Abstract
IRAK-M is a negative regulator of innate immunity signaling processes. Although attenuation of innate immunity may help to prevent excessive inflammation, it may also lead to compromised immune surveillance of tumor cells and contribute to tumor formation and growth. Here, we demonstrate that IRAK-M−/− mice are resistant to tumor growth upon inoculation with transplantable tumor cells. Immune cells from IRAK-M−/− mice are responsible for the anti-tumor effect, since adoptive transfer of splenocytes from IRAK-M−/− mice to wild type mice can transfer the tumor-resistant phenotype. Upon tumor cell challenge, there are elevated populations of CD4+ and CD8+ T cells and a decreased population of CD4+ CD25+Foxp3+ regulatory T cells in IRAK-M −/− splenocytes. Furthermore, we observe that IRAK-M deficiency leads to elevated proliferation and activation of T cells and B cells. Enhanced NFκB activation directly caused by IRAK-M deficiency may explain elevated activation of T and B cells. In addition, macrophages from IRAK-M−/− mice exhibit enhanced phagocytic function toward acetylated LDL and apoptotic thymocytes. Collectively, we demonstrate that IRAK-M is directly involved in the regulation of both innate and adaptive immune signaling processes, and deletion of IRAK-M enhances host anti-tumor immune response.
Keywords: Innate Immunity, cancer, IRAK-M, adaptive immunity, Signaling
1. Introduction
Increasing evidence suggests that tumor formation and growth benefit in part from suppression and/or alteration of host immune system (de Visser et al., 2006). The mammalian immune system is composed of two major arms: innate and adaptive immunity. Innate immunity is the first line of defense and senses abnormalities both outside and inside of host cells. Signals processed and released from innate immune cells can subsequently regulate T cell- and B cell-mediated adaptive immune responses. Compromising either innate or adaptive immunity may subject the host to chronic diseases such as tumor formation and progression. Indeed, extensive human clinical and animal tumor model studies indicate that tumor tissues as well as circulating immune cells within tumor-bearing hosts tend to have decreased CD8+ cytotoxic T lymphocytes, increased population of regulatory CD4+CD25+Foxp3+ T cells, and altered activities of macrophages and dendritic cells (Curiel et al., 2004; Ichihara et al., 2003; Klein et al., 2003; Mantovani et al., 2002; Vicari et al., 2002).
Toll-like-receptor (TLR) mediated signaling processes are critical for both innate and adaptive immune responses (Pasare and Medzhitov, 2005). Activation of TLR signaling pathways can lead to the production of cytokines and costimulatory molecules, which may further activate natural killer and tumor-reactive T cells, and enhance host anti-tumor response. Strategies using extra-cellular TLR agonists to boost innate immunity have been tested and shown in several cases to attenuate tumor growth (Akazawa et al., 2004; Zaks et al., 2006). However, the role of the TLR intracellular signaling network in tumor immune surveillance is poorly defined. Among the downstream signaling molecules, there is a family of interleukin-1 receptor associated kinases (IRAKs). IRAK-4, 2, and 1 are involved in positively regulating innate and adaptive immunity by facilitating the expression of various inflammatory mediators, and enhancing proliferation and activation of various immune cells (Day et al., 2004; Ohnuma et al., 2005; Rosati and Martin, 2002; Suzuki et al., 2003). In contrast, IRAK-M is a negative regulator counteracting these effects (Kobayashi et al., 2002; Nakayama et al., 2004). It is likely that IRAK-M may serve as a double-edged sword. The presence of IRAK-M helps to de-activate innate immunity signaling and prevent excessive inflammatory responses. On the other hand, cancer cells may exploit the inhibitory function of IRAK-M to evade host immune surveillance. Indeed, it has been reported that IRAK-M levels are elevated in tumor tissues and may compromise the human host immune defense and facilitate tumor progression (del Fresno et al., 2005; Deng et al., 2006).
In this study, we have therefore tested the hypothesis that IRAK-M deficient mice may have enhanced anti-tumor response. We compared the tumor growth in wild type and IRAK-M−/− mice following injection with transplantable murine tumor cells such as B16-F0 or TM-7. Furthermore, we analyzed the immune cell populations using flow cytometry in tumor cell-injected wild type and IRAK-M−/− mice. The contribution of IRAK-M to T cell proliferation and cytotoxicity, B cell expression of co-stimulatory molecules, and macrophage activation were subsequently examined. We conclude that IRAK-M deficiency leads to enhanced functions of immune cells including macrophages, T and B cells, which may collectively contribute to host resistance to tumor growth.
