and the Director, NHLS. The workshop was also to allow the participants to share their experiences and ideas.
Training evaluation
Each participant was provided with course evaluation sheet to record daily course performance. The results were kept for future reference to improve the training. Three to six months after the training, post-training follow-up visits were paid to the participants to assess the impact of the training.
Results
A total of 80 personnel were trained comprising 10 regional TB coordinators (3 medical officers, 6 disease control officers and 1 public health nurse) and 70 laboratory personnel (20 medical technologists, 25 laboratory technicians, 20 laboratory assistants and 5 orderlies).
Knowledge assessment
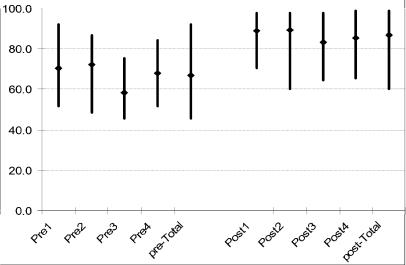
The participants were given the same questions to answer in both the initial and post assessments. There was an improvement in the performance of the participants in their post assessment as compared to initial assessment (Figure 1).
Figure 1.
Results of knowledge assessment
Skills assessment
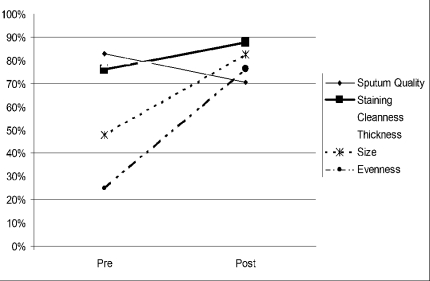
Improvements in smear preparation and staining as well as the reading ability of the participants were observed in the post-test. With the exception of the specimen quality, which decreased from pre-test average mark of 84% to 70%, all the other parameters of smear preparation: staining ability, smear cleanness, thickness, size, and evenness increased from 75%, 77%, 59%, 49%, and 23%, in the average pre-test marks to 88%, 80%, 80%, 82%, and 78% respectively in the post-test (Figure 2). The reading ability of the participants also improved as the overall agreement in positive and negative results with respect to the panel test increased from 60% to 99%.
Figure 2.
Assessment of smear preparation showing average pre and post marks
Training evaluation by participants
The course content as assessed by the participants based on parameters such as delivery of the lectures, relevance of the topic to their work and duration of the lectures indicated that the participants found all the topics relevant to their work and the delivery of the lectures was also rated as very good.
Post-training follow-up findings
A general improvement in SSM was observed in the microscopy centres of the trained personnel during the post-training follow-up visits. Documentation was rated as excellent as laboratory registers were filled out completely and accurately. In the area of biosafety, most laboratories had improved greatly. Laboratories had been arranged to maximize ventilation. The choice of disinfectant, use of laboratory coats, methods of disposal were commendable and there was a general change from the use of wire loop to wooden applicators in smear making.
Regional SSM training
By mid-2005, each of the 10 regions had run its own SSM training with those trained at the national level serving as facilitators. The trainings were supported by staff of the NTBRL in order to maintain its quality. This approach has resulted in another 100 district TB coordinators and 200 laboratory personnel being trained.
Discussion
The main aim of the NTP is to contribute to the improvement of the health status of the people of Ghana by reducing disability, morbidity and mortality caused by TB. The emphasis of the programme is on the early and accurate laboratory diagnosis and treatment of smear-positive pulmonary TB patients who are a great source of infection in the community. The laboratory is well known to be the cornerstone of the TB programme worldwide as it is involved in TB diagnosis, monitoring of patients and documenting cure at the end of treatment but much attention was not paid to the TB laboratory services since Ghana adopted the DOTS control strategy in 1994. International review of Ghana's programme in the late nineties identified the laboratory services as the weakest component of the TB control strategy4.
Before the training most microscopists in both the intermediate and peripheral-level laboratories felt neglected and isolated. The training was therefore a morale booster for the participants. Apart from the head of laboratories that were invited for the training, personnel who were actually performing SSM were also invited. This has helped a lot as enough trained bench personnel are currently working. The improvement in smear preparation, staining and microscopy as indicated by the post assessment results was an indication that learning took place during the training sessions. The decrease in sputum quality from 80% to 70% (Figure 2) confirmed the need to work on fresh specimen whenever possible as sputum specimen quality deteriorates with time.
The 380 laboratory personnel and coordinators trained from 2001 to 2005 have led to strengthening of the TB laboratory services in the public health sector and this has contributed to a large extent the steady increases in notified TB cases including smear positive pulmonary TB in the country (Table 3). The increases in smear negative pulmonary and extra-pulmonary TB cases may be due to HIV infection. However, unlike some African countries, the national median HIV prevalence has been relatively low over the years, the highest being 3.6% in 2003 and lowest 2.2% in 2008 (Table 3). The training has also led to the formulation of regional ined slides for blinded rechecking. There is now a TB QA teams that pays quarterly on-site evaluation prompt feedback system which allowed personnel at visit to peripheral-level laboratories and selects exam-the peripheral laboratories to improve in areas they were found to be lacking and also to follow-up recommendations formulated during the on-site evaluation visits. A functional TB laboratory network is now in place in the country with clearly defined roles at the central, intermediate and peripheral-level laboratories. The involvement of the TB coordinators who are able to interact with regional and district directors of health services has led to reduction in the rotation of QA assessors who are experienced in the management of the national QA system.
