Abstract
The incidence of gastric cancer is very high in Japan, Korea, and China. Reducing the morbidity and mortality associated with gastric cancer requires early diagnosis, which can be facilitated by applying gastroscopy more frequently in high-risk groups. A strategy of population screening for gastric cancer is currently being adopted in Korea, Japan, and the Matsu region of Taiwan, but using different screening methods. In addition, the history of pepsinogen (PG) in research as a gastric cancer biomarker has varied, in that the use of serum levels of PGI and PGII and the PGI/PGII ratio as gastric cancer screening tools was introduced in Japan before 1990, but in Korea the first research results were only reported in 2008. This review first evaluates the physiology of PG, followed by the usefulness or limitations of serum PG testing with regard to the detection of gastric cancer. Finally, the factors affecting the efficacy of PG tests as a gastric cancer biomarker (i.e., Helicobacter pylori infection status, gender, histopathologic features, and cancer location and depth) are evaluated. It was found that the strategies used to increase the efficacy of PG tests should be individualized in each country according to the seroprevalence of H. pylori.
Keywords: Pepsinogen, Gastric cancer, Atrophic gastritis, Helicobacter pylori
INTRODUCTION
Gastric cancer is the second leading cause of cancer-related death in the world.1 However, the risk of gastric cancer varies among the countries and populations in the world, and also in the Asian region. High risk areas include Korea, Japan and China, where the age standardized incidence rate (ASR) is higher than 20 per 100,000. Specifically, in Korea age standardized incidence rate of gastric cancer during 1990-2001 determined by the Cancer Registry at the Korean National Cancer Center in 2002, was 65.6 per 100,000 for men and 25.8 for women.2 In case of Japan the age-adjusted mortality has decreased from 69.9 to 34.5 per 100 000 in men and from 34.1 to 13.2 per 100,000 in women between 1980 and 2003.3 Intermediate risk countries (ASR 11-19/100,000) include Malaysia, Singapore and Taiwan, while low risk areas (ASR <10/100,000) include Australia, New Zealand, India and Thailand.1 The geographic variability in gastric cancer appears to be explained by a synergistic interaction between Helicobacter pylori (H. pylori) infection and environmental factors such as diet (salt, nitrates and low intake of fresh fruits and vegetables)4-9 or smoking.10 When diagnosed at an early stage, 5-year survival rates for gastric cancer exceed 90%.11,12 In addition, early gastric cancer, depending on the depth of mucosal infiltration and degree of differentiation, may also be suitable for endoscopic mucosal resection or endoscopic submucosal dissection, with lower morbidity, but similar efficacy, when compared to surgery.13 On the other hand, when diagnosed at an advanced stage, 5-year survival rates are below 50%.14 Thus, it is necessary to diagnose gastric cancer at an early stage to reduce the morbidity and mortality from gastric cancer. A strategy of population screening for gastric cancer has been adopted in Korea,15 Japan,16 and in Taiwan,17 which is different. That is, in Korea a health check-up program (upper endoscopy or upper gastrography) designed to detect gastric cancer has been implemented biannually for those over 40 years by the Korean government since 2001,15 but there has been criticism regarding the cost/benefit of this strategy. In Japan gastric cancer screening using photofluorography has been recommended for population-based and opportunistic screening.16 In case of Matzu, an offshore island between Taiwan and mainland China endoscopy was performed for participants who had shown positive test results from anti-H. pylori (immunoglobulin IgG) and pepsinogen I and II (PG I/II) or reporting a family history of gastric cancer or a personal history of peptic ulcer or other gastrointestinal disease from questionnaire.17 As the approach in Matzu if serum pepsinogen test could work as serum markers or an indicators which can identify those at high risk selective monitoring, then the costs of gastric cancer screening would be decreased.
There is a precancerous cascade, in which the gastric mucosa undergoes a series of changes resulting in gastritis, atrophy, intestinal metaplasia, and dysplasia, before developing into gastric cancer.4 H. pylori colonizes the gastric mucosa and triggers a series of inflammatory reactions, and considered as important cause of atrophic gastritis (AG).18 AG caused by H. pylori usually begins at the gastric antrum and extends proximally towards the cardia,19 resulting in decrease of gastric secretory function as the area of fundic gland mucosa also gets smaller. Although AG is a histopathological diagnosis the accurate quantification of the AG extent based on a few endoscopic biopsy samples is very difficult because AG is usually a multifocal process especially in the early stage.20 Serum PG has been found to be a marker of gastric mucosal status, including mucosal atrophy.21,22 A low PG I level and a low PG I/II ratio have been associated with severe gastric atrophy, and are frequently found in gastric cancer.18,23-32 In Japan measures of PG I and PG II levels were found to be a noninvasive and straightforward means of mass screening for gastric cancer, as compared with endoscopy.25,33,34 Most of these reports used immunoradiometric assay by PG I, II RIA BEAD kits from Dainabot, Tokyo.24-27,29,31 In contrast to these reports from Japan, the recent study which has been performed in Korea showed that the sensitivity and specificity of PG II ratio ≤3 for detection of gastric cancer was rather low, 59.2% and 61.0%, respectively using Latex enhanced turbidimetric immunoassay (L-TIA) (Shima Laboratories, Tokyo, Japan).32 In addition, a study from Singapore showed that the prevalence of low PG was highest in Indian subjects although gastric cancer incidence was lowest in this race than Chinese and Malay.35 These results suggest that the application of PG test should be cautious in the different condition or race.
The aim of this review is to evaluate the usefulness or limitation of serum PG in the detection of gastric cancer based on the literature. In addition, affecting factors (H. pylori infection status, gender, age, histopathologic features, cancer location, and depth) on the efficacy of PG tests were evaluated to find out a way to increase the efficacy of this gastric cancer biomarker.
SERUM PEPSINOGENS
1. Pepsinogen I and II
Two biochemically distinct pepsinogens are produced by gastric mucosa. PG I (also called as PGA) is exclusively produced by chief and mucous neck cells in the fundic glands, while PG II (also called as PGC) is secreted by these cells and also by the cells in the pyloric glands and Brunner's glands.36,37 Serum PG concentrations have been shown to reflect the morphological and functional status of the gastric mucosa. As the fundic gland mucosa reduces, PG I levels gradually decrease, whereas PG II levels remain constant.38 Thus, the serum PG I level as well as the PG I/II ratio, were positively correlated with maximal gastric output.39,40 However, there has been a report that did not show any significant relation between acid secretion and PG levels.41 For this reason Iijima et al.42 suggested that PG I was influenced not only parietal cell mass but also by gastric mucosal inflammation induced largely by H. pylori infection. H. pylori, which causes inflammation and peptic ulcer diseases, was found to be one of the most important factors that affect PG levels as well as gastric secretion. Finally, it was realized that the subjects should be divided into two groups according to their H. pylori status.