2. Materials and Methods
2.1. Mice and in vivo tumor model
IRAK-1−/− mice of C57BL6 background were a kind gift from Dr. James Thomas from the University of Texas Southwestern Medical School (Thomas et al., 1999). IRAK-M−/− mice of C57BL/6 background were obtained from Dr. Richard Flavell at Yale University (Kobayashi et al., 2002). IRAK-1−/− mice were screened by PCR using the forward primer 5′-gccttctatcgccttcttgacg-3′ and reverse primer 5′ - gcctctgtaagagatcaggtag - 3′. IRAK-M mice were screened by PCR using the forward primer 5′- cgttccataacacacctctctgc-3′ and reverse primer 5′-ttctatcgccttcttgacgagttc- 3′. Wild type C57BL/6 and BALB/c mice were purchased from The Charles River Laboratory. Mice were housed and bred at Derring Hall Animal Facility under pathogen-free conditions in accordance with established institutional guidance and approved protocols from Institutional Animal Care and Use Committees at Virginia Polytechnic Institute and State University (Blacksburg, VA). Mouse B16-F0 melanoma and TM-7 mouse fibrosarcoma cells were purchased from American Type Culture Collection and maintained in DMEM medium. For subcutaneous tumor challenge, 5×104 B16 or 2×106 TM-7 tumor cells were injected s.c. into the lower back of 12 week-old IRAK-1−/− , IRAK-M−/−, as well as age and sex matched wild-type C57BL/6 mice. Tumor growth was monitored every two days, and two perpendicular diameters of the tumor mass were recorded. Tumor volumes were calculated as Π/6×length×width2. After 21 days, mice were killed, and spleens as well as tumor specimens were harvested for subsequent analyses. For adoptive transfer experiments, two groups of 5 sex- and age-matched wild type C57BL/6 mice were injected via tail vain injection 1.5×107 splenocytes from either C57/BL6 or IRAK-M−/− mice. 24 hours later, each mouse was inoculated with 5 ×104 B16-F0 tumor cells s.c. as described above, and the tumor growth was monitored as described.
2.2. Flow cytometric analyses
Splenocytes were first depleted of red blood cells using ACK lysis buffer (Cambrex Bio Science, ,Walkersville, MD), and single cell suspensions were obtained by mechanic dispersion followed by filtration. Freshly excised tumor tissues were gently minced into small pieces, and incubated in 1mg/ml of collagenase D (Roche, Palo Alto, CA) solution for 30 min at 37 °C to obtain single cell suspensions. 1×106 freshly prepared cells were suspended in a mixture of 1 × PBS, 2% FCS and 0.02% (wt/vol) sodium azide with FcgIII/IIR-specific antibody to block nonspecific binding and were subsequently stained with different combinations of fluorochrome-coupled antibodies to F4/80 (eBioscience, San Diego, CA), CD4, CD8, CD19, CD11c, CD11b, Gr1, CD49b, CD25, CD40, CD80, CD86, MHC I-Ab (MHC class II) (BD Biosciences, San Jose, CA), as well as corresponding isotype control antibodies. In order to detect the T cell intra-cellular Foxp3, the cells were first stained with corresponding anti-CD4 and anti-CD25 antibodies, fixed with paraformaldehyde, and permeabilized with the cytoperm/Wash kit from BD Biosciences before labeling with anti-Foxp3 (eBioscience, San Diego, CA) antibody. The cells were then analyzed on a FACS Calibur flow cytometer using the CellQuest software (BD Biosciences, San Jose, CA).
2.3. In vitro splenocyte proliferation and activation assays
Single cell suspensions of whole splenocytes were prepared as described above. 1×106 splenocytes were suspended in 1 ml of PBS and supplemented with CFSE (Carboxylfluorescein diacetate succinimidyl ester), (Invitrogen, Carlsbad, CA) to achieve a final concentration of 3μM. The cells were incubated at 37 °C for 5 min and then washed twice with 10 ml of RPMI medium (GIBCO, Carlsbad, CA). The cells were resuspended at 1×106 cells/ml in complete RPMI culture medium supplemented with 10% FCS. CFSE-stained splenocytes were cultured in triplicate at 2 ×105 cells/well in 96-well round-bottom tissue culture plates in the presence of test mitogens, LPS, Tumor cells, or BSA as indicated(Gujar and Michalak, 2005). CFSE-labeled cells cultured with medium alone and cells not stained with CFSE but stimulated with test mitogens or antigens were used as controls. The plates were incubated at 37°C for 72h, and the cells from triplicate wells were pooled and stained with fluorescently conjugated anti-CD4 or anti-CD8 antibodies. Cells were analyzed in a FACSCalibur flow cytometer using CelQuest software (BD Biosciences, San Jose, CA). Proliferations of CD4+ T cells and B cells were evaluated by CFSE staining intensity gated with either CD4 positive or CD19 positive staining. For measuring the induction of co-stimulatory molecules on B cells, splenic B cells were purified through positive selection using Miltenyi magnetic beads. Purified B cells were stimulated with either 1 μg/ml BSA or 100 ng/ml LPS (Sigma-Aldrich, St. Louis, MO) for 16 h. Cell surface CD86 and I-Ab expressions were determined by staining with fluorescently conjugated anti-CD86 and I-Ab antibodies (BD Biosciences, San Jose, CA) and flow cytometry as described above.
2.4. In vivo cytotoxicity assay
Balb/c and C57BL/6 splenocytes were harvested, labeled with different concentrations of CFSE (CFSEhigh and CFSElow respectively), and used as target cells. Equal amount of target cells (1 × 107cells per mouse) were injected i.v. into wild type, IRAK-1−/−, and IRAK-M−/− mice. Recipient mice splenocytes were harvested 16 h after injection and were subjected to flow cytometry to measure the ratio of CFSEhigh : CFSElow cells. Cell lysis was calculated as follows: r = % CFSElow / % CFSEhigh and % lysis = [1 - (runprimed / rprimed)] × 100, where r is the ratio (Coles et al., 2002).
2.5. Cell culture, lyses and Western blot analyses
Cell lysates were prepared from indicated cells in buffer containing 150 mM NaCl, 20 mM HEPES (pH 7.0), 10% glycerol, 0.75% Triton X-100, protease and phosphatase inhibitors. Equal amounts of cell lysates were separated on 10% SDS-PAGE and proteins transferred onto a PVDF membrane. The membrane was incubated with primary antibodies against IκBα (Cell Signal, Beverly, MA ) or β-actin (Sigma-Aldrich, St. Louis, MO) at 4°C overnight, followed by incubation with horseradish peroxidase (HRP)-conjugated secondary Abs. Corresponding protein bands were visualized using enhanced chemiluminescence (Pharmacia, Piscata, NJ) and documented by Fuji 9000 digital imaging system.
