Abstract
Background/Aims
Endoscopic resection has proven to be a safe and effective alternative to surgery for duodenal adenomas. However, few data are available on the adequacy of resection and long-term outcomes. This study evaluated the efficacy and longterm endoscopic findings in a cohort of Korean patients who underwent endoscopic mucosal resection (EMR) of sporadic duodenal adenomas.
Methods
Seventeen patients with nonampullary duodenal adenomas without familial polyposis syndrome and who were treated by EMR between January 2001 and December 2007 were evaluated retrospectively. Their management, follow-up, and outcomes were reviewed.
Results
In total, seventeen lesions were removed from EMR in 17 patients (mean age, 59.3 years; 6 women, 11 men). The mean size of the tumors was 15.1 mm (median, 13 mm, range, 8-27 mm). Of these 17 adenomas, 16 adenomas were tubulous and 1 was tubulovillous. The EMR was performed successfully in all 17 patients in a single session. After a median follow-up period of 29 months (range, 13-72 months), all patients remained in remission. One patient had bleeding at the site of the EMR. There were no perforations after the EMR.
Conclusions
EMR for sporadic duodenal adenomas seemed to be a safe and effective treatment modality.
Keywords: Duodenal neoplasm, Adenoma, Endoscopic mucosal resection, Treatment efficacy
INTRODUCTION
Although duodenal adenomas are common in patients with familial adenomatous polyposis,1-3 sporadic duodenal adenomas are rare. In a large retrospective endoscopic series from Germany, only 6.9% of 378 duodenal polyps identified in the course of more than 25,000 esophagogastroduodenoscopy (EGD) procedures were adenomatous.4 One prospective study from Denmark reported that 0.4% of 584 patients had duodenal adenomas.5 With the increasing widespread use of endoscopy, these tumors are being diagnosed more frequently. These lesions are of particular interest because of the adenoma-carcinoma sequence accepted as the progression of colorectal tumors, which has also been postulated to be associated with that of the small bowel.6,7 Since 30-85% of duodenal adenomas undergo malignant transformation, surgical or endoscopic excision is mandatory.7-13 However, the optimal approach to management of these lesions remains to be determined. The standard treatment for duodenal adenomas has been either local excision or pancreaticoduodenectomy.9,10 Although local excision is an organ-preserving operation, it has been associated with high recurrence rates.9,12 By contrast, pancreaticoduodenectomy is a very effective treatment. However, it may be associated with perioperative morbidity, mortality, and long-term complications affecting the quality of life.7,12 In the early 1970s, the first endoscopic resection of a duodenal adenoma was performed.14 Since then several studies have reported endoscopic resection as a safe and effective alternative to surgery in patients with benign duodenal adenomas and for those that were poor surgical candidates.13,15-19 However, data on the adequacy of the resection and long-term outcomes are limited.
This study evaluated the efficacy and long-term endoscopy findings in a cohort of Korean patients that underwent endoscopic excision of sporadic nonampullary duodenal adenomas.
MATERIALS AND METHODS
1. Patients
From January 2001 to December 2007, 17 nonampullary duodenal adenomas in 17 patients were treated by endoscopic mucosal resection (EMR) at two teaching hospitals (Uijeongbu St. Mary's Hospital and St. Vincent's Hospital). All lesions were histopathologically diagnosed by preoperative endoscopic biopsy specimens. These adenomas were identified during EGD performed due to symptoms unrelated to adenomas in all of the patients. Patients were excluded if they had polyposis syndromes or if the lesion was in major or minor papilla. The diameter of polyp was estimated in relation to the opened biopsy forceps. Endoscopic ultrasonography (EUS) was rarely used. Instead, resectability was evaluated at the time of the EMR. This study was performed in accordance with guidelines of the Institutional Review Board, which approved the study. All the participants provided written informed consent before the procedure.
