Abstract
Adefovir dipivoxil (ADV) is commonly used as an antiviral agent in the treatment of chronic hepatitis B or human immunodeficiency virus infection. Nephrotoxicity has been shown to occur at daily dosages of 60-120 mg. Fanconi's syndrome is a generalized dysfunction of the renal proximal tubular cells, which is usually accompanied by complications. Here we report a case of Fanconi's syndrome in a chronic hepatitis B patient who had been treated with a prolonged regimen of ADV at 10 mg/day. A 47-year-old man complained of severe back and chest-wall pain. He had chronic hepatitis B and had been treated with ADV at a daily dose of 10 mg for 38 months. He was hospitalized because of severe bone pain, and laboratory and radiologic findings suggested a diagnosis of Fanconi's syndrome with osteomalacia. After discontinuation of the ADV, he recovered and was discharged from hospital. His laboratory findings had normalized within 2 weeks. This case indicates that Fanconi's syndrome can be acquired by a chronic hepatitis B patient taking ADV at a conventional dosage of 10 mg/day. Therefore, patients treated with long-term ADV should be checked regularly for the occurrence of ADV-induced Fanconi's syndrome.
Keywords: Fanconi syndrome, Adefovir, Chronic hepatitis B
INTRODUCTION
Fanconi's syndrome results from a generalized dysfunction of the proximal renal tubule causing impaired reabsorption of amino acids, glucose, urate, bicarbonate, and phosphate and increased excretion of these solutes into the urine. The resulting electrolyte imbalance and osteomalacia cause symptoms of muscle weakness, fatigue, bone pain, and fractures.1,2
Adefovir dipivoxil (ADV), the prodrug of adefovir and a structurally similar acyclic nucleoside analogue, is known to cause adverse events when used in the treatment of human immunodeficiency virus (HIV) infection at dosages of 60 to 120 mg/day and has been shown to exhibit nephrotoxicity, typically taking the form of a gradual increase in serum creatinine with a concomitant decrease in serum phosphorus.3 However, when used at a dose of 10 mg/day, ADV shows an adverse-event profile similar to that of a placebo over the course of 1 year.4
Here we report a case of Fanconi's syndrome in a hepatitis B virus (HBV) patient treated with ADV at a dose of 10 mg daily.
CASE REPORT
A 47-year-old man visited the hospital for evaluation of multiple joint pain. He complained of severe back and chest wall pain as well as knee joint pain over the previous 6 months. The pain was initially dull and aching but worsened during physical activity, and it had gotten worse to the point that he could walk only with the assistance of a cane. He was treated lamivudine during 20 months and lamivudine-resistance M204V mutation was found. After that, he had been taking adefovir during the previous 38 months. At the time he began taking adefovir, his laboratory findings were: alanine aminotransferase (ALT), 481 IU/L; HBe antigen positive; and HBV-DNA, 2.1×107 copies/mL (bDNA assay, detection limit 2000 copies/mL). Once ADV therapy began, the ALT was normalized at 3 months, and HBV-DNA was undetectable at 20 months. Medications at the time of evaluation included zonisamide, carbamazepine, valproic acid, and clonazepam prescribed for epilepsy, for which the patient had been diagnosed eight years earlier. Then there were no other health problems. On physical examination, he had normal muscle strength. He had tenderness over the lumbar spine area and anterior chest wall, but he did not show any neurologic symptoms.