2. H. pylori infection and the effect of H. pylori eradication on the pepsinogen levels
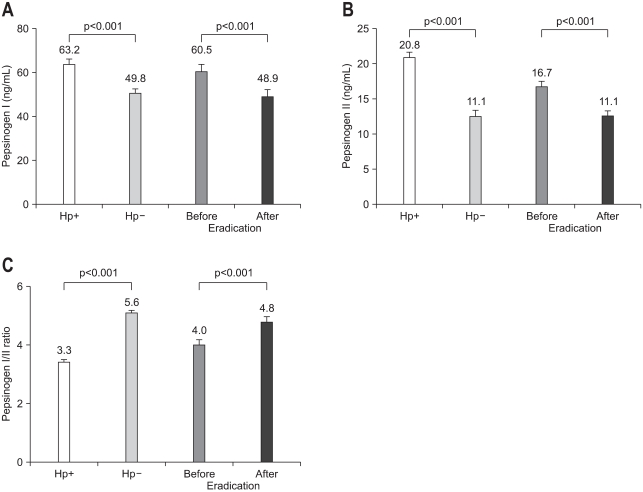
Serum PG I and PG II levels are known to increase in the H. pylori-associated non-atrophic superficial gastritis. However, as PG II exhibits a greater rise relative to PG I,43-45 the PG I/II ratio decreases in the presence of H. pylori-infection (Fig. 1).32 The value of PG II is different depending on H. pylori-infection. That is, in H. pylori-positive subjects there is little correlation between PG II and gastric acid secretion because of the wide variety of PG II levels. In contrast, in H. pylori-negative subjects PG II is a relatively constant value and correlates with acid secretion. There have been several reports regarding the increase of PG levels in case of H. pylori-infection. Cave et al.46 showed that H. pylori sonicate and H. pylori lipopolysaccharide stimulate PG release from isolated rabbit gastric glands, suggesting of a direct stimulatory effect of H. pylori on chief cells. In addition, purified H. pylori lipopolysaccharide increased PG secretion 50-fold while the E. coli lipopolysaccharide raised this secretion 12-fold, suggesting the specificity of H. pylori lipopolysaccharide on gastric secretion.47 In addition to this direct effect of H. pylori the H. pylori-associated gastric inflammation status also increases the PG levels.44
Fig. 1.
Comparison of the serum pepsinogen (PG) I level (A), PG II level (B), and the PG I/PG II ratio (C) in Helicobacter-pylori-positive and -negative cases before and 1 year after H. pylori eradication. Data are presented as mean and standard error values (Reproduced with permission from Kang JM, et al. Helicobacter 2008;13:146-156.32).
Hp, Helicobacter pylori.
These elevated PG levels were found to be decreased 1 year after the eradication of H. pylori (Fig. 1A and B).32 This decrease originates from the decreases of the severity of H. pylori-associated gastritis as well as from the clearing of H. pylori lipopolysaccharide. Although H. pylori-eradication reduced both of PG I and PG II, but PG II exhibited a greater decrease relative to PG I (Fig. 1A and B), thus elevated the PG I to PG II ratio similar to the pre-eradication level (Fig. 1C).32 A significant decrease in basal PG II levels occurred immediately (one month) after completion of the treatment but the decrease of PG I level occurs progressively for 6 months.48
3. The effect of other factors on pepsinogen levels
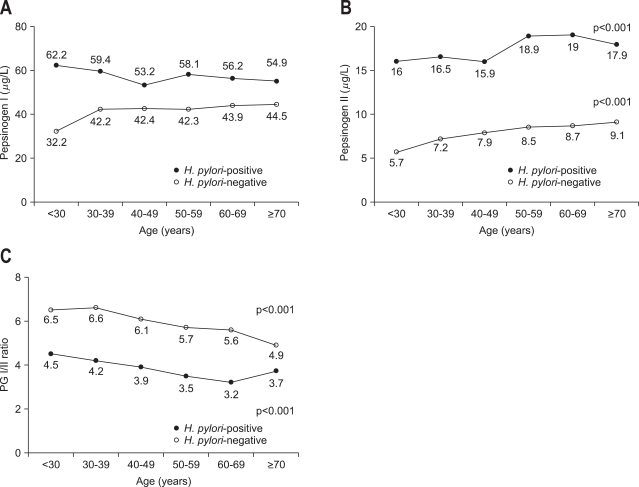
Other factors such as age, gender, height, body weight, body surface area, smoking, and drinking habits have been suggested to be related with PG I and PG II levels in the literature.43,49-52 Kim et al.43 showed that male gender was associated with higher PG I than female but there was no correlation in case of PG II or PG I/II ratio in 1,485 healthy subjects (964 [64.9%] H. pylori positive cases). However, PG I was not associated with body mass index (BMI). The only independent significant factor associated with gender was smoking, which was more common in men than in women in this study; however, there was no significant correlation between the PG levels and the smoking status. These results suggest that the difference in the PG I levels observed between genders could be related to hormonal effects. For H. pylori infection and age, PG I did not show any significant change (Fig. 2A). However, PG II increased two-fold, a change much greater than PG I, in H. pylori-positive subjects (Fig. 2B), and this reduced the PG I/II ratio (Fig. 2C). In addition, the PG II increased significantly with age regardless of H. pylori (Figs. 2B, 3A), different from the PG I observations, resulting in the decrease of PG I/II ratio proportion to age regardless of H. pylori (Figs. 2C, 3B). These results suggest that gender and age are affecting factors on the levels of PG I, PG II, and PG I/II ratio, which might be confounding factors for the interpretation of cut off values of PG levels in case of atrophic gastritis or gastric cancer.
Fig. 2.
Distributions of pepsinogen (PG) I (A), PGII (B), and PGI/PGII ratio (C) with patient age according to the presence of H. pylori infection (Reproduced with permission from Kim HY, et al. Eur J Gastroenterol Hepatol 2009;21:606-612.43).
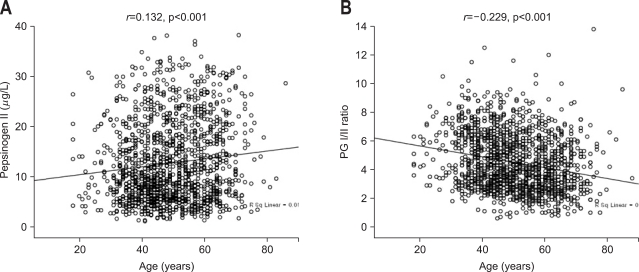
Fig. 3.
Correlations between pepsinogen (PG) II, PG I/II ratio, and patient age. There was a positive correlation between PG II and age (Pearson's correlation coefficient r=0.132) (A) and a negative correlation between the PG I/II ratio and age (Pearson's correlation coefficient r=-0.229) (B) (Reproduced with permission from Kim HY, et al. Eur J Gastroenterol Hepatol 2009;21:606-612.43).
4. Measurement method of serum pepsinogen levels
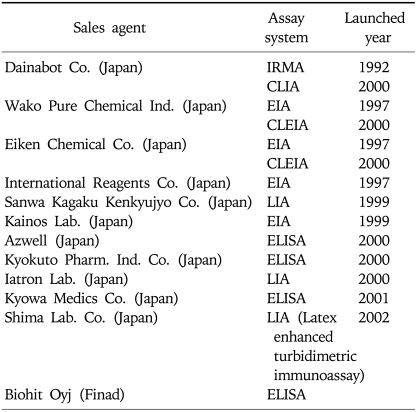
There are several methods of measuring serum pepsinogens (Table 1),23 which could be another factor for causing different results among reports. The radioimmunoassay method which has been usually used to measure serum PG levels,53,54 is no longer available. Instead, immunoradiometric assay (PG I/II RIA BEAD; Dainabot Co. Ltd., Tokyo, Japan), a modified method of radioimmunoassay became popular.24-31 Soon after latex enhanced turbidimetric immunoassay (LIA), a biochemical test, which is easy to perform, and makes it possible to measure serum pepsinogen levels in large numbers, thus suitable for mass screening became available.32,55 Finally, enzyme-linked immunosorbent assays (ELISA) (Bihit ELISA kit; Biohit, Helsinki, Finland) became used.56,57 Several determinations of a suitable cut off point for gastric cancer screening have been reported in case of immunoradiometric assay (IRMA). That is, PG I concentration ≤70 ng/mL and PG I/II ratio ≤3 has been widely accepted as a cut off value of AG in Japan.24-27,29 However, in the other countries such as USA and Thailand the cut off value for PG I/II ratio was set as 2 and 3.3, respectively, for gastric cancer diagnosis although they used the same method (Table 2).28,30 Furthermore, the normal value of pepsinogen levels could be different depending on test methods. For instance the mean PG I level checked by using LIA was found to be 42.2 ng/mL in the 521 H. pylori-negative healthy check up subjects in comparison to the 56.3 ng/mL in the 964 H. pylori-positive subjects,43 which is quite lower than the normal value of PG I by IRMA. However, the results of PG I/II ratio by LIA were similar to other reports using IRMA method.24-31 That is, the mean PG I/II ratio in 521 H. pylori-negative healthy check up subjects was 6.0 in comparison to the 3.7 in the 964 H. pylori-positive subjects.43 In addition, the PG I/II cut off value for atrophic gastritis was 6.0 for H. pylori-negative subjects and 3.0 for H. pylori-positive individuals.43 Taken together it might be reasonable to conclude that to increase the efficacy of the PG I/II ratio for the detection of atrophic gastritis or gastric cancer the cut off value of the PG I or PG I/II ratio could be stratified according to the H. pylori status or the measurement methods of PG levels in each country.