2. EMR technique
Midazolam was administered intravenously for sedation, and the cadiorespiratory functions was monitored. The EMR procedure was performed using a single-channel gastroduodenoscope (GIF XQ240, Q240 and Q260; Olympus, Tokyo, Japan). The relationship between the lesion and the papilla was routinely assessed, using a side-viewing instrument if necessary. Before the resection, normal saline solution in combination with dilute epinephrine (1 in 10,000) was injected into the submucosa near the tumor through the needle forceps until the tumor was completely elevated, as part of an artificial wound. For lesions extending over a fold, the distal part of the tumor was injected first to maintain visualization of the lesion. The tumor was then captured with a snare device and removed by electrocoagulation with a blended current (Fig. 1). Two different electrosurgical generators were used during the study. Prior to 2005, a Olympus UES-10 (Olympus), with a blended current of 60 watts cut and 30 watts coagulation, was used in nine cases. After 2005, an ERBE electrosurgical unit (VIO 300; ERBE, Tübingen, Germany) set to Endocut Q, Effect 3, delivering a cut duration of 2 milliseconds and a cut interval of 1,200 milliseconds was used. The techniques were individualized based on the morphology and the location of the lesions. The lesions that could not be resected, as a whole, underwent a series of resections (piecemeal technique). Occasionally, argon plasma coagulation (APC2; ERBE) was used to eradicate residual adenoma tissue. To control bleeding during the EMR or to prevent possible bleeding from visible vessels in the artificial ulcer, immediately after the procedure, either an injection of 1:10000 epinephrine solution, clipping with hemoclips (HX-600-135, 135 S hemoclip; Olympus, Tokyo, Japan), monopolar electrocoagulation, or argon plasma coagulation were used. After the endoscopic intervention, the patients were monitored for 48 hours to rule out any complications resulting from the procedure. Delayed bleeding was defined as clinical evidence of bleeding, as evidenced by melena within 0-30 days after EMR and a need for endoscopic treatment. When delayed bleeding occurred, the patient underwent repeat endoscopy. If macroscopic or histological evidence of residual adenomatous tissue was detected in the resected area, at the next control endoscopy (within 3 months), a second mucosal resection was performed. Complete resection of the adenoma was defined as no residual adenomatous tissue observed.
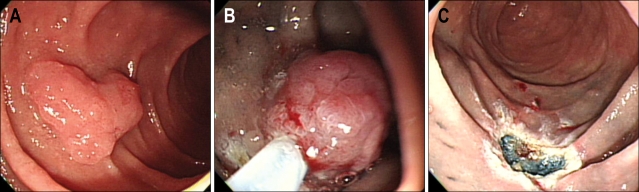
Fig. 1.
Endoscopic images of the endoscopic mucosal resection (EMR) procedure. (A) Adenoma in the second part of the duodenum. (B) EMR procedure. (C) The site after the EMR.
3. Histological analysis
All tissues were retrieved, and pinned on a corkboard for pathological assessment. In cases with piecemeal-resected lesions, the dominant part was pinned on the corkboard and the remaining pieces were analyzed separately. All specimens were reviewed by pathologists that specialized in GI pathology. The adenoma was classified as low- or high-grade according to the Vienna classification.20 A complete resection was considered to have a lesion-free margin when both the lateral and basal tissues were free of the lesion.
4. Follow-up assessment
Patients that had a complete resection with endoscopically clear margins were scheduled for follow up within six months and then annually. At subsequent endoscopies, "remission" of the adenoma was defined as a normal endoscopic appearance with no visible residual adenomatous tissue and negative results from biopsy specimens; a "recurrence" was defined as macroscopic or histological evidence of the lesion.
RESULTS
Seventeen patients were included in this retrospective study. The demographic characteristics are presented in Table 1. There were 11 men and six women with a mean age of 59.3 years. Eight of the seventeen patients (47%) underwent gastroscopy for mass screening of gastric cancer. Six patients presented with abdominal discomfort, two presented with anemia, and one with weight loss.
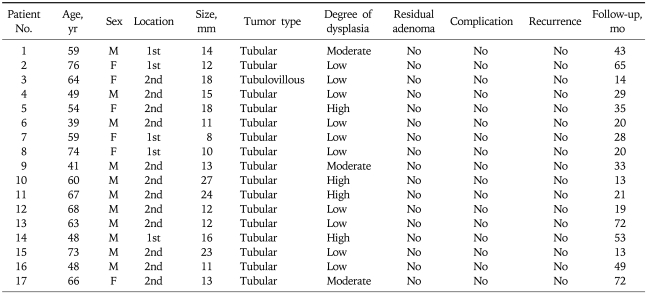
Table 1.