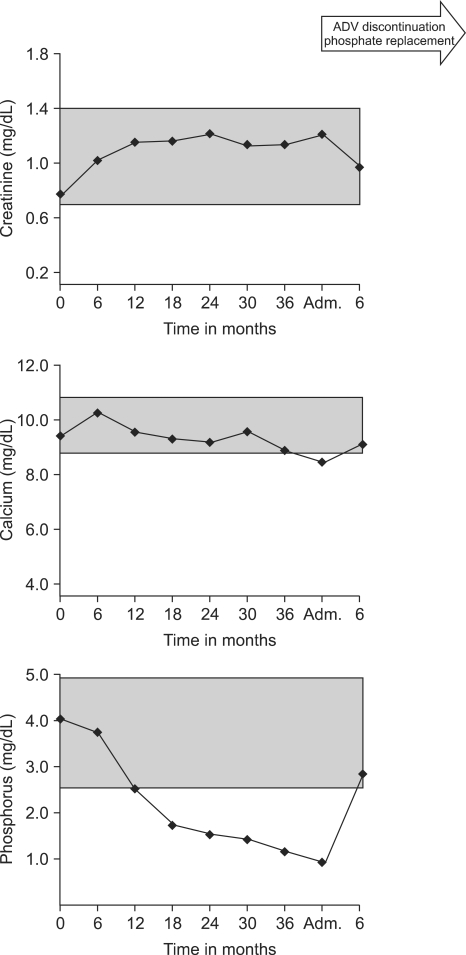
Analysis of his laboratory data revealed a serum sodium of 135 meq/L and chloride of 104 meq/L. He had a slightly elevated creatinine level of 1.35 mg/dL (normal range, 0.7-1.3 mg/dL). Serum bicarbonate was low normal (22.7 mEq/L), with an anion gap of 8.3, demonstrating a hyperchloremic non-anion gap metabolic acidosis. Mild hypokalemia (K of 3.3 mM; normal range, 3.5-5.0 mM) was found. Serum albumin was 4.3 g/dL, calcium was low normal (8.5 mg/dL; normal range, 8.4-10.2 mg/dL). A decline in serum phosphate to 1.3 mg/dL (normal range, 2.5-4.5 mg/dL) and a rise in ALP activity to 321 U/L (normal range, 38-126 U/L) was noted. In addition, we analyzed serial levels of serum phosphate and calcium by using stored samples with the consent of patient (Fig. 1). Urine studies revealed polyuria (over 4 L/day). Glucosuria and proteinuria in the absence of hyperglycemia was noted. Therefore, given the presence of a hyperchloremic nonanion gap metabolic acidosis, hypokalemia, hypophosphatemia, glucosuria, and proteinuria, we suspected acquired Fanconi's syndrome in this patient.
Fig. 1.
Laboratory test results before and after hospital admission. The graph shows a dramatic change in electrolytes after adefovir dipivoxil (ADV) discontinuation and phosphate replacement. The shaded area indicates the normal reference range.
Additional serologic and radiologic tests were performed to confirm the diagnosis. The PTH level was normal (20 pg/mL; normal range, 10-65 pg/mL). Vitamin D levels were as follows: the 25(OH) vitamin D level was 31.8 ng/mL (normal range, 9-52 ng/mL), and the 1,25(OH)2 vitamin D level was 19.1 pg/mL (normal range, 15-60 pg/mL). A 24-hour urinary study showed an increased phosphate of 440 mg/day (normal range, 70-220 mg/day) and increased glucose excretion of up to 5,068 mg/day (normal range, 500-1,500 mg/day).
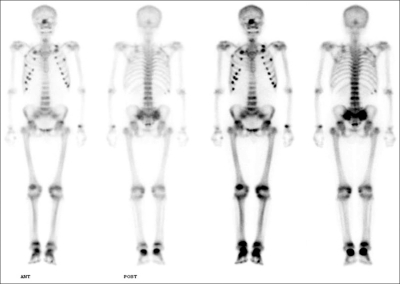
The radiological evaluation included a technetium pertechnetate bone scan, which showed increased uptake in the lumbar spine, multiple ribs, both knees, and both ankle joints (Fig. 2). X-rays of these areas showed lumbar spine spondylosis and multiple rib fractures. Dual-energy X-ray absorptiometry (DXA) showed a decreased lumbar spine bone mineral density (BMD) of 0.316 g/cm2 (T-score, -3.4) and a total hip BMD of 0.50 g/cm2 (T-score, -3.2).
Fig. 2.
Technetium pertechnetate bone scan showing multifocal osteoblastic lesions involving the bilateral ribs, lumbar spine, hip joints, and ankle joints.
Because we excluded other causes, it was thought to be caused by interstitial nephritis secondary to the antiviral medication. So, the patient stopped adefovir and was treated with phosphate. Several laboratory parameters improved, and the joint pain also decreased so that the patient no longer needed a cane for ambulation, and pain medication requirements decreased dramatically.
Sixteen days after admission, the patient left the hospital with an oral phosphorus supplement. Two weeks after discharge, he was given follow-up blood tests, which showed that the serum creatinine was 0.92 mg/dL, potassium was 4.8 meq/L, phosphorus was 2.4 mg/dL, serum bicarbonate was 25 meq/L, and the metabolic acidosis had resolved. The patient no longer reported bone pain.