Table 1.
List of Pepsinogen Test Kits Available in Japan and Korea
Reproduced with permission from Miki K, et al. Am J Gastroenterol 2003;98:735-739.33
CLEIA, chemiluminescent enzyme immunoassay; CLIA, chemiluminescent immunoassay; EIA, enzyme immunoassay; ELISA, enzyme-linked immunosorbent assay; IRMA, immunoradiometric assay; LIA, latex immunoassay.
Table 2.
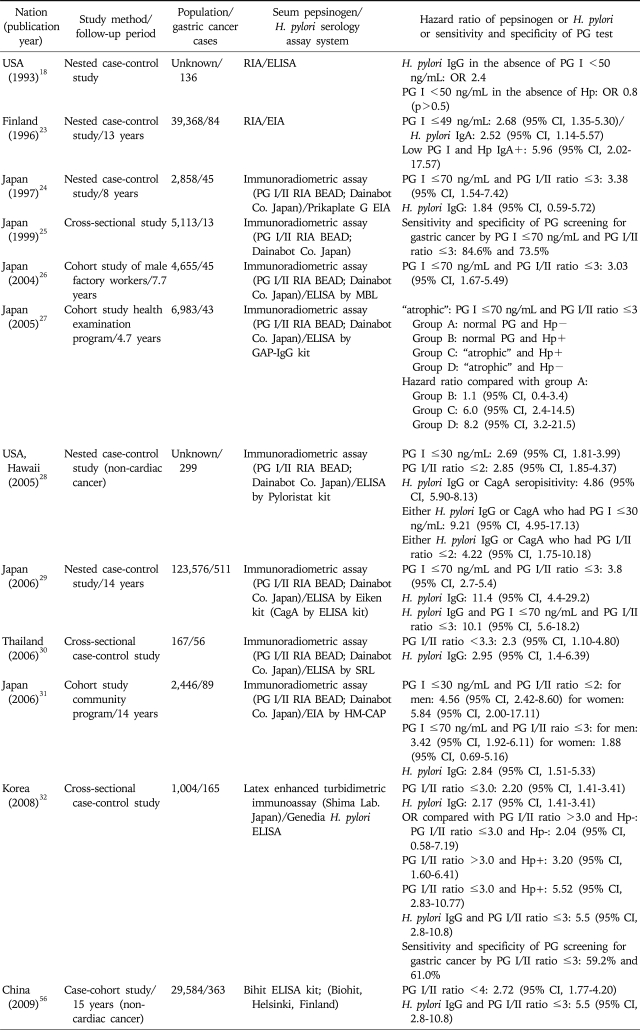
The Hazard Ratios of Serum Pepsinogen Levels and/or H. pylori Serology for the Risk of Gastric Cancer
CI, confidence interval; PG, pepsinogen; Hp, Helicobacter pylori; RIA, radioimmunoassay; ELISA, enzyme-linked immunosorbent assay; EIA, enzyme immunoassay; OR, odds ratio.
ATROPHIC GASTRITIS AND INTESTINAL METAPLASIA
1. Serum pepsinogen test as a biomarker of atrophic gastritis and intestinal metaplasia
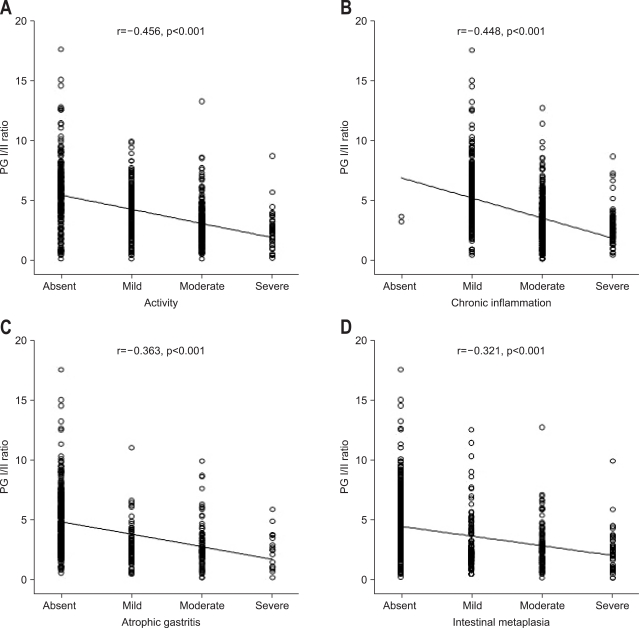
The clinical importance of atrophic gastritis (AG) with intestinal metaplasia (IM) is related with the fact that it increases the risk of gastric cancer development.18,58,59 In the process of gastric carcinogenesis, especially, for the intestinal type of gastric carcinomas it has been proposed that the gastric mucosa evolves through the stages if chronic active gastritis, glandular atrophy, IM, and dysplasia before developing into gastric adenocarcinoma.58 The risk of gastric neoplasia rises with the increasing grade and extent of AG.60 AG is usually diagnosed by endoscopic biopsies. However, there is a significant potential sampling errors in identifying AG or IM by a random biopsy because the AG or IM of the gastric mucosa could be patchy, especially in the early stage.20 Instead low serum pepsinogen has been used as a surrogate marker for AG.21,22,61 In addition, the differential changes in PG levels are indicative of the histological state of the gastric mucosa. That is, as gastritis progresses PG I and PG II are positively correlated with activity and chronic inflammation of gastritis in the antrum and corpus.32 However, PG I/II ratios are inversely correlated with activity and chronic inflammation of gastritis in the corpus (Fig. 4A and B, respectively), which was similar in the antrum. This phenomenon was attributed to a greater increase in PG II than PG I with increasing activity and chronic inflammation.32,43-45 However, as disease severity increases further to atrophic gastritis, chief cells are replaced by pyloric glands resulting in decrease of PG I but the level of PG II remains elevated, thus the PG I/II ratio are further reduced reflecting atrophy and intestinal metaplasia (Fig. 4C and D, respectively). As atrophic gastritis is acknowledged to be a precancerous condition or frequently associated findings especially in case of intestinal type of gastric cancer this decrease of PG I concentration (≤70 ng/mL) and PG I/II ratio (≤3) has been widely accepted as a cut off value of gastric cancer in Japan.24-27,29
Fig. 4.