The Clinical and Pathological Characteristics of 17 Duodenal Adenomas Treated by Endoscopic Mucosal Resection
Five of the adenomas (29%) were found in the first part of the duodenum, and 12 (71%) in the second part of the duodenum. In all cases, the macroscopic appearance of the tumor was an elevated type without ulcerations. The mean size of the tumors was 15.1 mm (median, 13 mm; range, 8-27 mm). EMR was performed on 17 lesions; 14 en bloc resections (mean size, 13.1 mm) and three piecemeal (mean size, 24.7 mm) resections. The completeness of the resection was first evaluated by histological analysis and was stated to be complete 12 EMR procedures (71%) and indeterminate in 5 (29%), either due to coagulation necrosis (12%) or because spatial reconstruction was not performed after piecemeal resection (17%). Sixteen adenomas were tubulous and one was tubulovillous. The degree of dysplasia was low-grade in 10 cases, moderate in three cases and high-grade in four cases. None of the resected specimens showed any evidence of carcinoma. The EMR was performed successfully in all 17 patients during a single session. Additional argon plasma coagulation was used for residual adenomatous tissue in three patients whose lesions were treated by piecemeal resection. There were neither perforations nor clinically significant immediate bleeding. One case with early bleeding developed within 48 hours after the procedure and was successfully treated with combination therapy of epinephrine injection and hemoclipping. Even if three patients complained of abdominal pain or discomfort after the procedure, they had no local abdominal tenderness or radiographic evidence of free air in the abdomen. No blood transfusions were required.
All 17 patients underwent at least one follow-up endoscopy after the lesion was resected. The first follow-up endoscopy did not show any residual adenomatous tissue either macroscopically or histologically on the biopsies taken at the resection sites. After a median endoscopic follow-up of 29 months (range, 13-72 months), all patients remained in remission. Among these patients, 12 are still under surveillance, five had no signs of recurrence and decided not to continue with follow-up.
DISCUSSION
The results of this study demonstrate the safety and long-term efficacy of EMR of sporadic nonampullary duodenal adenomas. EMR is an effective alternative to open surgery for patients with superficial neoplastic lesions of the gastrointestinal tract not amenable to endoscopic resection with standard electrocautery techniques.16 EMR has the advantage over open surgery in that it provides the benefits of less invasiveness, shorter hospitalized time, and lower costs.21,22 Sporadic duodenal adenomas are uncommon, and their endoscopic resection has traditionally been considered as of high risk due to the thin duodenal wall.23 Therefore, there have been only a few studies on duodenal EMR over a small number of patients.13,15-19
In the present study, the standard inject-and-cut EMR technique was used to treat the duodenal adenomas. The largest tumor resected by this method was 27 mm in diameter. During the initial treatment phase, remnant adenomatous tissue at the site of the EMR was observed in three patients (17.6%) whose lesions were resected by the piecemeal technique. Adjuvant ablative therapies such as the use of APC or electrocoagulation might be used to destroy residual or recurrent adenomatous tissue not removed during primary snare resection attempts.13,24 In this study, all three patients with remnant adenomatous tissue were subsequently treated with APC, and at a median follow-up of 29 months they had no evidence of remnant adenomas. Alexander et al.19 described 23 patients with large duodenal adenomas treated with EMR; an EMR was performed on 21 lesions, including eight en bloc resections and 13 piecemeal resections with remnant tissue found in 23.8% of cases. Although they have shown that patients with minor residual adenomas were treated successfully with additional snare resection and/or APC, piecemeal resection might be associated with higher rates of residual tumor or recurrence. In addition, the precise histopathological assessment of piecemeal resections could not be determined. These results suggest that en bloc EMR is more effective in preventing recurrence. Another means of minimizing adenoma recurrence or residual tumor application of the new technologies such as narrowband imaging or high-magnification chromoendoscopy at the time of EMR to examine the resected margin. By using high-magnification chromoendoscopy after EMR of colonic flat lesions more than 2 cm, Hurlstone et al.25 showed a reduction of local neoplastic recurrence from 8.7% to 0.5%. Further long-term follow-up studies specifically examining this technique in the duodenum are required. Recurrence from piecemeal resection can perhaps be eliminated by using endoscopic submucosal dissection (ESD). A Japanese group has performed ESD in nine superficial duodenal neoplasms;26 the ESD required a long operation time and was associated with a high risk for complications such as perforation and delayed bleeding. ESD for duodenal lesions is technically difficult because the duodenum is anatomically fixed to the retroperitoneum. In addition, abundant blood vessels in the submucosal layer and a thin muscle layer are thought to be related to a serious risk for bleeding and perforation. Further development of techniques and instruments are needed for safe and successful ESD procedures in the duodenum.