Table 3.
Officially reported cases of TB and median HIV prevalence in Ghana (1996–2008)
| Year | Pulmonary TB | Extrapulmonary | Total | Median HIV prevalence % |
||
| New smear positive |
Relapse smear positive |
New smear negative |
||||
| 1996 | 4809 | 340 | 1921 | 355 | 7425 | 2.4 |
| 1997 | 7254 | 356 | 2598 | 541 | 10749 | 2.3 |
| 1998 | 7757 | 383 | 2621 | 591 | 11352 | 3.4 |
| 1999 | 6877 | 401 | 2443 | 665 | 10386 | 2.2 |
| 2000 | 7316 | 502 | 2500 | 615 | 10933 | 2.3 |
| 2001 | 7712 | 575 | 2770 | 816 | 11873 | 2.9 |
| 2002 | 7732 | 504 | 2694 | 793 | 11723 | 3.4 |
| 2003 | 7714 | 558 | 2864 | 760 | 11896 | 3.6 |
| 2004 | 7259 | 542 | 3122 | 904 | 11827 | 3.1 |
| 2005 | 7584 | 540 | 3076 | 1020 | 12220 | 2.7 |
| 2006 | 7826 | 497 | 3139 | 1049 | 12511 | 3.2 |
| 2007 | 7649 | 463 | 3759 | 1092 | 12963 | 2.6 |
| 2008 | 7814 | 559 | 4323 | 1326 | 14022 | 2.2 |
The morale of the laboratory personnel is now high in the country as was observed during the post training follow-up and quarterly supervision visits. The perception that SSM is hazardous, cumbersome and “nasty” to perform is no more5. Laboratory personnel are now motivated to perform SSM. The certificates awarded at the end of the training are now a plus during promotion of laboratory personnel in their career progression by the Ghana Health Service.
In conclusion, the training has led to strengthening of TB laboratory services in the country and has contributed among other factors to increases in officially reported TB cases. Training and re-training of laboratory personnel in both public and private health sector on SSM and QA at regular intervals are therefore highly recommended to ensure continued high quality TB laboratory services.
Acknowledgement
We are most grateful to the Infectious Diseases Project of the Noguchi Memorial Institute for Medical Research, funded by the Japan International Cooperation Agency, the Department For International Development (DFID) (contract number AG 2071) as part of the West African TB Research Initiative and the Global Fund Against AIDS, Tuberculosis and Malaria for their support of the training. We are also thankful to the participants for their wonderful cooperation during the training sessions.
References
- 1.World Health Organization, author. Global Tuberculosis Control Report. 2005. [Google Scholar]
- 2.World Health Organization, author. Global Tuberculosis Control Report. 2006. [Google Scholar]
- 3.National Tuberculosis Programme Annual Report, author. Ghana Health Service/Ministry of Health. 2008. [Google Scholar]
- 4.Addo KK, Owusu-Darko K, Dan-Dzide M, Yeboah-Manu D, Ablordey A, Caulley P, Minamikawa M, Bonsu F, Lienhardt C, Akpedonu P, Ofori-Adjei D. Situation analysis of TB microscopy centres in Ghana. Int J Tubercle Lung Dis. 2006;8:870–875. [PubMed] [Google Scholar]
- 5.Ridderhof J, Angra P, Gilpin C, Aziz M, Van Deun A, Lumb R, Jost K, Laszlo A, Vincent V, Fujiki A, Urbanczik R, Ryszewska K, Madison B, Kim SA. Acid-Fast Direct Smear Microscopy Training Manual. World Health Organization/International Union Against Tuberculosis and Lung Disease/Centres for Disease Control and Prevention/United States Agency for International Development/Association of Public Health Laboratories/Research Institute of Tuberculosis. 2006. [Google Scholar]
- 6.Rieder HL, Chonde TM, Myking H. The Public Health Services National Tuberculosis Reference Laboratory and the National Laboratory Network, minimum requirements, role and operation in a low-income country. Paris, France: The International Union Against Tuberculosis and Lung Disease; 1998. [Google Scholar]
- 7.Fujiki A. AFB Microscopy Training. Tokyo, Japan: The Research Institute of Tuberculosis; 2005. [Google Scholar]
- 8.World Health Organization/International Union Against Tuberculosis and Lung Disease/Centres for Disease Control and Prevention/Pan American Health Organization/Instituto Nacional de Dignóstico y Referencia Epidemiológicos/ Association of Public Health Laboratories, author. The video “Acid-Fast Direct Smear Microscopy”. 2000. [Google Scholar]