Correlations between serum pepsinogen (PG) I/II ratio and histological features in the corpus: activity (A), chronic inflammation (B), atrophic gastritis (C), and intestinal metaplasia (D) (Reproduced with permission from Kang JM, et al. Helicobacter 2008;13:146-156.32).
r, correlation coefficient.
2. Different value of pepsinogen test as a biomarker of atrophic gastritis depending on each country
However, several studies have yielded conflicting results regarding the values of pepsinogens as biomarkers of AG. Broutet et al. showed that only PG I/II ratio among PG I, PG II, and PG I/II ratio was a reliable marker of AG in the corpus with a sensitivity of 77% and a specificity of 87% at a cutoff value of 5.6.62 Similarly, Korean study showed that a PG I of ≤70 ng/mL was found to have a sensitivity of around 80% for detecting corpus AG/IM, but very low specificity, around 30%.32 In contrast, a PG I/II ratio of ≤3 showed moderate sensitivity (66.8% and 67.5%, respectively) and high specificity (74.7 and 70.1%, respectively) for detecting corpus AG/IM.32 Furthermore, in 147 asymptomatic Italian subjects PG I, PG II, and PGI/II ratio were not reliable for predicting antral predominant atrophic gastritis,63 suggesting that the value of PG levels as a biomarker of AG could be variable in each country or each population.
EFFICACY OF PEPSINOGEN TEST FOR GASTRIC CANCER BIOMARKER
1. Pepsinogen test as a biomarker of gastric cancer
In Japan gastric cancer screening using photofluorography was started in Miyagi prefecture around 1960, and this approach has been adopted nationwide.16 In 1983, under the Health Service Law for the Aged, gastric cancer screening was introduced for all residents aged 40 years and over.16 Photofluorography was recommended for gastric cancer screening based on the results of several case-control and cohort studies. However, small asymptomatic cancers are relatively difficult to detect using photofluorography, raising the necessity of compensatory tool. Thus, other methods including endoscopy, serum pepsinogen and H. pylori antibody testing have been tried in the clinical setting for opportunistic screening.16 That is, PG as a marker for atrophic gastritis has been incorporated into gastric cancer screening programs to identify people who would benefit most from gastric cancer screening (Table 2).24-27,29,31 This trial has been also performed in other countries (Table 2).18,23,28,30,32 In general all of these studies have shown that PG testing is useful in detecting gastric cancer. Miki et al.33 performed a meta-analysis of the sensitivity and specificity results from 42 individual studies. In this meta-analysis PG I level ≤70 ng/mL and PG I/II ratio ≤3 had a sensitivity of 77% and false positive rate of 27%. The positive predictive value was varied between 0.77% and 1.25%, and the negative predictive value varied between 99.08% and 99.90%. However, studies from several countries the OR of PG I/II ratio has been reported to be around 2-3, which is lower than those from Japanese reports, from 2.8 to 9.7.23,24,26,27,31 Specifically, in a case control study from Thailand, the odds ratio (OR) for gastric cancer was 2.3 (95% confidence interval [CI], 1.10-4.80, Table 2) for PG I/II ratio ≤3.3.30 In case of China OR for gastric cancer was 2.72 (95% CI, 1.77-4.20, Table 2) for PG I/II ratio ≤4,56 and in Korea it was 2.20 (95% CI, 1.41-3.41) for PG I/II ratio ≤3.0.32 Taken together gastric cancer was more frequent and the hazard ratio was significantly higher in the group with a lower PG I/II ratio although the degree was different in each country. These results are suggestive of a dose-response correlation between cancer development and the progression of atrophic gastritis.
2. Different value of pepsinogen test as a biomarker of gastric cancer depending on country
Kitahara et al.25 reported that the sensitivity and specificity of PG I/II ≤3 alone for gastric cancer are 84.6% and 67.2%, respectively, and that those of PG I ≤70 ng/mL and a PG I/II ratio of ≤3.0 are 84.6% and 73.5%, respectively. When PG I ≤70 ng/mL and a PG I/II ratio of ≤3.0 are further categorized into PG I ≤50 ng/mL and a PG I/II ratio of ≤2.0 as strong positive then the hazard ratio for gastric cancer further increased.31 In contrast to Japan, in Korean study a PG I of ≤70 ng/mL showed sufficient sensitivity (72.4%), but a low specificity (20.2%), and the sensitivity and specificity of a PG I/II ratio cut off of ≤3 were found to be 59.2-61.7% and 61.0%, respectively, in gastric cancer or dysplasia when the latex enhanced turbidimetric immunoassay method (Shima Laboratories) was used.32 Furthermore there is other example with respect to the utility of serum pepsinogen levels. That is, a study from Singapore examined whether racial differences in the prevalence of H. pylori and serum PG could account for a racial difference in gastric cancer incidence.35 The H. pylori seroprevalence was similar between Chinese and Indian subjects, but significantly lower among Malay subjects. The gastric cancer incidence rates correlated with H. pylori seropositivity for the Chinese and Malay subjects, but not for the Indian subjects. The prevalence of low PG was highest in Indian subjects even when adjusted for gender and the presence of H. pylori. Altogether these results suggest that the usefulness of PG I and PG I/II ratio may different by country, thus these biomarkers should be validated before they are used practically for the screening of gastric cancer.
3. The affecting factors on the efficacy of pepsinogen test as a biomarker of gastric cancer
There might be several reasons for the different values of pepsinogen test as a biomarker of gastric cancer among several countries. The candidates are H. pylori infection, gender, histological type of gastric cancer, distribution of gastric cancer, gastric cancer stage or age when the gastric cancer is diagnosed.
1) H. pylori infection
H. pylori infection of the gastric mucosa is closely related to the level of serum PG and has been shown to increase the risk of gastric cancer. Thus it is quite natural that H. pylori infection affects on the PG test and it is necessary to analyze the PG tests depending on H. pylori positivity. Parsonnet et al. reported no significant difference in gastric cancer risk between H. pylori-negative subjects with a low PG level (PG I <50 ng/mL) and subjects with a normal PG level (PG I ≥50 ng/mL) but most of reports showed a significant association between serum PG levels and development of gastric cancer in H. pylori-negative as well as H. pylori-positive subjects.26,31 The difficulty of diagnosis of H. pylori infection especially in the background of severe AG/IM might be one of causes of this difference. That is, sometimes it is difficult to exclude the H. pylori-false negative subjects in the H. pylori-negative group because both AG and IM significantly affected the validity of H. pylori diagnostic tests.64 At first, the sensitivity of the Campylobacter like organism test, which is a very simple and practical test for clinicians, is markedly reduced with progression of AG or IM, which drives the bacterium out of the gastric mucosa.65 As time further goes on after the disappearance of H. pylori from mucosa then the serology slowly be converted into negative, and the subjects could be categorized as H. pylori-negative.66 A Japanese report that the hazard ratio was the highest in the group of H. pylori-negative and PG-positive (PG I level ≤70 ng/mL and PG I/II ratio ≤3) could be explained from this background (Table 2).27 However, Korean report did not show similar results. That is, the OR of gastric cancer was highest in case of H. pylori-positive and PG I/II ratio ≤3 (Table 2).32 Taken together these reports suggest that cut off value of PG test or the efficacy of PG test could be variable depending on H. pylori prevalence and the severity of AG in each country.