The recognized complications of EMR include bleeding, pain, perforation, and stricture formation. Bleeding is the most common complication and usually occurs during or within 24 hours of the procedure. Early bleeding in the duodenum has been reported in up to 33% of patients, and it is the most common complication associated with this procedure.16 The reported frequency of bleeding during an EMR for a duodenal adenoma range from 4% to 33%.13,15-19 In the present study, early bleeding occurred in 6% of the cases and was treated accordingly with epinephrine injection and the use of clips. In addition, perforation or delayed bleeding rarely occurred during the removal of duodenal adenomas in this study. The frequency of delayed bleeding can be reduced by prevention measures such as APC or clipping. Primary closure of the resected area by clips is preferable to APC because it does not increase tissue injury after EMR. Moreover, primary closure might also treat perforation in difficult cases. These efficacies need to be confirmed in a prospective and randomized study. Also, closure of the resected area is probably not possible for very large adenomas. One limitation of this study was the retrospective design with the potential for both the underreporting of complications and selection bias. The selection bias was minimized by including all patients with large, sessile, sporadic non-ampullary duodenal adenomas identified within the database over the study period.
In conclusion, the results of this study showed that the majority of lesions in the duodenum can be resected using the standard EMR technique. This treatment method helps to reduce the need for open surgery and offers an acceptable complication rate that can be managed by endoscopy. Careful endoscopic follow-up is necessary to treat recurrent or residual lesions.
References
- 1.Yao T, Ida M, Ohsato K, Watanabe H, Omae T. Duodenal lesions in familial polyposis of the colon. Gastroenterology. 1977;73:1086–1092. [PubMed] [Google Scholar]
- 2.Sarre RG, Frost AG, Jagelman DG, Petras RE, Sivak MV, McGannon E. Gastric and duodenal polyps in familial adenomatous polyposis: a prospective study of the nature and prevalence of upper gastrointestinal polyps. Gut. 1987;28:306–314. doi: 10.1136/gut.28.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Domizio P, Talbot IC, Spigelman AD, Williams CB, Phillips RK. Upper gastrointestinal pathology in familial adenomatous polyposis: results from a prospective study of 102 patients. J Clin Pathol. 1990;43:738–743. doi: 10.1136/jcp.43.9.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Höchter W, Weingart J, Seib HJ, Ottenjann R. Duodenal polyps. Incidence, histologic substrate and significance. Dtsch Med Wochenschr. 1984;109:1183–1186. doi: 10.1055/s-2008-1069345. [DOI] [PubMed] [Google Scholar]
- 5.Jepsen JM, Persson M, Jakobsen NO. Prospective study of prevalence and endoscopic and histopathologic characteristics of duodenal polyps in patients submitted to upper endoscopy. Scand J Gastroenterol. 1994;29:483–487. doi: 10.3109/00365529409092458. [DOI] [PubMed] [Google Scholar]
- 6.Sellner F. Investigations on the significance of the adenoma-carcinoma sequence in the small bowel. Cancer. 1990;66:702–715. doi: 10.1002/1097-0142(19900815)66:4<702::aid-cncr2820660419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Sakorafas GH, Friess H, Dervenis CG. Villous tumors of the duodenum: biologic characters and clinical implications. Scand J Gastroenterol. 2000;35:337–344. doi: 10.1080/003655200750023877. [DOI] [PubMed] [Google Scholar]
- 8.Perzin KH, Bridge MF. Adenomas of the small intestine: a clinicopathologic review of 51 cases and a study of their relationship to carcinoma. Cancer. 1981;48:799–819. doi: 10.1002/1097-0142(19810801)48:3<799::aid-cncr2820480324>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 9.Krukowski ZH, Ewen SW, Davidson AI, Matheson NA. Operative management of tubulovillous neoplasms of the duodenum and ampulla. Br J Surg. 1988;75:150–153. doi: 10.1002/bjs.1800750221. [DOI] [PubMed] [Google Scholar]