2.6. Isolation of peritoneal macrophages, lipid uptake, and phagocytosis assay
Peritoneal macrophages were elicited from 8-week-old male C57BL/6 wild type or IRAK-M−/− mice by thioglycollate. Cells were resuspended in DMEM containing penicillin /streptomycin and 10% FBS and plated at a density of 1×106 cells/ml in either 96-well plates or 8-chamber glass slides (Lab-Teck, Naperville, IL). After overnight incubation at 37 °C, non-adherent cells were removed by gentle washing for three times with serum-free DMEM (Whitman et al., 2000). Adherent macrophages were then cultured in medium supplemented with 10% FBS for 24 h prior to phagocytosis assay. BODIPY FL-conjugated acetylated LDL was purchased from Molecular Probes. Apoptotic murine thymocytes were prepared by treating isolated thymocytes from 8-week-old C57BL/6 mice with 10−7 M dexamethasone in 10 ml RPMI medium at a concentration of 5×106 cell/ml for 16 h (Bianchini et al., 2006). Either acLDL (6 μg/ml) or apoptotic thymocytes (5:1) were added to macrophage cultures and co-incubated for 2 h. Un-bound free acLDL or thymocytes were removed by washing twice with ice-cold PBS, and the macrophages were subsequently fixed with paraformaldehyde (4% in PBS, pH 7.4) for 15 min on ice. The fluorescence intensities of the incorporated acLDL within macropahges were recorded and quantitated by a fluorescent plate reader (Molecular Devices, Downingtown, PA). The uptake of apoptotic thymocytes were visualized by microscopy and over 500 macrophages were counted to evaluate macrophage phagocytosis of apoptotic thymocytes.
2.7. Statistics
Statistical significance was determined using the unpaired 2-tailed Student’s t-test or 1-way ANOVA corrected for multiple comparisons where appropriate. P values less than 0.05 were considered statistically significant. All calculations were performed using the Prism 4.03 software program for Windows (GraphPad Software).
3. Results
3.1. IRAK-M deficient mice are resistant to tumor growth following inoculation with transplantable tumor cells
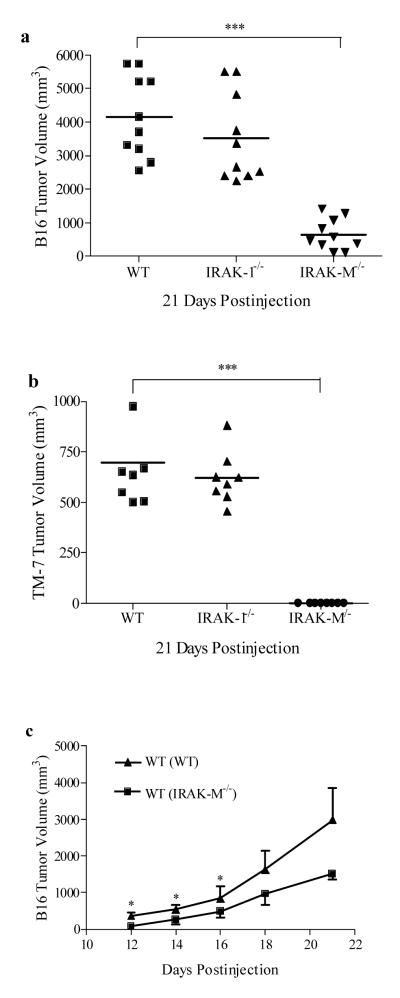
To determine whether IRAK-M deficiency renders enhanced anti-tumor response, we examined tumor formation and growth in wild type C57BL6 and IRAK-M−/− mice following injection of transplantable murine B16-F0 or TM-7 tumor cells. Subcutaneous injection of either B16-F0 or TM-7 tumor cells to wild type C57BL6 mice can lead to tumor formation. Both IRAK-M−/− and IRAK-1−/− mice are of C15BL6 background. First, we injected 5×104 B16 cells/per mouse s.c. into the lower back of the C57/BL6 wild type, IRAK-1−/−, and IRAK-M−/− mice (each group with 10 mice of 12 weeks old). As shown in Fig. 1a, wild type and IRAK-1−/− mice grew comparably large, palpable tumors 21 days after the tumor cell injection. In contrast, the tumor sizes of IRAK-M−/− mice were significantly (p=0.000094 and substantially smaller. The results using TM-7 cells were even more striking, in that IRAK-M−/− mice did not exhibit any visible tumor growth as compared to large tumors in wild type and IRAK-1−/− mice (Fig. 1b).
Fig. 1.
IRAK-M−/− mice exhibit enhanced anti-tumor response. (a) B16 tumor development was significantly inhibited in IRAK-M−/− mice 21 days post injection. Shown are means+s.e.m of the representative results of three independent experiments. n=10, *** p<0.0001. (b) TM-7 tumor growth was abrogated in IRAK-M−/− mice 21 days post injection. Shown are means+s.e.m of the results representative of the two independent experiments; n=8, *** p<0.0001. (c) B16 tumor growth was significantly attenuated in wild type mice upon adoptive transfer of IRAK-M−/− splenocytes. Tumor challenge was performed 1d after reconstitution. Shown are means+s.e.m of the representative results of two independent experiments; n=5 for each experiment, *p<0.05.