DISCUSSION
We have reported a case of Fanconi's syndrome with complications in a chronic hepatitis B patient taking adefovir at a dose of 10 mg per day. The patient demonstrated several features of acquired Fanconi's syndrome, including bone pain, pathologic fractures, electrolyte and mineral abnormalities, and evidence of renal tubular dysfunction.
Fanconi's syndrome results from a generalized dysfunction of the proximal renal tubule causing impaired reabsorption of amino acids, glucose, urate, bicarbonate, and phosphate and an increased excretion of these solutes into the urine. The electrolyte abnormalities and osteopenia cause the symptoms of muscle weakness, fatigue, bone pain, and pseudofractures.1,2 Osteomalacia is common in Fanconi's syndrome.5 Normal calcium, low phosphate, normal 25(OH) vitamin D and 1,25(OH)2 vitamin D levels, normal PTH, and elevated ALP imply renal phosphorus loss, which is characteristic of Fanconi's syndrome. The bone scan pattern in this patients was focal and multiple, similar to the typical pattern of osteomalacia.6
Drug-induced Fanconi's syndrome is associated with the use of a number of medications and chemicals, including aminoglycosides,7 valproate,8 methyl-3-chromone,9 paraquat,10 L-lysine,11 tetracycline,12 and ifosfamide.13 There have also been several cases reported of Fanconi's syndrome in HIV patients treated with antinucleoside antiretrovirals.14-17
Some mechanisms of drug-induced Fanconi's syndrome have been suggested. The multidrug-resistance-associated protein 2 (Mrp2) superfamily of transporters has been localized on the luminal membrane of proximal tubule cells. Molecular biology has also identified organic anion transporters (OATs).18,19 Adefovir and cidofovir enter the renal cells on OAT1 and exit on Mrp2.20 The ATP-driven drug efflux pump Mrp2 is one transporter responsible for nucleoside phosphonate efflux from renal cells.21 Because Mrp2 mediates the efflux of adefovir from proximal tubule cells, competitive interactions at the transporter may result in a reduced efflux, intracellular accumulation, and increased toxicity.22
Nephrotoxicity associated with high doses of adefovir (60-120 mg) typically has a late onset and is dose dependent. Data from a randomized double-blind comparison of ADV, at doses of 60 and 120 mg once daily, suggest that nephrotoxicity is less frequent with the lower dosage. Of a group of patients treated with 60 or 120 mg/day, 27% and 50%, respectively, had a serum phosphate level less then 2.0 mg/dL after 42 weeks of treatment, with an associated serum creatinine level increase of 0.5 mg/dL or more above baseline.23 On the other hand, it had been established that adefovir at a dose of 10 mg/day does not appear to produce clinically significant nephrotoxicity. A long-term safety and efficacy study that examined a 144-week trial showed only 4 patients (3%) with confirmed increases in serum creatinine that were greater than or equal to 0.5 mg/dL above pretreatment values. But no patient had confirmed serum hypophosphatemia while on ADV therapy.24
In our literature review, we found a few report of severe hypophosphatemia resulting from a conventional dose of 10 mg per day.25 For this reason, most clinicians do not include calcium and phosphorus in routine clinical monitoring for adefovir therapy. However, in this case, we found that Fanconi's syndrome did occur with a conventional dose. So all clinicians treating chronic hepatitis B patients with adefovir over a long period of time should carefully observe the patient's symptoms and monitor renal function and electrolytes, including calcium and phosphorus; if an adverse reaction like this is noted, an alternative drug should be considered (excluding tenofovir, which can cause similar problems).
In addition to the antiviral agent, the patient had been taking valproic acid, which can cause Fanconi's syndrome by causing proximal tubular damage by the intracellular accumulation and concentration of valproate before its secretion into the tubular lumen.26 Thus it is difficult to determine with certainty whether the adverse reaction we observed occurred secondary to therapy with one of the drugs or possibly because of the combination of the two agents. We do not know why this patient experienced Fanconi's syndrome with osteomalacia, but this may in part have been due to the delay in diagnosis despite early hypophophatemia. In addition, the use of valproate for the previous 8 years may have led to an underlying osteomalacia and synergistic nephrotoxicity. However, most of patients treated with valproate reported on in published studies have been severely disabled, bedridden, or wheelchair-bound children.27 With respect to this issue, perhaps the most important fact of our case is that the patient's clinical data and symptoms improved greatly after discontinuing adefovir while he was still taking valproic acid because of his epilepsy.
