Abstract
Background/Aims
The incidence of treatment failure or recurrence of Clostridium difficile-associated diarrhea (CDAD) following metronidazole treatment has increased recently. We studied the treatment failure, recurrence rate, and risk factors predictive of treatment failure and recurrence after metronidazole treatment for CDAD.
Methods
We retrospectively identified consecutive patients who were admitted and treated for CDAD at a single tertiary institution in Korea over a recent 10-year period (i.e., 1998-2008).
Results
Metronidazole was administered as the initial treatment to 111 of 117 patients (94.9%) with CDAD. Fourteen patients (12.6%) had no clinical response to the metronidazole treatment, and in 13 patients (13.4%) CDAD recurred after successful metronidazole treatment. Diabetes mellitus (p=0.014) and sepsis (p=0.002) were independent risk factors for metronidazole treatment failure. Patients who had received surgery within 1 month before CDAD developed were more likely to experience a recurrence after metronidazole treatment (p=0.032). Vancomycin exhibited a higher response rate after treatment failure, and metronidazole showed a reasonable response rate in the treatment of recurrence. Treatment failure and recurrence rates increased with time after metronidazole treatment for CDAD over the 10-year study period.
Conclusions
Our data suggest that diabetes mellitus and sepsis are independent risk factors for metronidazole treatment failure, and that operation history within 1 month of development of CDAD is a predictor of a recurrence after metronidazole treatment.
Keywords: Clostidium difficile-associated diarrhea, Metronidazole, Vancomycin
INTRODUCTION
Clostidium difficile (C. difficile) is one of the most common causes of nosocomial infectious diarrhea, which accounts for 15-20% of antibiotic-induced diarrhea.1,2 Metronidazole has been the drug of choice in the treatment of C. difficile-associated diarrhea (CDAD). Metronidazole is less expensive, but it has been shown to be as effective as vancomycin. However, treatment failure or recurrence with the metronidazole treatment has increased.3,4 Explanations for this include an increased use of broad-spectrum antibiotic and the emergence of more virulent and highly antibiotic-resistant strains of C. difficile.5 However, there have been few studies concerning the data on the clinical risk factors predictive of treatment failure or recurrence after metronidazole treatment. Moreover, most investigations regarding CDAD exclusively came from Western countries. The effectiveness or the resistance to antibiotics for CDAD varies by region or country.5 Therefore, we evaluated the CDAD treatment failure rate and recurrence rate over the past 10 years at a single tertiary hospital in South Korea (hereafter Korea) and examined the clinical risk factors associated with the treatment failure and recurrences.
MATERIALS AND METHODS
A retrospective review was performed to identify patients who had been admitted to Severance Hospital between March 1, 1998 and August 1, 2008 with a diagnosis of CDAD. Severance Hospital is a 2,000-bed tertiary hospital, affiliated with Yonsei University College of Medicine in Seoul, Korea. The diagnosis of CDAD was confirmed by a positive enzyme linked immunoassay for cytotoxin A (TOX A/B QUIK CHEK; Techlab, Blacksburg, VA, USA) in patients with diarrhea or documented pseudomembranous colitis (PMC) which was seen on a sigmoidscopy or colonoscopy.6 PMC was defined as yellow-white plagues loosely adherent to the inflamed colorectal mucosa.6 No outpatients were included in this study. Patients were excluded from the study if: 1) they did not complete five days of initial antibiotic treatment for CDAD; 2) they had a coexistent gastrointestinal disease; or 3) clinical patient data was not completely recorded. In this study, treatment response was defined as the complete resolution of all symptoms (bowel movement >3 times per day, fever >38℃, and abdominal pain) and no positive toxin assay after the treatment.7 Treatment failure was defined as the persistence or the incomplete resolution of the symptoms, or positive toxin assay after 10 days of the treatment.3 Cytotoxin assay or culture was followed up only in the case of incomplete resolution of the symptoms. Recurrence was defined as the reappearance of either a symptom or a positive toxin assay within 90 days of the treatment response.4 Additional information about age, sex, combined comorbidity, place of residence, intensive care unit (ICU) admission, antibiotics used, symptom (fever, abdominal pain, bowel movement, and etc.), and laboratory findings (including WBC count, albumin level, liver function, and renal function) were obtained from the patients' charts. The severity of CDAD was assessed using the previously described criteria.6 CDAD severity was defined to be severe if pseudomembranous colitis was found in endoscopic examination, or the patient was treated in the intensive care unit, or two or more of the following conditions took place: age >60 years, temperature >38.3℃, WBC >15,000 cells/mm3, albumin level <2.5 mg/dL. For the metronidazole treatment, our clinic administered a dose of 1.5 g/day of oral metronidazole, and administered intravenous metronidazole to six patients because of their inability to be treated orally. For the vancomycin treatment, a dose of 250 mg of oral vancomycin was administered to all patients four times a day.