To directly test whether the immune cells from IRAK-M−/− mice may confer tumor resistance, we performed adoptive transfer experiments. Two groups of wild type C57BL6 mice were adoptively transferred with splenocytes from either C57BL6 or IRAK-M−/− mice via tail vein injection. Both groups were subsequently challenged with 5×104 B16 tumor cells. Tumor growth was monitored for a three week period. As shown in Fig. 1c, transfusion of IRAK-M−/−, but not wild type, splenocytes caused nearly a twofold decrease in tumor mass during the observation period.
3.2. IRAK-M−/− mice inoculated with tumor cells exhibit elevated CD4 and CD8 T cell population
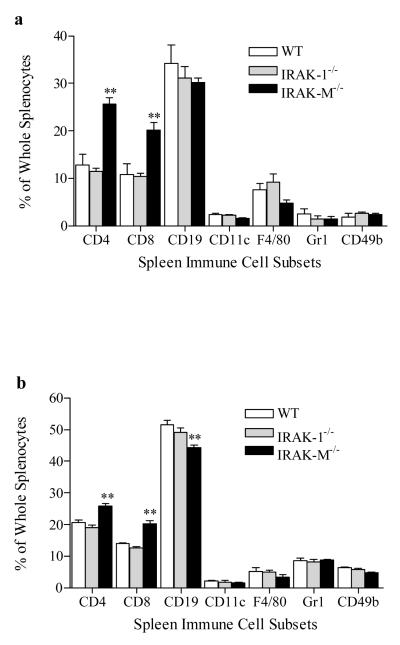
Since splenocytes from IRAK-M−/− mice attenuated tumor growth in wild type mice, we further examined the immune cell subsets in mice inoculated with tumor cells. Splenocytes from wild type, IRAK-1−/−, and IRAK-M−/− mice inoculated with either B16-F0 or TM-7 tumor cells for 21 days were harvested and stained with antibodies against various immune cell surface markers. As shown in Fig. 2a and 2b, there were significantly elevated populations of CD4+ (p=0.0065) and CD8+ (p=0.0028) T cells in IRAK-M−/− spleens compared with spleens from wild type and IRAK-1−/− mice. Since T regulatory cells are involved in suppressing anti-tumor immune surveillance, we further measured the levels of CD4+CD25+Foxp3+ regulatory cells in B16 tumor-bearing mice. As shown in the bottom panel of Fig. 2c, CD25+ cells within CD4+ T cell populations were comparable (~14%) between tumor-bearing wild type, IRAK-1−/− and IRAK-M−/− splenocytes. Intriguingly, compared with wild type or IRAK-1 mice, the percentages of Foxp3+ cells within CD4+CD25+ T cell populations in IRAK-M−/− splenocytes were significantly (p=0.0071) less. There was no appreciable difference in populations of dendritic cells, macrophages, neutrophils, and NK cells as measured by staining with CD11c, F4/80, CD11b/Gr1 and CD11b/CD49b. Naive mice without tumor cell injection exhibited no significant difference in immune cell types (data not shown). We further analyzed immune cells harvested from tumor tissues. Upon tumor tissue dissociation, leukocytic cells (CD45+) could be distinguished by FACS analysis from malignant cells by their size and morphology (Preynat-Seauve et al., 2006). Consistent with the finding from splenocytes, 21 days post injection of tumor cells, there were significantly higher percentages of CD4+ (p=0.0053) and CD8+ (p=0.0029) T cells in B16 tumor environment from IRAK-M−/− mice compared with wild type or IRAK-1−/− mice (Fig. 2d). The infiltrating levels of other immune cells were not significantly different among IRAK-1−/−, M−/− and wild type mice.
Fig. 2.
Increased T cell populations in IRAK-M−/− tumor-bearing mice. (a) Percentages of splenic immune cell subsets in B16 TM-7 (right) tumor-bearing mice as measured by flow cytometry. Results shown are mean+s.e.m; n=6, ** p<0.01. (b) Percentages of splenic immune cell subsets in TM-7 tumor-bearing mice as measured by flow cytometry. Results shown are mean+s.e.m; n=6, ** p<0.01. (c) Reduced population of CD4+CD25+Foxp3+ regulatory cells in splenocytes from B16 tumor-bearing IRAK-M−/− mice. The top panel represents triple positive cells, and the bottom panel represents CD4+CD25+ double positive cells. (d) Percentages of immune cell subsets in tumor tissues of B16 tumor-bearing mice. Data are means+s.e.m; n=6, ** p<0.01.