2) Gender
The age-adjusted standardized incidence ratios of gender for gastric cancer are usually reported to be 3-4 times frequent in the men than women.31,32 In addition, the efficacy of PG test were found to be different between genders. Oishi et al.31 showed that adjusted hazard ratio of PG I level ≤70 ng/mL and PG I/II ratio ≤3 (positive) was 3.42 (95% CI, 1.92-6.11) in men but there was no significance in women 1.88 (95% CI, 0.69-5.16). However, when the cut off value increased up to PG I level ≤50 ng/mL and PG I/II ratio ≤2 (strong positive) then both of genders were showed high hazard ratio (in men 4.13 [95% CI, 2.18-7.82]; in women 5.77 [95% CI, 1.91-17.39]). Several clinical and epidemiological studies have reported that compared with women, men have a higher risk of developing gastric cancer after severe gastric atrophy.60,67 In the animal experiments, this tolerance of women toward carcinogenesis has been explained in terms of their sex hormones.68 Thus, if women proportion is increased among gastric cancer patients then the efficacy of PG test will be decreased. To increase the efficacy of PG test in screening of gastric cancer, it might be necessary to reduce the cut off level of PG I and PG I/II ratio in women than in men.
3) Histology of gastric cancer
Regarding the gastric cancer subtype as determined by the Lauren classification,69 it has been hypothesized that the intestinal type and diffuse type of gastric cancer have different etiologies because of their different epidemiological features such as gender, age, and geographical patterns. In addition, Correa's hypothesis,58 which applies to the intestinal type of gastric cancer, implies that there is a different pathophysiology for these two subtypes of gastric cancer. The intestinal type has been more commonly identified in males, and in older age groups, and in high risk areas for gastric cancer. On the other hand, the diffuse type is more frequent in younger age groups, and the gender ratio is close to one.70,71 Thus, it has been proposed that the intestinal type is more dependent on environmental factors, whereas the diffuse type is more related to host factors such as genetic susceptibility. PG I and PG I/II ratio were found to be more valuable for intestinal type than diffuse type cancer, which is probably related with different grades of AG and IM according to histologic type. It is generally accepted that intestinal type gastric adenocarcinoma arises through a multistep process that from chronic gastritis that progresses through stages of atrophy, intestinal metaplasia, and dysplasia, and finally results in intestinal-type cancer.58 In contrast, diffuse type gastric cancer does not progress through severe atrophic gastritis.72 Thus, it appears to be reasonable that pepsinogen, a marker of chronic atrophic gastritis, is closely associated with intestinal type gastric cancer. For instance, Oishi et al.31 showed that multivariate-adjusted hazard ratio of PG I level ≤70 ng/mL and PG I/II ratio ≤3 (positive) was 3.75 (95% CI, 2.07-6.80) in intestinal type but there was no significance in diffuse type 1.69 (95% CI, 0.66-4.30). Even the cut off value increased up to PG I level ≤50 ng/mL and PG I/II ratio ≤2 (strong positive) this was not so changed (intestinal type 5.85 [95% CI, 3.14-10.93]; diffuse type 1.85 [95% CI, 0.51-6.72]). This is also supported by the findings of several clinical and epidemiologic studies.26,32,73-75 Thus if the ratio of diffuse type among gastric cancers is higher then the efficacy of PG test could be lower, which has been shown in Korean report.32 That is, in this report the proportions with diffuse or intestinal type turned out to be 42.1% (160 patients) and 57.9% (220 patients) among 380 gastric cancer patients. This is quite different from that found in Japanese studies, which reported that the diffuse type proportion is relatively low at 16-33%.26,27,31,68
4) Cancer location
When the gastric cancer is divided into cardiac and non-cardiac cancer PG test was significant only in the non-cardiac cancer in USA28 and China report,56 probably because the pathogenesis of gastric cancer is different. Furthermore when the cancer is divided into proximal one third and distal two thirds of the stomach the magnitude of the association was stronger in the distal cancer.18,28 Oishi et al.31 also showed that adjusted hazard ratio of PG I level ≤70 ng/mL and PG I/II ratio ≤3 (positive) was 2.98 (95% CI, 1.69-5.23) in the distal two thirds but there was no significance in proximal one third (OR, 1.91; 95% CI, 0.68-5.35). However, when the cut off value increased up to PG I level ≤50 ng/mL and PG I/II ratio ≤2 (strong positive) then both of gastric cancer location showed high hazard ratios (in the proximal one third 4.11 [95% CI, 1.42-11.94]; in the distal two thirds 4.71 [95% CI, 2.55-8.70]). This result could be related with the fact that atrophic change in gastric mucosa spreads from the antrum into body side.19 Thus if the portion of proximal one third gastric cancer is increased it could be one reason of decrease of the efficacy of PG test.
5) Depth of invasion
To be useful as a cancer marker it should be sensitive enough for the detection of precancerous lesion as well as cancer. In addition, it should detect early gastric cancer (EGC) as well as advanced gastric cancer (AGC). In regard to precancerous lesion Korean report showed that the sensitivity and specificity of PG I/II ratio ≤3 were similar in both of dysplasia and cancer group.32 That is, in cancer group the sensitivity and specificity were 59.2% and 61.0%, respectively and in dysplasia group they were 61.7% and 61.0%, respectively.32 In the regard to gastric cancer, Japanese report31 showed that adjusted hazard ratio of PG I level ≤70 ng/mL and PG I/II ratio ≤3 (positive) was 2.70 (95% CI, 1.50-4.87) in EGC and it was 4.42 (95% CI, 1.68-11.64) in AGC. This report confirmed that PG test can predict not only AGC but also EGC.
6) Age
Age was found to be one of the significant risk factors of AG and IM.59 If gastric cancer develops in the aged persons whose stomach showed severe AG and IM then PG test is very useful. Thus the cancer developing age could affect the usefulness of PG test as a gastric cancer biomarker. Actually, in the Korean report mean age at diagnosis of gastric cancer cases was 54.9±12.3 years,32 which was substantially less than the 64 to 66 years of the previous Japanese studies.25,76 Moreover, as compared with Korean cancer patients, elderly cancer patients in the Japanese studies may have had more severe AG/IM, and that this could be a cause of false negative result for H. pylori in Japan. This background might be a reason why the hazard ratio of H. pylori -, PG test + group highest in Japan in contrast to the H. pylori +, PG test + is highest in Korea.32 Thus, it is possible that gastric cancers in the Korean population might develop more frequently before conversion to seronegative for H. pylori than those in the Japanese population.
COMBINATION OF PEPSINOGEN TEST AND HELICOBACTER PYLORI INFECTION IN THE DETERMINATION OF GASTRIC CANCER RISK
In Japan, where the morbidity and mortality from gastric cancer are considerably high, a serum PG test based on the combination of the serum PG I level and the PG I/II ratio has been implemented to screen for gastric cancer.21,23,25 H. pylori infection of the gastric mucosa is closely related to the level of serum PG77 and has been shown to increase the risk of gastric cancer.78 However, there have been arguments regarding the value of PG test in the absence of H. pylori seropositivity. For instance Parsonnet et al.18 reported that H. pylori in the absence of low PG I (<50 ng/mL) was independently associated with cancer (OR, 2.4; p=0.04) but low PG I in the absence of H. pylori infection was not associated with cancer, concluding that infection rather than gastric atrophy is the determining factor in cancer risk. Similarly the OR of gastric cancer in the case of H. pylori + and PG I/II ratio >3 was significant (OR, 1.6; 95% CI, 1.60-6.41) based on H. pylori - and PG I/II ratio >3 but there was no significance in case of H. pylori - and PG I/II ratio ≤ 3 (OR, 2.04; 95% CI, 0.58-7.19) in Korea.32 These results suggest that H. pylori infection could be a more important factor than PG test in the determination of gastric cancer risk in USA or Korea. However, as the seroprevalence is rather high, 59.6% in the age over 16 years in Korea79 the H. pylori infection alone is not helpful for the localization of high risk group for gastric cancer, thus it needs another factor such as PG I/II ratio ≤3. In Japan PG test was very useful for the detection of gastric cancer regardless of H. pylori positivity.31 Furthermore the hazard ratio of H. pylori -, PG test + group was highest27 suggesting that these two tests can also localize the highest risk group for gastric cancer in Japan, which is different from Korea.