1. Statistical analysis
Univariate comparison of the groups was performed using the independent Student's t test for continuous variables and Fisher's exact test for categorical variables. To estimate independent risk factors for the treatment failure and recurrence, the multiple logistic regression analysis was used. A p value of <0.05 was considered to be significant. Data are expressed as means±standard deviation. The statistical package used was SPSS version 12.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
1. Patient characteristics and clinical courses
We identified 208 patients who were diagnosed as CDAD by reviewing medical records. Among these patients, a total of 117 patients qualified for this study, while the other patients were excluded because of insufficient information available from medical records (n=71), insufficient evidence of CDAD (n=12), or no treatment (n=8). The mean age of the patients was 62.5 years±13.86 (range, 23-88). Fifty-four patients were male and 63 were female (ratio, 1:1.6). PMC developed in 58 patients (49.6%), and 59 patients (50.4%) had positive toxin assay. Fifteen patients (12.8%) had both of PMC findings and positive toxin assay. Fourteen patients (12%) were diagnosed of sepsis and patients with sepsis were statistically more likely to maintain their previous antibiotics during CDAD (p=0.002). Durations of precipitating antibiotics use in sepsis were not different between metronidazole response (12.2±6.5 days) and failure group (13.0±5.5 days) (p=0.882). Types of antibiotics were not related to metronidazole failure in patients with sepsis (p=0.532). All patients (6 patients) with sepsis in metronidazole failure group had severe CDAD, while 4 of 6 (66.7%) in metronidazole response group had severe disease (p=0.078). Nine patients (7.7%) were treated in ICU. Twenty eight patients had diabetes mellitus, and the mean of glycated hemoglobin (HbA1c) was 6.3±1.7%.
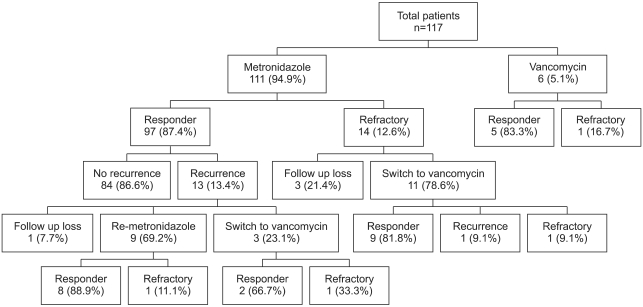
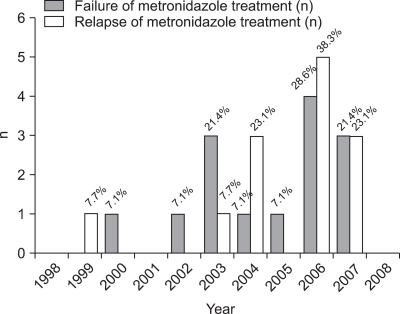
A flow chart of the clinical courses taken after CDAD treatment is shown in Fig. 1. Fourteen patients (12.6%) showed no positive reaction to the metronidazole treatment, and 10 out of 11 patients (90.9%) responded to vancomycin after the initial metronidazole treatment failed. After a successful metronidazole treatment, recurrence occurred in 13 out of 97 patients (13.4%). Among nine patients who were treated with metronidazole again, eight patients (88.9%) responded to the treatment. The other three patients were treated with vancomycin after the recurrence, and two patients responded (66%). The metronidazole treatment failure and recurrence after the treatment are compared according to years in Fig. 2. The rate of metronidazole treatment failure over the last three years (2006-2008) was 13.7 % (7 of 51 patients) while the period between 1998-2005 recorded 11.7% (7 of 60 patients). Moreover, the recurrence rate after successful metronidazole treatment over the last three years was 19.0% (8 of 42 patients), and that between 1998-2005 was 9.1% (5 of 55 patients).