3.3. Enhanced CD4 T cell proliferation and CD8 cytotoxic function in IRAK-M−/− mice inoculated with tumor cells
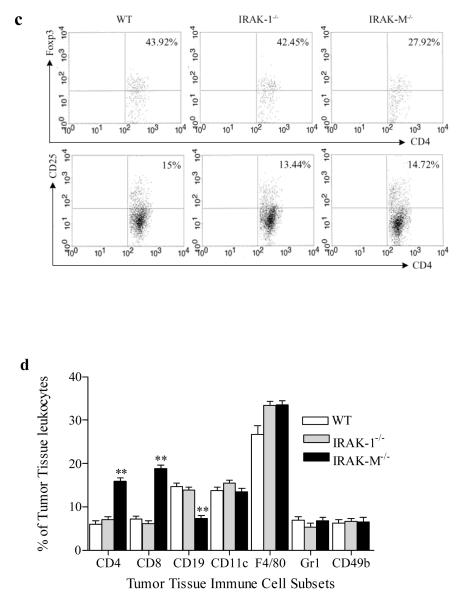
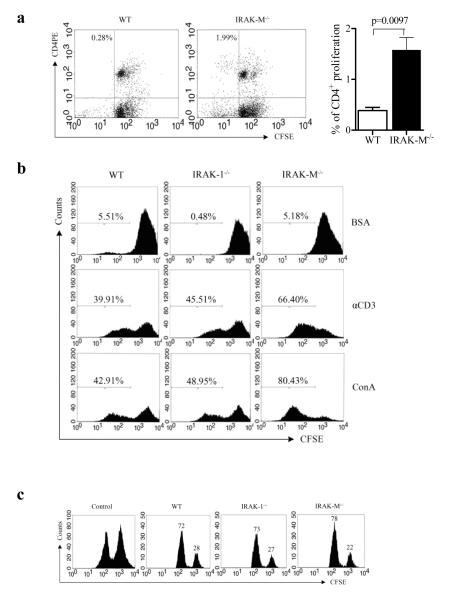
Given the fact that T cell populations were elevated in tumor-bearing IRAK-M−/− mice, we tested whether IRAK-M deficiency contributes to elevated functions of effector T cells. Splenocytes were harvested from wild type and IRAK-M−/− mice inoculated with B16 tumor cells for 21 days, labeled with CFSE, and stimulated with mitomycin-inactivated B16 cells in vitro for 72 hrs (Sfondrini et al., 2006). As shown in Fig. 3a, splenocytes from IRAK-M−/− tumor-bearing mice displayed significantly (p=0.036) increased CD4+ T cell proliferation, which is consistent with the elevated CD4+ T cell population observed in vivo. In addition, we observed that CD4+ T cells from naïve IRAK-M−/− mice exhibited higher in vitro proliferation upon ConA or anti-CD3 stimulation (Fig. 3b).
Fig. 3.
Activation of T cells and B cells in IRAK-M−/− mice. (a) T cells from IRAK-M−/− mice bearing B16 tumor were able to mount a stronger response against whole B16 tumor cell antigen than their wild-type counterparts, as assessed by in vitro T cell proliferation. Splenocytes harvested from B16 tumor-bearing mice were labeled with CFSE (3μM), and co-incubated with mitomycin-inactivated whole B16 tumor cells for 72 hours. Proliferation was assessed by flow cytometry. (b) Enhanced proliferation in splenocytes upon mitogen (ConA) and TCR stimulation from IRAK-M−/− mice. Splenocytes were stimulated with mitogen (ConA) or αCD3 Abs. T cell proliferation assays were the same as described above. (c) Enhanced in vivo cytotoxicity by IRAK-M−/− splenocytes. Splenocytes from Balb/c and C57BL/6 mice were labeled with different concentrations of CFSE ( CFSEhigh for Balb/c and CFSElow for C57BL/6). The 1:1 mixture of CFSEhigh(5μM) and CFSElow (0.5μM) splenocytes as target cells were injected into wild type and IRAK-M−/− mice. Tissue culture mixture of CFSEhigh and CFSElow splenocytes were used as control. The percentages of CFSEhigh and CFSElow in splenocytes were analyzed by flow cytometry and indicated in the figure.
Next, we addressed whether IRAK-M is involved in the function of cytotoxic T cells (CTLs). In vivo cytolytic activity was determined using Balb/c and C57BL/6 splenocytes as target cells, which had been differentially labeled with different concentrations of CFSE (CFSEhigh and CFSElow). These target cells were injected i.v.(1 × 107cells) into C57BL6 wild type, IRAK-1−/− and IRAK-M−/− mice. After 15h, spleens were removed and the ratios of CFSEhigh : CFSElow cells were determined by flow cytometry. As expected, enhanced lyses of target cells were found in IRAK-M−/− mice compared with wild type and IRAK-1−/− mice (Fig. 3c).
3.4. Elevated expression of co-stimulatory molecules on B cells from IRAK-M−/− mice
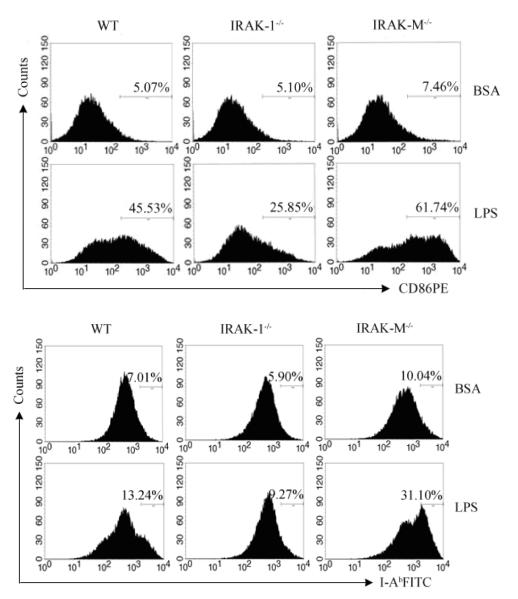
Cross-talk among B cells and T cells is necessary for T cell function, and elevated expression of co-stimulatory molecules on B cells may contribute to enhanced T cell proliferation and activation. We therefore studied the expression of CD86 and MHC molecule I-Ab expression on splenic B cells. As shown in Fig. 4, compared with wild type and IRAK-1−/− B cells, B cells from IRAK-M−/− mice exhibited elevated CD86 and MHC molecule I-Ab expression upon in vitro LPS challenge.