- 10.Galandiuk S, Hermann RE, Jagelman DG, Fazio VW, Sivak MV. Villous tumors of the duodenum. Ann Surg. 1988;207:234–239. doi: 10.1097/00000658-198803000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghilain JM, Dive C. Endoscopic laser therapy for small villous adenomas of the duodenum. Endoscopy. 1994;26:308–310. doi: 10.1055/s-2007-1008973. [DOI] [PubMed] [Google Scholar]
- 12.Farnell MB, Sakorafas GH, Sarr MG, et al. Villous tumors of the duodenum: reappraisal of local vs. extended resection. J Gastrointest Surg. 2000;4:13–21. doi: 10.1016/s1091-255x(00)80028-1. [DOI] [PubMed] [Google Scholar]
- 13.Apel D, Jakobs R, Spiethoff A, Riemann JF. Follow-up after endoscopic snare resection of duodenal adenomas. Endoscopy. 2005;37:444–448. doi: 10.1055/s-2005-861287. [DOI] [PubMed] [Google Scholar]
- 14.Haubrich WS, Johnson RB, Foroozan P. Endoscopic removal of a duodenal adenoma. Gastrointest Endosc. 1973;19:201. doi: 10.1016/s0016-5107(73)74009-8. [DOI] [PubMed] [Google Scholar]
- 15.Hirasawa R, Iishi H, Tatsuta M, Ishiguro S. Clinicopathologic features and endoscopic resection of duodenal adenocarcinomas and adenomas with the submucosal saline injection technique. Gastrointest Endosc. 1997;46:507–513. doi: 10.1016/s0016-5107(97)70005-1. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad NA, Kochman ML, Long WB, Furth EE, Ginsberg GG. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc. 2002;55:390–396. doi: 10.1067/mge.2002.121881. [DOI] [PubMed] [Google Scholar]
- 17.Oka S, Tanaka S, Nagata S, et al. Clinicopathologic features and endoscopic resection of early primary non-ampullary duodenal carcinoma. J Clin Gastroenterol. 2003;37:381–386. doi: 10.1097/00004836-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Lépilliez V, Chemaly M, Ponchon T, Napoleon B, Saurin JC. Endoscopic resection of sporadic duodenal adenomas: an efficient technique with a substantial risk of delayed bleeding. Endoscopy. 2008;40:806–810. doi: 10.1055/s-2008-1077619. [DOI] [PubMed] [Google Scholar]
- 19.Alexander S, Bourke MJ, Williams SJ, Bailey A, Co J. EMR of large, sessile, sporadic nonampullary duodenal adenomas: technical aspects and long-term outcome (with videos) Gastrointest Endosc. 2009;69:66–73. doi: 10.1016/j.gie.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 20.Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–255. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanabe S, Koizumi W, Mitomi H, et al. Clinical outcome of endoscopic aspiration mucosectomy for early stage gastric cancer. Gastrointest Endosc. 2002;56:708–713. doi: 10.1067/mge.2002.129085. [DOI] [PubMed] [Google Scholar]
- 22.Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc. 2003;57:567–579. doi: 10.1067/mge.2003.130. [DOI] [PubMed] [Google Scholar]
- 23.Ponchon T. Endoscopic mucosal resection. J Clin Gastroenterol. 2001;32:6–10. doi: 10.1097/00004836-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Standards of, Adler DG, Qureshi W, et al. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc. 2006;64:849–854. doi: 10.1016/j.gie.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 25.Hurlstone DP, Cross SS, Brown S, Sanders DS, Lobo AJ. A prospective evaluation of high-magnification chromoscopic colonoscopy in predicting completeness of EMR. Gastrointest Endosc. 2004;59:642–650. doi: 10.1016/s0016-5107(04)00156-7. [DOI] [PubMed] [Google Scholar]
- 26.Honda T, Yamamoto H, Osawa H, et al. Endoscopic submucosal dissection for superficial duodenal neoplasms. Dig Endosc. 2009;21:270–274. doi: 10.1111/j.1443-1661.2009.00908.x. [DOI] [PubMed] [Google Scholar]