Fig. 1.
Of 117 patients with C. difficile-associated diarrhea (CDAD), 111 (94.9%) received an initial treatment of metronidazole. Of these, 14 patients (12.6%) had no positive reaction. Of the responders, 13 (13.4%) experienced CDAD recurrence after successful metronidazole treatment. After failure of metronidazole failed, 10 out of 11 patients (90.9%) subsequently responded to vancomycin. In the cases of recurrence, eight out of nine patients (88.9%) responded to repeated metronidazole treatment.
Fig. 2.
Metronidazole treatment failure rates over a recent 3-year period (2006-2008) were 13.7% (7 of 51 patients), compared with 11.7% (7 of 60 patients) between 1998 and 2005. Moreover, the recurrence rate after successful metronidazole treatment over the 3-year period from 2006 to 2008 was 19.0% (8 of 42 patients), and the rate between 1998 and 2005 was 9.1% (5 of 55 patients).
2. Factors associated with metronidazole failure
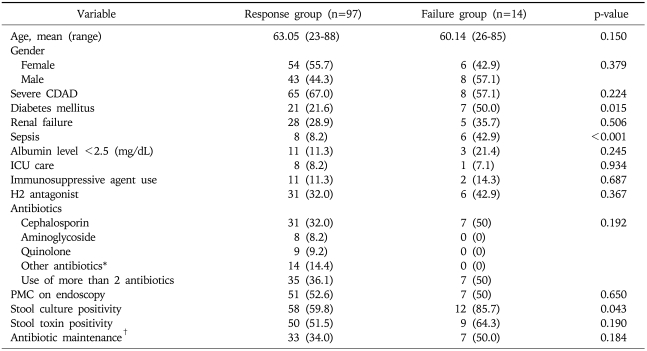
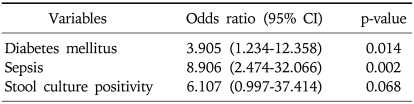
One of the major objectives in our study is to uncover risk factors for metronidazole treatment failure. Patients with diabetes mellitus and those with sepsis were less likely show a positive response to the metronidazole treatment for CDAD (p=0.015 and p<0.01, respectively) (Table 1). Multivariate analysis still supports a positive correlation between diabetes mellitus/sepsis and treatment failure (p=0.014 with an odd ratio of 3.905 [95% confidence interval, 1.234-12.358] and p=0.002 with an odd ratio of 8.906 [95% confidence interval, 2.474-32.066], respectively). Patients whose stool culture was positive for C. difficile were more likely to fail in the metronidazole treatment (p=0.043) in univariate analysis, but this correlations was not significant in the regression analysis correlation (p=0.068) (Table 2). Age, gender, renal failure, leukocytosis, albumin level <2.5 g/dL, stool toxin, finding of PMC on endoscopy, ICU admission, place of residence, use of immunosuppressive agent, use of histamine-2 receptor antagonists, use of proton pump inhibitor, kinds of antibiotic used for previous infection, duration of antibiotics, antibiotics maintenance during CDAD or any abdominal symptoms were not related to the failure of metronidazole treatment. In the analysis of patients with diabetes mellitus, HbA1c level was not significantly different between metronidazole response group and metronidazole failure group (8.11±1.12 vs 7.45±1.84%). Moreover, well controlled diabetes mellitus (HbA1c<7%) was not associated with metronidazole treatment response (p=0.615).
Table 1.