Fig. 4.
Enhanced expression of costimulatory molecules in splenic B cells from IRAK-M−/− mice. Splenic B cells from wild type and IRAK-M−/− mice were left treated (BSA) or stimulated with LPS for 18 h and stained for the surface expression of activation markers CD86 (top) and MHCII (bottom). The numbers represent the percentages of positively stained cell populations. Shown are means+s.e.m of the representative results of three independent experiments.
3.5. IRAK-M deficiency leads to increased NFκB pathway activation in both T and B cells
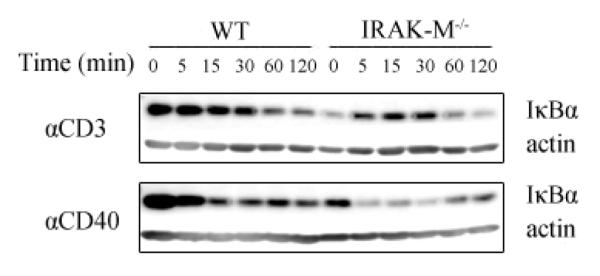
To understand the contribution of IRAK-M in regulating T and B cell function at the biochemical level, we examined the NFκB pathway activation status. Degradation of IκBα is the hallmark reflecting activated NFκB signaling pathway. As shown in Fig. 5, upon either anti-CD3 or anti-CD40 challenge, IκBα degradation was much more dramatic in isolated T and B cells harvested from IRAK-M−/− mice compared with wild type mice, indicating that IRAK-M is directly involved in mediating CD3 and CD40 downstream signaling.
Fig. 5.
Pronounced IκBα degradation in splenic T or B cells from IRAK-M−/− mice. Wild type or IRAK-M−/− splenic B cells (top) or T cell (bottom) were stimulated with αCD40 or αCD3 Abs respectively. Whole cell lysates were analyzed by SDS-PAGE and blotted with anti-IκBα and anti-actin Abs. Results are mean+s.e.m of the represent of three independent experiments.
3.6. Enhanced phagocytic function of macrophages from IRAK-M−/− mice
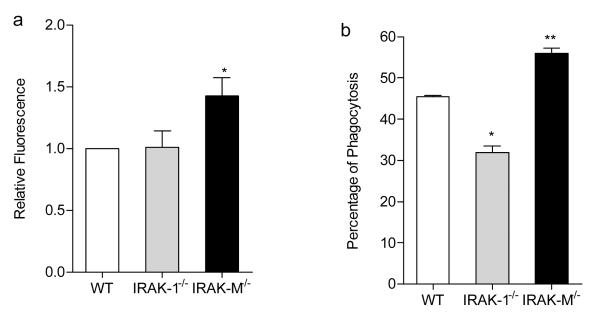
Although there was no significant difference in macrophage numbers among wild type and IRAK-M−/− tumor-bearing mice, there could be a functional difference. We therefore compared the phagocytic function of peritoneal macrophages harvested from different mice strains. As shown in Fig. 6, IRAK-M−/− macrophages exhibited enhanced uptake of acetylated LDL (Fig. 6a; p=0.023) as well as apoptotic thymocytes (Fig. 6b; p=0067) compared with wild type or IRAK-1−/− macrophages.
Fig. 6.
IRAK-M−/− macrophages exhibit enhanced uptake toward acLDL as well as apoptotic thymocytes. (a) IRAK-M−/− macrophages have increased capacity uptaking acLDL. Fluorescent intensities of acLDL associated with various macrophages were quantitated and plotted. * p<0.05 (b) Percentages of macrophages involved in engulfing apoptotic thymocytes were evaluated and plotted. The data is means+s.e.m of the represent of four independent experiments. ** p<0.01
4. Discussion
In this study, we have demonstrated that IRAK-M deficiency leads to enhanced host protection against tumor growth. Elevated immune cell functions, including increased T cell proliferation and activation, decreased regulatory T cell population, increased expression of co-stimulatory markers on B cells, and enhanced phagocytosis of macrophages, may collectively contribute to anti-tumor immunity observed in IRAK-M−/− mice.
It is likely that IRAK-M deficiency may lead to increased expression of cytokines and other cellular mediators, as well as co-stimulatory molecules, which in turn contribute to enhanced proliferation and function of effector T cells. Indeed, several studies show that IRAK-M−/− macrophages have elevated expression of IL-12, IL-6, CD40, CD80, and CD86 following stimulation with TLR ligands or microbes (Deng et al., 2006; Kobayashi et al., 2002). Our current report indicates that IRAK-M−/− B cells have increased CD86 expression following LPS challenge. Our unpublished data concur with previous findings and also reveal increased GM-CSF expression in IRAK-M−/− macrophages following either LPS or Pam3CSK4 challenge. Extensive studies have demonstrated that IL-12 contributes to tumor regression by promoting Th1 responses, increasing CD8+ T cell cytotoxicity, and inhibiting angiogenesis (Cavallo et al., 1999; Trinchieri, 2003). IL-6, on the other hand, was shown to be critical for effector T cell proliferation and activation by suppressing the inhibitory function of regulatory T cells (Medzhitov and Janeway, 2002). GM-CSF augments the generation and recruitment of dendritic cells, macrophages, granulocytes, and NKT cells (Dranoff, 2004) Collectively, the elevated expression of cytokines and costimulatory molecules due to IRAK-M deficiency may contribute to enhanced anti-tumor immune responses observed in this report.