FUTURE DIRECTIONS
Gastric cancer is the leading cause of morbidity and mortality in Japan, Korea and China but mass screening method is different. Two non-invasive tests, serum PG levels and H. pylori-positivity, could provide a tool for selecting those at high risk of having gastric cancer, and its adoption would reduce numbers of endoscopy candidates during national gastric cancer screening. However, as the sensitivity and specificity of PG test is different in each country further research is necessary to increase the efficacy of PG test as a gastric cancer biomarker. As the efficacy is different depending on gender, age, cancer histology, location and cancer depth, this research should include targeting of PG test in which the efficacy is maximized by subcategorization in a large population with long-term follow-up. In addition, the limitations of PG test should be clarified.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Shin HR, Won YJ, Jung KW, et al. Nationwide cancer incidence in Korea, 1999~2001; first result using the national cancer incidence database. Cancer Res Treat. 2005;37:325–331. doi: 10.4143/crt.2005.37.6.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nomura K, editor. Cancer statistics in Japan 2005. Tokyo: Foundation for Promotion Cancer Research; 2006. [Google Scholar]
- 4.Correa P, Piazuelo MB, Camargo MC. The future of gastric cancer prevention. Gastric Cancer. 2004;7:9–16. doi: 10.1007/s10120-003-0265-0. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs CS, Mayer RJ. Gastric carcinoma. N Engl J Med. 1995;333:32–41. doi: 10.1056/NEJM199507063330107. [DOI] [PubMed] [Google Scholar]
- 6.Hill MJ. Salt and gastric cancer. Eur J Cancer Prev. 1998;7:173–175. doi: 10.1097/00008469-199806000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Sandor J, Kiss I, Farkas O, Ember I. Association between gastric cancer mortality and nitrate content of drinking water: ecological study on small area inequalities. Eur J Epidemiol. 2001;17:443–447. doi: 10.1023/a:1013765016742. [DOI] [PubMed] [Google Scholar]
- 8.Serafini M, Bellocco R, Wolk A, Ekström AM. Total anti-oxidant potential of fruit and vegetables and risk of gastric cancer. Gastroenterology. 2002;123:985–991. doi: 10.1053/gast.2002.35957. [DOI] [PubMed] [Google Scholar]
- 9.Terry P, Nyrén O, Yuen J. Protective effect of fruits and vegetables on stomach cancer in a cohort of Swedish twins. Int J Cancer. 1998;76:35–37. doi: 10.1002/(sici)1097-0215(19980330)76:1<35::aid-ijc7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 10.Shin CM, Kim N, Yang HJ, et al. Stomach cancer risk in gastric cancer relatives: interaction between Helicobacter pylori infection and family history of gastric cancer for the risk of stomach cancer. J Clin Gastroenterol. 2010;44:e34–e39. doi: 10.1097/MCG.0b013e3181a159c4. [DOI] [PubMed] [Google Scholar]
- 11.Kikuchi S, Katada N, Sakuramoto S, et al. Survival after surgical treatment of early gastric cancer: surgical techniques and long-term survival. Langenbecks Arch Surg. 2004;389:69–74. doi: 10.1007/s00423-004-0462-2. [DOI] [PubMed] [Google Scholar]
- 12.Onodera H, Tokunaga A, Yoshiyuki T, et al. Surgical outcome of 483 patients with gastric cancer: prognosis, postoperative morbidity and mortality, and gastric remnant cancer. Hepatogastroenterology. 2004;51:82–85. [PubMed] [Google Scholar]
- 13.Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1–11. doi: 10.1007/s10120-006-0408-1. [DOI] [PubMed] [Google Scholar]
- 14.Kim JP, Nam SJ, Yang HK, Chung M. Clinical analysis of early gastric cancer. Korean J Gastroenterol. 1991;23:428–435. [Google Scholar]
- 15.Choi IJ. Gastric cancer screening and diagnosis. Korean J Gastroenterol. 2009;54:67–76. doi: 10.4166/kjg.2009.54.2.67. [DOI] [PubMed] [Google Scholar]
- 16.Hamashima C, Shibuya D, Yamazaki H, et al. The Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol. 2008;38:259–267. doi: 10.1093/jjco/hyn017. [DOI] [PubMed] [Google Scholar]
- 17.Liu CY, Wu CY, Lin JT, Lee YC, Yen AM, Chen TH. Multistate and multifactorial progression of gastric cancer: results from community-based mass screening for gastric cancer. J Med Screen. 2006;13(Suppl 1):S2–S5. [PubMed] [Google Scholar]
- 18.Parsonnet J, Samloff IM, Nelson LM, et al. Helicobacter pylori, pepsinogen, and risk of gastric adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 1993;2:461–466. [PubMed] [Google Scholar]
- 19.Kimura K. Chronological transition of the fundic-pyloric border determined by stepwise biopsy of the lesser and greater curvatures of the stomach. Gastroenterology. 1972;63:584–592. [PubMed] [Google Scholar]
- 20.Kuipers EJ. Review article: relationship between Helicobacter pylori, atrophic gastritis and gastric cancer. Aliment Pharmacol Ther. 1998;12(Suppl 1):25–36. doi: 10.1111/j.1365-2036.1998.00009.x. [DOI] [PubMed] [Google Scholar]
- 21.Miki K, Ichinose M, Kawamura N, et al. The significance of low serum pepsinogen levels to detect stomach cancer associated with extensive chronic gastritis in Japanese subjects. Jpn J Cancer Res. 1989;80:111–114. doi: 10.1111/j.1349-7006.1989.tb02276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samloff IM, Varis K, Ihamaki T, Siurala M, Rotter JI. Relationships among serum pepsinogen I, serum pepsinogen II, and gastric mucosal histology. A study in relatives of patients with pernicious anemia. Gastroenterology. 1982;83(1 Pt 2):204–209. [PubMed] [Google Scholar]
- 23.Aromaa A, Kosunen TU, Knekt P, et al. Circulating anti-Helicobacter pylori immunoglobulin A antibodies and low serum pepsinogen I level are associated with increased risk of gastric cancer. Am J Epidemiol. 1996;144:142–149. doi: 10.1093/oxfordjournals.aje.a008901. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe Y, Kurata JH, Mizuno S, et al. Helicobacter pylori infection and gastric cancer. A nested case-control study in a rural area of Japan. Dig Dis Sci. 1997;42:1383–1387. doi: 10.1023/a:1018833819860. [DOI] [PubMed] [Google Scholar]
- 25.Kitahara F, Kobayashi K, Sato T, Kojima Y, Araki T, Fujino MA. Accuracy of screening for gastric cancer using serum pepsinogen concentrations. Gut. 1999;44:693–697. doi: 10.1136/gut.44.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohata H, Kitauchi S, Yoshimura N, et al. Progression of chronic atrophic gastritis asssociated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer. 2004;109:138–143. doi: 10.1002/ijc.11680. [DOI] [PubMed] [Google Scholar]