Comparisons between the Response and Failure Groups Following Metronidazole Treatment
Values are presented as mean (range) or number (%).
CDAD, C. Difficile-associated diarrhea; H2 antagonist, histamine-2 receptor antagonist; ICU, intensive care unit; PMC, pseudomembranous colitis.
*Other antibiotics indicate all kind of antibiotics except cephalosporin, aminoglycoside and quinolone; †Antibiotic maintenance indicates receiving previous antibiotics during CDAD.
Table 2.
Multivariate Analysis of Risk Factor for Failure of Metronidazole Treatment Failure
CI, confidence interval.
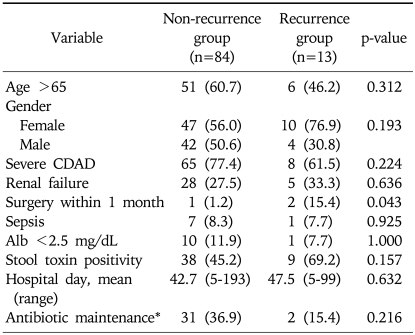
3. Factors associated with recurrence after metronidazole treatment
Patients who had surgery within a month before developing CDAD are more likely to recur after treatment, which was significant (p=0.043). An age greater than 65 years, female gender, length of hospital stay, place of residence, renal failure, white blood cell count, maintenance of antibiotics, or any other clinical symptoms were not found to be related to a recurrence after the metronidazole treatment (Table 3). In multivariate analysis, a history of surgery within a month before the treatment was found to be positively correlated with the recurrence of CDAD (p=0.032) with an odd ratio of 14.667 (95% confidence interval, 1.234-174.273).
Table 3.
Comparisons between the Recurrence and Nonrecurrence Groups after Successful Metronidazole Treatment
Values are presented as mean (range) or number (%).
CDAD, C. difficile-associated diarrhea.
*Indicate receiving previous antibiotics during CDAD.
DISCUSSION
C. difficile is associated with an uneven balance between normal colon bacterial flora and pathogenic bacteria. As a treatment of CDAD, metronidazole and vancomycin have been estimated to be more efficient than other antibiotics.8 A randomized trial comparing the efficacy of metronidazole and vancomycin for CDAD illustrated equivalent efficiency of the two antibiotics.9 The appearance of vancomycin-resistant enterococci and a low cost have made metronidazole the drug of choice in CDAD treatment since the late 1980s. However, increasing rates of treatment failure and recurrence have been reported since the late 1990s. In two recent studies, treatment failure rates of metronidazole were reported to be 38% and 78%, respectively.3,10 Pepin et al. reported the recurrence rate to be as high as 28.9% in their large-scale retrospective study. Moreover, another study reported the recurrence rate as 28% after using metronidazole.7,10 Therefore, our retrospective review focused on the results of metronidazole in the treatment of CDAD and the risk factors predictive of metronidazole treatment failure. This information is important because it enhances understanding in selecting patients who might benefit from vancomycin as an initial treatment of CDAD.
In our study, most patients (94.9%) were initially treated with metronidazole. The response rate of metronidazole (87.4%) was high compared to the reports mentioned above. Based on our study, it is too early to generalize whether metronidazole is sufficient to be the first treatment option for CDAD. Our results were consistent with a recent study conducted in Korea. Byun et al.11 reported that metronidazole seemed to be effective for CDAD treatment in a retrospective study on 86 Korean patients. However, we found a recently rising trend of metronidazole treatment failure. Complementary to our results, Pepin et al.10 reported increased treatment failures in the 2000s comparing to the 1990s. In North America, there were outbreaks of CDAD after the emergence of C. difficile ribotype 027/NAP-1 pulsotype in the early 2000s, and both the metronidazole treatment failure rate and the recurrence rate increased compared to the 1990s.10 Currently, the reason of increasing tendency in the treatment failure rate is not clear, but one possible explanation is an increase in the emergence of strains resistant to antibiotics. The recurrence of CDAD should be carefully monitored in the future, and a strain assay should be performed if treatment failure increases continuously.