In this case, we found that Fanconi's syndrome can be acquired in a chronic hepatitis B patient taking adefovir at a conventional dose of 10 mg per day. We do not know exactly why only this patient has developed Fanconi's syndrome with osteomalacia in response to the drug treatment. However, we propose that chronic hepatitis B patients who take adefovir over a long period of time should be checked with regular laboratory monitoring, including tests of serum creatinine, phosphorus, and calcium. If the laboratory tests indicate that Fanconi's syndrome is possible, we suggest replacing adefovir with a different antiviral agent other than tenofovir in order to prevent complications.
ACKNOWLEDGEMENTS
The authors declare no conflict of interest.
References
- 1.Earle KE, Seneviratne T, Shaker J, Shoback D. Fanconi's syndrome in HIV+ adults: report of three cases and literature review. J Bone Miner Res. 2004;19:714–721. doi: 10.1359/jbmr.2004.19.5.714. [DOI] [PubMed] [Google Scholar]
- 2.Laing CM, Toye AM, Capasso G, Unwin RJ. Renal tubular acidosis: developments in our understanding of the molecular basis. Int J Biochem Cell Biol. 2005;37:1151–1161. doi: 10.1016/j.biocel.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Kahn J, Lagakos S, Wulfsohn M, et al. Efficacy and safety of adefovir dipivoxil with antiretroviral therapy: a randomized controlled trial. JAMA. 1999;282:2305–2312. doi: 10.1001/jama.282.24.2305. [DOI] [PubMed] [Google Scholar]
- 4.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med. 2003;348:800–807. doi: 10.1056/NEJMoa021812. [DOI] [PubMed] [Google Scholar]
- 5.Clarke BL, Wynne AG, Wilson DM, Fitzpatrick LA. Osteomalacia associated with adult Fanconi's syndrome: clinical and diagnostic features. Clin Endocrinol (Oxf) 1995;43:479–490. doi: 10.1111/j.1365-2265.1995.tb02621.x. [DOI] [PubMed] [Google Scholar]
- 6.Fogelman I, McKillop JH, Bessent RG, Boyle IT, Turner JG, Greig WR. The role of bone scanning in osteomalacia. J Nucl Med. 1978;19:245–248. [PubMed] [Google Scholar]
- 7.Melnick JZ, Baum M, Thompson JR. Aminoglycoside-induced Fanconi's syndrome. Am J Kidney Dis. 1994;23:118–122. doi: 10.1016/s0272-6386(12)80820-1. [DOI] [PubMed] [Google Scholar]
- 8.Yoshikawa H, Watanabe T, Abe T. Fanconi syndrome caused by sodium valproate: report of three severely disabled children. Eur J Paediatr Neurol. 2002;6:165–167. doi: 10.1053/ejpn.2002.0585. [DOI] [PubMed] [Google Scholar]
- 9.Otten J, Vis HL. Acute reversible renal tubular dysfunction following intoxication with methyl-3-chromone. J Pediatr. 1968;73:422–425. doi: 10.1016/s0022-3476(68)80124-6. [DOI] [PubMed] [Google Scholar]
- 10.Vaziri ND, Ness RL, Fairshter RD, Smith WR, Rosen SM. Nephrotoxicity of paraquat in man. Arch Intern Med. 1979;139:172–174. [PubMed] [Google Scholar]
- 11.Lo JC, Chertow GM, Rennke H, Seifter JL. Fanconi's syndrome and tubulointerstitial nephritis in association with L-lysine ingestion. Am J Kidney Dis. 1996;28:614–617. doi: 10.1016/s0272-6386(96)90476-x. [DOI] [PubMed] [Google Scholar]
- 12.Varavithya W, Chulajata R, Ayudthya PS, Preeyasombat C. Fanconi syndrome caused by degraded tetracycline. J Med Assoc Thai. 1971;54:62–67. [PubMed] [Google Scholar]
- 13.Negro A, Regolisti G, Perazzoli F, Davoli S, Sani C, Rossi E. Ifosfamide-induced renal Fanconi syndrome with associated nephrogenic diabetes insipidus in an adult patient. Nephrol Dial Transplant. 1998;13:1547–1549. doi: 10.1093/ndt/13.6.1547. [DOI] [PubMed] [Google Scholar]