- 27.Watabe H, Mitsushima T, Yamaji Y, et al. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: a prospective endoscopic cohort study. Gut. 2005;54:764–768. doi: 10.1136/gut.2004.055400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Normura AM, Kolonel LN, Miki K, et al. Helicobacter pylori, pepsinogen, and gastric adenocarcinoma in Hawaii. J Infect Dis. 2005;191:2075–2081. doi: 10.1086/430353. [DOI] [PubMed] [Google Scholar]
- 29.Sasazuki S, Inoue M, Iwasaki M, et al. Effect of Helicobacter pylori infection combined with CagA and pepsinogen status on gastric cancer development among Japanese men and women: a nested case-control study. Cancer Epidemiol Biomarkers Prev. 2006;15:1341–1347. doi: 10.1158/1055-9965.EPI-05-0901. [DOI] [PubMed] [Google Scholar]
- 30.Yamada S, Matsuhisa T, Makonkawkeyoon L, et al. Helicobacter pylori infection in combination with the serum pepsinogen I/II ratio and interleukin-1beta-511 polymorphisms are independent risk factors for gastric cancer in Thais. J Gastroenterol. 2006;41:1169–1177. doi: 10.1007/s00535-006-1951-6. [DOI] [PubMed] [Google Scholar]
- 31.Oishi Y, Kiyohara Y, Kubo M, et al. The serum pepsinogen test as a predictor of gastric cancer. The Hisayama study. Am J Epidemiol. 2006;163:629–637. doi: 10.1093/aje/kwj088. [DOI] [PubMed] [Google Scholar]
- 32.Kang JM, Kim N, Yoo JY, et al. The role of serum pepsinogen and gastrin test for the detection of gastric cancer in Korea. Helicobacter. 2008;13:146–156. doi: 10.1111/j.1523-5378.2008.00592.x. [DOI] [PubMed] [Google Scholar]
- 33.Miki K, Morita M, Sasajima M, Hoshina R, Kanda E, Urita Y. Usefulness of gastric cancer screening using the serum pepsinogen test method. Am J Gastroenterol. 2003;98:735–739. doi: 10.1111/j.1572-0241.2003.07410.x. [DOI] [PubMed] [Google Scholar]
- 34.Miki K. Gastric cancer screening using the serum pepsinogen test method. Gastric Cancer. 2006;9:245–253. doi: 10.1007/s10120-006-0397-0. [DOI] [PubMed] [Google Scholar]
- 35.Ang TL, Fock KM, Dhamodaran S, Teo EK, Tan J. Racial differences in Helicobacter pylori, serum pepsinogen and gastric cancer incidence in an urban Asian population. J Gastroenterol Hepatol. 2005;20:1603–1609. doi: 10.1111/j.1440-1746.2005.03898.x. [DOI] [PubMed] [Google Scholar]
- 36.Huang SC, Miki K, Sano J, et al. Pepsinogens I and II in gastric cancer: an immunohistochemical study using monoclonal antibodies. Jpn J Cancer Res. 1988;79:1139–1146. doi: 10.1111/j.1349-7006.1988.tb01537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samloff IM. Cellular localization of group I pepsinogens in human gastric mucosa by immunofluorescence. Gastroenterology. 1971;61:185–188. [PubMed] [Google Scholar]
- 38.Miki K, Ichinose M, Shimizu A, et al. Serum pepsinogens as a screening test of extensive chronic gastritis. Gastroenterology Jpn. 1987;22:133–141. doi: 10.1007/BF02774209. [DOI] [PubMed] [Google Scholar]
- 39.Feldman M, Richardson CT, Lam SK, Samloff IM. Comparison of gastric acid secretion rates and serum pepsinogen I and II concentrations in occidental and oriental duodenal ulcer patients. Gastroenterology. 1988;95:630–635. doi: 10.1016/s0016-5085(88)80008-8. [DOI] [PubMed] [Google Scholar]
- 40.Kinoshita Y, Kawanami C, Kishi K, Nakata H, Seino Y, Chiba T. Helicobacter pylori in dependent chronological change in gastric acid secretion in the Japanese. Gut. 1997;41:452–458. doi: 10.1136/gut.41.4.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yasunaga Y, Shinomura Y, Kanayama S. Serum pepsinogen I levels and acid secretion in Helicobacter pylori associated enlarged fold gastritis. Ital J Gastroenterol. 1996;28:457–461. [PubMed] [Google Scholar]
- 42.Iijima K, Sekine H, Koike T, Imatani A, Ohara S, Shimosegawa T. Serum pepsinogen concentrations as a measure of gastric acid secretion in Helicobacter pylori-negative and -positive Japanese subjects. J Gastroenterol. 2005;40:938–944. doi: 10.1007/s00535-005-1677-x. [DOI] [PubMed] [Google Scholar]
- 43.Kim HY, Kim N, Kang JM, et al. Clinical meaning of pepsinogen test and Helicobacter pylori serology in the health check-up population in Korea. Eur J Gastroenterol Hepatol. 2009;21:606–612. doi: 10.1097/MEG.0b013e3283086757. [DOI] [PubMed] [Google Scholar]
- 44.Wagner S, Haruma K, Gladziwa U, et al. Helicobacter pylori infection and serum pepsinogen A, pepsinogen C, and gastrin in gastritis and peptic ulcer: significance of inflammation and effect of bacterial eradication. Am J Gastroenterol. 1994;89:1211–1218. [PubMed] [Google Scholar]
- 45.Asaka M, Kimura T, Kudo M, et al. Relationship of Helicobacter pylori to serum pepsinogens in asymptomatic Japanese population. Gastroenterology. 1992;102:760–766. doi: 10.1016/0016-5085(92)90156-s. [DOI] [PubMed] [Google Scholar]
- 46.Cave TR, Cave DR. Helicobacter pylori stimulates pepsin secretion from isolated rabbit gastric glands. Scand J Gastroenterol. 1991;181(Suppl):9–14. doi: 10.3109/00365529109093202. [DOI] [PubMed] [Google Scholar]
- 47.Young GO, Stemmet N, Lastovica A, et al. Helicobacter pylori lipopolysaccharide stimulates gastric mucosal pepsinogen secretion. Aliment Pharmacol Ther. 1992;6:169–177. doi: 10.1111/j.1365-2036.1992.tb00260.x. [DOI] [PubMed] [Google Scholar]
- 48.Gisbert JP, Boixeda D, Al-Mostafa A, et al. Basal and stimulated gastrin and pepsinogen levels after eradication of Helicobacter pylori: a 1-year follow-up study. Eur J Gastroenterol Hepatol. 1999;11:189–200. doi: 10.1097/00042737-199902000-00022. [DOI] [PubMed] [Google Scholar]
- 49.Knight T, Greaves S, Wilson A, et al. Variability in serum pepsinogen levels in an asymptomatic population. Eur J Gastroenterol Hepatol. 1995;7:647–654. [PubMed] [Google Scholar]
- 50.Kikuchi S, Inaba Y, Wada O, et al. The association of smoking and drinking habits with serum pepsinogens. Int J Epidemiol. 1995;24:346–353. doi: 10.1093/ije/24.2.346. [DOI] [PubMed] [Google Scholar]
- 51.Denda K, Fujibayashi S, Seko C, Nakamura K, Ehata Y, Yagami T. Relationship between factors examined at health examination and serum pepsinogen levels in healthy adults. Physical measurements, blood chemical tests, drinking and smoking. Nippon Koshu Eisei Zasshi. 1998;45:336–342. [PubMed] [Google Scholar]
- 52.Hokkanen S, Kosunen TU, Sarna S, et al. Normal serum pepsinogen I levels in adults: a population-based study with special reference to Helicobacter pylori infection and parietal cell antibodies. Scand J Clin Lab Invest. 2005;65:291–299. doi: 10.1080/00365510510013848. [DOI] [PubMed] [Google Scholar]