Furthermore, our results indicate that IRAK-M is directly involved in mediating both innate and adaptive immune signaling. IRAK-M has been shown to modulate TLR-mediated innate immunity signaling (Harada et al., 2006; Kobayashi et al., 2002) and regulate macrophage and osteoclast function (Kuzmin et al., 2005; Pathak et al., 2005). Our data agree with these published studies and provide additional information indicating that IRAK-M plays a key role in regulating macrophage phagocytosis. Strikingly, the role of IRAK-M in directly regulating adaptive immunity signaling process is evident from our data showing enhanced IκB degradation in IRAK-M−/− T and B cells following either anti-CD3 or anti-CD40 stimulation. This indicates that IRAK-M may directly relay signals from either TCR or CD40. This is not surprising, considering that its close homologue IRAK-4 has recently been shown to directly participate in TCR-mediated adaptive immunity signaling (Suzuki et al., 2006). Collectively, these studies demonstrate that IRAK-M molecule plays a critical role in modulating the close cross-talk between innate and adaptive immunity signaling processes. However, further studies are needed to define the molecular mechanism explaining the role and regulation of IRAK-M in these processes.
It is interesting to note that IRAK-M may pose a double-edged sword to the host. Although IRAK-M deficiency can clearly enhance host anti-tumor immunity, it can lead to other diseases such as bone loss and osteoporosis due to elevated macrophage and osteoclast function (Li et al., 2005). A similar paradigm has been noticed with other genes related to tumor surveillance. For example, a p53 mutant mouse strain exhibiting augmented p53 function was developed and shown to have enhanced resistance to tumor growth (Tyner et al., 2002). Intriguingly, this mutant mouse strain also displays severe osteoporosis and organ atrophy. Further biochemical analyses are needed to better understand the molecular mechanism for IRAK-M activation and regulation. Strategies that can temporarily inactivate IRAK-M may hold promise for treating cancer and avoiding side effects such as bone loss.
Acknowledgement
We are grateful to the technical help of Andrew Becker in our laboratory. This work was supported by grants from US NIH to L.L.
Footnotes
Disclosures The authors have no financial conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akazawa T, Masuda H, Saeki Y, Matsumoto M, Takeda K, Tsujimura K, Kuzushima K, Takahashi T, Azuma I, Akira S, Toyoshima K, Seya T. Adjuvant-mediated tumor regression and tumor-specific cytotoxic response are impaired in MyD88-deficient mice. Cancer Res. 2004;64:757–764. doi: 10.1158/0008-5472.can-03-1518. [DOI] [PubMed] [Google Scholar]
- Bianchini R, Nocentini G, Krausz LT, Fettucciari K, Coaccioli S, Ronchetti S, Riccardi C. Modulation of pro- and anti-apoptotic molecules in double positive (CD4+CD8+) thymocytes following dexamethasone treatment. J. Pharmacol. Exp.Ther. 2006;319:887–897. doi: 10.1124/jpet.106.108480. [DOI] [PubMed] [Google Scholar]
- Cavallo F, Di Carlo E, Butera M, Verrua R, Colombo MP, Musiani P, Forni G. Immune events associated with the cure of established tumors and spontaneous metastases by local and systemic interleukin 12. Cancer Res. 1999;59:414–421. [PubMed] [Google Scholar]
- Coles RM, Mueller SN, Heath WR, Carbone FR, Brooks AG. Progression of armed CTL from draining lymph node to spleen shortly after localized infection with herpes simplex virus 1. J. Immunol. 2002;168:834–838. doi: 10.4049/jimmunol.168.2.834. [DOI] [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- Day N, Tangsinmankong N, Ochs H, Rucker R, Picard C, Casanova JL, Haraguchi S, Good R. Interleukin receptor-associated kinase (IRAK-4) deficiency associated with bacterial infections and failure to sustain antibody responses. J. Pediatr. 2004;144:524–526. doi: 10.1016/j.jpeds.2003.11.025. [DOI] [PubMed] [Google Scholar]
- de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- del Fresno C, Otero K, Gomez-Garcia L, Gonzalez-Leon MC, Soler-Ranger L, Fuentes-Prior P, Escoll P, Baos R, Caveda L, Garcia F, Arnalich F, Lopez-Collazo E. Tumor cells deactivate human monocytes by up-regulating IL-1 receptor associated kinase-M expression via CD44 and TLR4. J. Immunol. 2005;174:3032–3040. doi: 10.4049/jimmunol.174.5.3032. [DOI] [PubMed] [Google Scholar]
- Deng JC, Cheng G, Newstead MW, Zeng X, Kobayashi K, Flavell RA, Standiford TJ. Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J. Clin. Invest. 2006;116:2532–2542. doi: 10.1172/JCI28054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat. Rev. Cancer. 2004;4:11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- Gujar SA, Michalak TI. Flow cytometric quantification of T cell proliferation and division kinetics in woodchuck model of hepatitis B. Immunol. Invest. 2005;34:215–236. [PubMed] [Google Scholar]