Next, we evaluated risk factors for the metronidazole treatment failure. In our study, risk factors with metronidazole failure were the presence of diabetes mellitus and sepsis. Diabetic patients are more vulnerable to infection and more likely to have gastrointestinal motility problems.12 However, to our knowledge, there has been no evidence that the status of hyperglycemia is related to the metronidazole refractory CDAD, which indicates that this is a novel finding in our study. Future studies are needed to validate the relationship and pathophysiology between diabetes mellitus and drug resistant CDAD.
There are several possible explanations for the association between sepsis and treatment failure. First, the tendency of prolonged use of previous antibiotics during CDAD could be a possible reason. In our study, patients with sepsis were more likely to continue using the previously used antibiotics while treating CDAD. The maintenance of the previous antibiotics or prolonged use of antibiotics continues to delay normal flora from recovering.4,13 However, duration of antibiotics use and specific types of antibiotics that were administered to patients with sepsis were not related with metronidazole treatment failure in our study. Considering small number of patients in our study, the clarification of these relationships need further investigations. Second, in most patients with sepsis in our study, CDAD severity was quite high (85.7%). Moreover, all patients with sepsis in metronidazole failure group had severe CDAD in our study. In a previous study, severe CDAD was more likely to cause metronidazole treatment failure than moderate or mild CDAD.6 Although we found no direct relationship between CDAD severity and metronidazole failure in our study, severity of CDAD could be one explanation of our findings.
After metronidazole treatment failure, vancomycin seemed to be efficient in most patients in our study. Patients who were treated with vancomycin after metronidazole failure showed a significant reduction of C. difficile concentration.14 Vancomycin could be considered an effective treatment option when metronidazole fails to treat CDAD.
Third, we tried to determine the risk factors for recurrence after metronidazole treatment. In our study, only the surgical history a month before the treatment was found to be a related risk factor of recurrence after metronidazole treatment. Gastrointestinal surgery has been reported as a risk factor for developing CDAD, but no specific kind of surgery was reported to be related with recurrence.15 In our study, two patients underwent gastrointestinal tract surgery - one patient underwent colectomy for cancer, and another small bowel resection for perforation - and one orthopedic surgery (amputation of the diabetic foot). It is perhaps understood that surgery of the gastrointestinal tract or pelvis changes normal flora and may cause CDAD. However, the reason for its relation to the treatment recurrence is not necessarily certain.15 In our study, only a small number of patients had a history of surgery. Therefore, a larger confirmative study would be necessary to draw a solid conclusion concerning this issue.
After the documentation of treatment recurrence, 69.2% of our patients were treated with metronidazole again, and the response rate was 88.9% and was considered efficient. However, vancomycin has a broad spectrum for gastrointestinal flora16 and tapering of vancomycin was efficient in treating recurred CDAD.17 Proper antibiotics for recurred CDAD should be chosen with careful consideration of the clinical situation. In our study, only six patients were initially treated with vancomycin, and CDAD recurred in one patient. The recurrence rates between metronidazole and vancomycin treatments were similar in our study (12.6% vs 16.7%, respectively). Correspondingly, in another study there was no difference in the recurrence rate between the initial treatment of vancomycin and that of metronidazole.18 Considering our small number of vancomycin treatment group, further studies regarding the relationship between recurrence and the initial treatment of CDAD are needed.
Our study has several limitations. This study can of course be affected by all the limitations of a retrospective design. The number of patients was relatively small and collected in a single institution. Finally, no strain of C. difficille was identified in this study.
In conclusion, treatment failure and recurrence rates increase after metronidazole treatment for CDAD, as indicated in our study. Vancomycin showed a higher response rate after treatment failure, and metronidazole still showed a reasonable response rate in the treatment of recurrence. Our data suggest that diabetes mellitus and sepsis are risk factors for the failure of metronidazole therapy of CDAD, and the history of surgery within a month before the treatment is a risk factor of recurrence.