- 53.Ichinose M, Miki K, Furihata C, et al. Radioimmunoassay of serum group I and group II pepsinogens in normal controls and patients with various disorders. Clin Chim Acta. 1982;126:183–191. doi: 10.1016/0009-8981(82)90034-1. [DOI] [PubMed] [Google Scholar]
- 54.Samloff IM. Pepsinogen I and II: purification from gastric mucosa and radioimmunoassay in serum. Gastroenterology. 1982;82:26–33. [PubMed] [Google Scholar]
- 55.Mukoubayashi C, Yanaoka K, Ohata H, et al. Serum pepsinogen and gastric cancer screening. Intern Med. 2007;46:261–266. doi: 10.2169/internalmedicine.46.6181. [DOI] [PubMed] [Google Scholar]
- 56.Ren JS, Kamangar F, Qiao YL, et al. Serum pepsinogens and risk of gastric and oesophgeal cancers in the General Population Nutrition Intervention Trial Cohort. Gut. 2009;58:636–642. doi: 10.1136/gut.2008.168641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ijima K, Abe Y, Kikuchi R, et al. Serum biomarker tests are useful in delineating between patients with gastric atrophy and normal, healthy stomach. World J Gastroenterol. 2009;15:853–859. doi: 10.3748/wjg.15.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Correa P. Helicobacter pylori and gastric carcinogenesis. Am J Surg Pathol. 1995;19(Suppl 1):S37–S43. [PubMed] [Google Scholar]
- 59.Kim N, Park YS, Cho SI, et al. Prevalence and risk factors of atrophic gastritis and intestinal metaplasia in a Korean population without significant gastroduodenal disease. Helicobacter. 2008;13:245–255. doi: 10.1111/j.1523-5378.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 60.Sipponen P, Kekki M, Haapakoski J, Ihamäki T, Siurala M. Gastric cancer risk in chronic atrophic gastritis: statistical calculations of cross-sectional data. Int J Cancer. 1985;35:173–177. doi: 10.1002/ijc.2910350206. [DOI] [PubMed] [Google Scholar]
- 61.Bodger K, Wyatt JI, Heatley RV. Variation in serum pepsinogens with severity and topography of Helicobacter pylori-associated chronic gastritis in dyspeptic patients referred for endoscopy. Helicobacter. 2001;6:216–224. doi: 10.1046/j.1523-5378.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- 62.Broutet N, Plebani M, Sakarovitch C, Sipponen P, Megraud F. Pepsinogen A, pepsinogen C, and gastrin as markers of atrophic chronic gastritis in European dyspeptics. Br J Cancer. 2003;88:1239–1247. doi: 10.1038/sj.bjc.6600877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ricci C, Vakil N, Rugge M, et al. Serological markers for gastric atrophy in asymptomatic patients infected with Helicobacter pylori. Am J Gastroenterol. 2004;99:1910–1915. doi: 10.1111/j.1572-0241.2004.40614.x. [DOI] [PubMed] [Google Scholar]
- 64.Shin CM, Kim N, Lee HS, et al. Validation of diagnostic tests for Helicobacter pylori with regard to grade of atrophic gastritis and/or intestinal metaplasia. Helicobacter. 2009;14:512–519. doi: 10.1111/j.1523-5378.2009.00726.x. [DOI] [PubMed] [Google Scholar]
- 65.Lee JH, Kim N, Chung JI, et al. Long-term follow-up of Helicobacter pylori IgG serology after eradication and reinfection rate of H. pylori in South Korea. Helicobacter. 2008;13:288–294. doi: 10.1111/j.1523-5378.2008.00616.x. [DOI] [PubMed] [Google Scholar]
- 66.Kang HY, Kim N, Park YS, et al. Progression of atrophic gastritis and intestinal metaplasia drives Helicobacter pylori out of the gastric mucosa. Dig Dis Sci. 2006;51:2310–2315. doi: 10.1007/s10620-006-9276-0. [DOI] [PubMed] [Google Scholar]
- 67.Webb PM, Hengels KJ, Moller H, et al. EUROGAST Study Group. The epidemiology of low serum pepsinogen A levels and an international association with gastric cancer rates. Gastroenterology. 1994;107:1335–1344. doi: 10.1016/0016-5085(94)90535-5. [DOI] [PubMed] [Google Scholar]
- 68.Yoshihara M, Sumii K, Haruma K, et al. Correlation of ratio of serum pepsinogen I and II with prevalence of gastric cancer and adenoma in Japanese subjects. Am J Gastroenterol. 1998;93:1090–1096. doi: 10.1111/j.1572-0241.1998.00335.x. [DOI] [PubMed] [Google Scholar]
- 69.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 70.Seoane A, Bessa X, Balleste B, et al. Helicobacter pylori and gastric cancer: relationship with histological subtype and tumor location. Gastroenterol Hepatol. 2005;28:60–64. doi: 10.1157/13070701. [DOI] [PubMed] [Google Scholar]
- 71.Trajkov D, Stardelova K, Dimitrova M, Mishevski J, Serafimoski V. Helicobacter pylori and gastric carcinoma. Prilozi. 2007;28:39–46. [PubMed] [Google Scholar]
- 72.Nardone G. Reveiw article: molecular basis of gastric carcinogenesis. Aliment Pharmacol Ther. 2003;17(Suppl 2):75–81. doi: 10.1046/j.1365-2036.17.s2.10.x. [DOI] [PubMed] [Google Scholar]
- 73.Fujishiro M, Oka M, Yahagi N, et al. Correlation of serum pepsinogens and gross appearances combined with histology in early gastric cancer. J Exp Clin Cancer Res. 2006;25:207–212. [PubMed] [Google Scholar]
- 74.Hansen S, Vollset SE, Derakhshan MH, et al. Two distinct aetiologies of cardia cancer: evidence from premorbid serological markers of gastric atrophy and Helicobacter pylori status. Gut. 2007;56:918–925. doi: 10.1136/gut.2006.114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kokkola A, Louhimo J, Puolakkainen P, Alfthan H, Haglund C, Rautelin H. Helicobacter pylori infection and low serum pepsinogen I level as risk factors for gastric carcinoma. World J Gastroenterol. 2005;11:1032–1036. doi: 10.3748/wjg.v11.i7.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kitahara F, Shimazaki R, Sato T, Kojima Y, Morozumi A, Fujino MA. Severe atrophic gastritis with Helicobacter pylori infection and gastric cancer. Gastric Cancer. 1998;1:118–124. doi: 10.1007/s101200050005. [DOI] [PubMed] [Google Scholar]
- 77.Kiyohira K, Yoshihara M, Ito M, Haruma K, Tanaka S, Chayama K. Serum pepsinogen concentration as a marker of Helicobacter pylori infection and the histologic grade of gastritis; evaluation of gastric mucosa by serum pepsinogen levels. J Gastroenterol. 2003;38:332–338. doi: 10.1007/s005350300060. [DOI] [PubMed] [Google Scholar]
- 78.Yamagata H, Kiyohara Y, Aoyagi K, et al. Impact of Helicobacter pylori infection on gastric cancer incidence in a general Japanese population: the Hisayama study. Arch Intern Med. 2000;160:1962–1968. doi: 10.1001/archinte.160.13.1962. [DOI] [PubMed] [Google Scholar]
- 79.Yim JY, Kim N, Choi SH, et al. Seroprevalence of Helicobacter pylori in South Korea. Helicobacter. 2007;12:333–340. doi: 10.1111/j.1523-5378.2007.00504.x. [DOI] [PubMed] [Google Scholar]