- 14.Gaspar G, Monereo A, García-Reyne A, de Guzmán M. Fanconi syndrome and acute renal failure in a patient treated with tenofovir: a call for caution. AIDS. 2004;18:351–352. doi: 10.1097/00002030-200401230-00035. [DOI] [PubMed] [Google Scholar]
- 15.Kazory A, Singapuri S, Wadhwa A, Ejaz AA. Simultaneous development of Fanconi syndrome and acute renal failure associated with cidofovir. J Antimicrob Chemother. 2007;60:193–194. doi: 10.1093/jac/dkm143. [DOI] [PubMed] [Google Scholar]
- 16.Mathew G, Knaus SJ. Acquired Fanconi's syndrome associated with tenofovir therapy. J Gen Intern Med. 2006;21:C3–C5. doi: 10.1111/j.1525-1497.2006.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parsonage MJ, Wilkins EG, Snowden N, Issa BG, Savage MW. The development of hypophosphataemic osteomalacia with myopathy in two patients with HIV infection receiving tenofovir therapy. HIV Med. 2005;6:341–346. doi: 10.1111/j.1468-1293.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 18.Schaub TP, Kartenbeck J, König J, et al. Expression of the conjugate export pump encoded by the mrp2 gene in the apical membrane of kidney proximal tubules. J Am Soc Nephrol. 1997;8:1213–1221. doi: 10.1681/ASN.V881213. [DOI] [PubMed] [Google Scholar]
- 19.Sweet DH, Miller DS, Pritchard JB. Localization of an organic anion transporter-GFP fusion construct (rROAT1-GFP) in intact proximal tubules. Am J Physiol. 1999;276(6 Pt 2):F864–F873. doi: 10.1152/ajprenal.1999.276.6.F864. [DOI] [PubMed] [Google Scholar]
- 20.Masereeuw R, Russel FG, Miller DS. Multiple pathways of organic anion secretion in renal proximal tubule revealed by confocal microscopy. Am J Physiol. 1996;271(6 Pt 2):F1173–F1182. doi: 10.1152/ajprenal.1996.271.6.F1173. [DOI] [PubMed] [Google Scholar]
- 21.König J, Nies AT, Cui Y, Leier I, Keppler D. Conjugate export pumps of the multidrug resistance protein (MRP) family: localization, substrate specificity, and MRP2-mediated drug resistance. Biochim Biophys Acta. 1999;1461:377–394. doi: 10.1016/s0005-2736(99)00169-8. [DOI] [PubMed] [Google Scholar]
- 22.Izzedine H, Launay-Vacher V, Isnard-Bagnis C, Deray G. Drug-induced Fanconi's syndrome. Am J Kidney Dis. 2003;41:292–309. doi: 10.1053/ajkd.2003.50037. [DOI] [PubMed] [Google Scholar]
- 23.Servais A, Lechat P, Zahr N, et al. Tubular transporters and clearance of adefovir. Eur J Pharmacol. 2006;540:168–174. doi: 10.1016/j.ejphar.2006.04.047. [DOI] [PubMed] [Google Scholar]
- 24.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131:1743–1751. doi: 10.1053/j.gastro.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 25.Lee HJ, Choi JW, Kim TN, Eun JR. A case of severe hypophosphatemia related to adefovir dipivoxil treatment in a patient with liver cirrhosis related to hepatitis B virus. Korean J Hepatol. 2008;14:381–386. doi: 10.3350/kjhep.2008.14.3.381. [DOI] [PubMed] [Google Scholar]
- 26.Graf R, Gossrau R, Merker HJ, Schwabe R, Stahlmann R, Nau H. Enzyme cytochemistry combined with electron microscopy, pharmacokinetics, and clinical chemistry for the evaluation of the effects of steady-state valproic acid concentrations on the mouse. Histochemistry. 1985;83:347–358. doi: 10.1007/BF00684382. [DOI] [PubMed] [Google Scholar]
- 27.Knorr M, Schaper J, Harjes M, Mayatepek E, Rosenbaum T. Fanconi syndrome caused by antiepileptic therapy with valproic Acid. Epilepsia. 2004;45:868–871. doi: 10.1111/j.0013-9580.2004.05504.x. [DOI] [PubMed] [Google Scholar]