References
- 1.Barbut F, Corthier G, Charpak Y, et al. Prevalence and pathogenicity of Clostridium difficile in hospitalized patients. A French multicenter study. Arch Intern Med. 1996;156:1449–1454. [PubMed] [Google Scholar]
- 2.Bartlett JG. Clostridium difficile: history of its role as an enteric pathogen and the current state of knowledge about the organism. Clin Infect Dis. 1994;18(Suppl 4):S265–S272. doi: 10.1093/clinids/18.supplement_4.s265. [DOI] [PubMed] [Google Scholar]
- 3.Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40:1586–1590. doi: 10.1086/430311. [DOI] [PubMed] [Google Scholar]
- 4.Nair S, Yadav D, Corpuz M, Pitchumoni CS. Clostridium difficile colitis: factors influencing treatment failure and relapse--a prospective evaluation. Am J Gastroenterol. 1998;93:1873–1876. doi: 10.1111/j.1572-0241.1998.00541.x. [DOI] [PubMed] [Google Scholar]
- 5.Kuijper EJ, Wilcox MH. Decreased effectiveness of metronidazole for the treatment of Clostridium difficile infection? Clin Infect Dis. 2008;47:63–65. doi: 10.1086/588294. [DOI] [PubMed] [Google Scholar]
- 6.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302–307. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez A, Anand G, Friedenberg F. Factors associated with failure of metronidazole in Clostridium difficile-associated disease. J Clin Gastroenterol. 2004;38:414–418. doi: 10.1097/00004836-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 8.McFarland LV. Epidemiology, risk factors and treatments for antibiotic-associated diarrhea. Dig Dis. 1998;16:292–307. doi: 10.1159/000016879. [DOI] [PubMed] [Google Scholar]
- 9.Teasley DG, Gerding DN, Olson MM, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile-associated diarrhoea and colitis. Lancet. 1983;2:1043–1046. doi: 10.1016/s0140-6736(83)91036-x. [DOI] [PubMed] [Google Scholar]
- 10.Pepin J, Alary ME, Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40:1591–1597. doi: 10.1086/430315. [DOI] [PubMed] [Google Scholar]
- 11.Byun TJ, Han DS, Ahn SB, et al. Clinical characteristics and changing epidemiology of Clostridium difficile-associated disease (CDAD) Korean J Gastroenterol. 2009;54:13–19. doi: 10.4166/kjg.2009.54.1.13. [DOI] [PubMed] [Google Scholar]
- 12.Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N Engl J Med. 1999;341:1906–1912. doi: 10.1056/NEJM199912163412507. [DOI] [PubMed] [Google Scholar]
- 13.Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med. 2002;346:334–339. doi: 10.1056/NEJMcp011603. [DOI] [PubMed] [Google Scholar]
- 14.Al-Nassir WN, Sethi AK, Nerandzic MM, Bobulsky GS, Jump RL, Donskey CJ. Comparison of clinical and microbiological response to treatment of Clostridium difficile-associated disease with metronidazole and vancomycin. Clin Infect Dis. 2009;47:56–62. doi: 10.1086/588293. [DOI] [PubMed] [Google Scholar]
- 15.Barbut F, Petit JC. Epidemiology of Clostridium difficile-associated infections. Clin Microbiol Infect. 2001;7:405–410. doi: 10.1046/j.1198-743x.2001.00289.x. [DOI] [PubMed] [Google Scholar]
- 16.Edlund C, Barkholt L, Olsson-Liljequist B, Nord CE. Effect of vancomycin on intestinal flora of patients who previously received antimicrobial therapy. Clin Infect Dis. 1997;25:729–732. doi: 10.1086/513755. [DOI] [PubMed] [Google Scholar]
- 17.McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97:1769–1775. doi: 10.1111/j.1572-0241.2002.05839.x. [DOI] [PubMed] [Google Scholar]
- 18.Pépin J, Routhier S, Gagnon S, Brazeau I. Management and outcomes of a first recurrence of Clostridium difficile-associated disease in Quebec, Canada. Clin Infect Dis. 2006;42:758–764. doi: 10.1086/501126. [DOI] [PubMed] [Google Scholar]