- Harada K, Isse K, Sato Y, Ozaki S, Nakanuma Y. Endotoxin tolerance in human intrahepatic biliary epithelial cells is induced by upregulation of IRAK-M. Liver Int. 2006;26:935–942. doi: 10.1111/j.1478-3231.2006.01325.x. [DOI] [PubMed] [Google Scholar]
- Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin. Cancer Res. 2003;9:4404–4408. [PubMed] [Google Scholar]
- Klein L, Trautman L, Psarras S, Schnell S, Siermann A, Liblau R, von Boehmer H, Khazaie K. Visualizing the course of antigen-specific CD8 and CD4 T cell responses to a growing tumor. Eur. J. Immunol. 2003;33:806–814. doi: 10.1002/eji.200323800. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Hernandez LD, Galan JE, Janeway CA, Jr., Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- Kuzmin I, Geil L, Gibson L, Cavinato T, Loukinov D, Lobanenkov V, Lerman MI. Transcriptional regulator CTCF controls human interleukin 1 receptor-associated kinase 2 promoter. J. Mol. Biol. 2005;346:411–422. doi: 10.1016/j.jmb.2004.11.066. [DOI] [PubMed] [Google Scholar]
- Li H, Cuartas E, Cui W, Choi Y, Crawford TD, Ke HZ, Kobayashi KS, Flavell RA, Vignery A. IL-1 receptor-associated kinase M is a central regulator of osteoclast differentiation and activation. J. Exp. Med. 2005;201:1169–1177. doi: 10.1084/jem.20041444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway CA., Jr. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Okugawa S, Yanagimoto S, Kitazawa T, Tsukada K, Kawada M, Kimura S, Hirai K, Takagaki Y, Ota Y. Involvement of IRAK-M in peptidoglycan-induced tolerance in macrophages. J. Biol. Chem. 2004;279:6629–6634. doi: 10.1074/jbc.M308620200. [DOI] [PubMed] [Google Scholar]
- Ohnuma K, Yamochi T, Uchiyama M, Nishibashi K, Iwata S, Hosono O, Kawasaki H, Tanaka H, Dang NH, Morimoto C. CD26 mediates dissociation of Tollip and IRAK-1 from caveolin-1 and induces upregulation of CD86 on antigen-presenting cells. Mol. Cell Biol. 2005;25:7743–7757. doi: 10.1128/MCB.25.17.7743-7757.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Adv. Exp. Med. Biol. 2005;560:11–18. doi: 10.1007/0-387-24180-9_2. [DOI] [PubMed] [Google Scholar]
- Pathak SK, Basu S, Bhattacharyya A, Pathak S, Kundu M, Basu J. Mycobacterium tuberculosis lipoarabinomannan-mediated IRAK-M induction negatively regulates Toll-like receptor-dependent interleukin-12 p40 production in macrophages. J. Biol. Chem. 2005;280:42794–42800. doi: 10.1074/jbc.M506471200. [DOI] [PubMed] [Google Scholar]
- Preynat-Seauve O, Schuler P, Contassot E, Beermann F, Huard B, French LE. Tumor-infiltrating dendritic cells are potent antigen-presenting cells able to activate T cells and mediate tumor rejection. J. Immunol. 2006;176:61–67. doi: 10.4049/jimmunol.176.1.61. [DOI] [PubMed] [Google Scholar]
- Rosati O, Martin MU. Identification and characterization of murine IRAK-2. Biochem. Biophys. Res. Commun. 2002;297:52–58. doi: 10.1016/s0006-291x(02)02130-7. [DOI] [PubMed] [Google Scholar]
- Sfondrini L, Rossini A, Besusso D, Merlo A, Tagliabue E, Menard S, Balsari A. Antitumor activity of the TLR-5 ligand flagellin in mouse models of cancer. J. Immunol. 2006;176:6624–6630. doi: 10.4049/jimmunol.176.11.6624. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Suzuki S, Eriksson U, Hara H, Mirtosis C, Chen NJ, Wada T, Bouchard D, Hwang I, Takeda K, Fujita T, Der S, Penninger JM, Akira S, Saito T, Yeh WC. IL-1R-associated kinase 4 is required for lipopolysaccharide-induced activation of APC. J. Immunol. 2003;171:6065–6071. doi: 10.4049/jimmunol.171.11.6065. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Suzuki S, Millar DG, Unno M, Hara H, Calzascia T, Yamasaki S, Yokosuka T, Chen NJ, Elford AR, Suzuki J, Takeuchi A, Mirtsos C, Bouchard D, Ohashi PS, Yeh WC, Saito T. A critical role for the innate immune signaling molecule IRAK-4 in T cell activation. Science. 2006;311:1927–1932. doi: 10.1126/science.1124256. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Allen JL, Tsen M, Dubnicoff T, Danao J, Liao XC, Cao Z, Wasserman SA. Impaired cytokine signaling in mice lacking the IL-1 receptor-associated kinase. J. Immunol. 1999;163:978–984. [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, Lu X, Soron G, Cooper B, Brayton C, Park S. Hee, Thompson T, Karsenty G, Bradley A, Donehower LA. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- Vicari AP, Caux C, Trinchieri G. Tumor escape from immune surveillance through dendritic cell inactivation. Semin. Cancer Biol. 2002;12:33–42. doi: 10.1006/scbi.2001.0400. [DOI] [PubMed] [Google Scholar]
- Whitman SC, Daugherty A, Post SR. Regulation of acetylated low density lipoprotein uptake in macrophages by pertussis toxin-sensitive G proteins. J. Lipid Res. 2000;41:807–813. [PubMed] [Google Scholar]
- Zaks K, Jordan M, Guth A, Sellins K, Kedl R, Izzo A, Bosio C, Dow S. Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J. Immunol. 2006;176:7335–7345. doi: 10.4049/jimmunol.176.12.7335. [DOI] [PubMed] [Google Scholar]