Abstract
Inflammatory myofibroblastic tumors (IMTs) are solid neoplastic mesenchymal proliferations composed of myofibroblastic spindle cells admixed with inflammatory infiltrates. The documented sites in the gastrointestinal tract include the esophagus, small intestine, colon, appendix, rectum, pancreas, spleen, liver, and Meckel's diverticulum. Biliary IMTs are rare, and IMTs arising from the ampulla of Vater have not been reported previously. Herein we report the case of a 65-year-old woman with an extrahepatic biliary obstruction due to IMT of the ampulla of Vater, and a successful therapeutic approach using endoscopic ultrasonography and endoscopic papillectomy.
Keywords: Inflammatory myofibroblastic tumor, Ampulla of Vater, Endoscopic papillectomy
INTRODUCTION
Inflammatory myofibroblastic tumors (IMTs) or inflammatory pseudotumors are solid neoplastic mesenchymal proliferations composed of myofibroblastic spindle cells admixed with the inflammatory infiltrates of plasma cells, lymphocytes, eosinophils and histiocytes within a collagenous or myxoid matrix.1 However, the etiology and cellular origin of IMTs remain unknown. The most of IMTs often affect children and young adults, with a slight female predominance. IMTs usually have more aggressive behavior in the young patients and are not easy to distinguish from malignancy such as fibrosarcoma histopathologically.1-4 Although the treatment of IMTs is controversial, recently, the lesion is regarded as a neoplasm with a wide spectrum of biological behavior and has been treated surgically because of diagnostic uncertainty and presumed risk of progression.1,5,6 We report the case of a 65-year-old woman with extra-hepatic biliary obstruction (EHBO) due to IMT of the ampulla of Vater, which was evaluated by endoscopic ultrasonography (EUS) and successfully treated by endoscopic papillectomy.
CASE REPORT
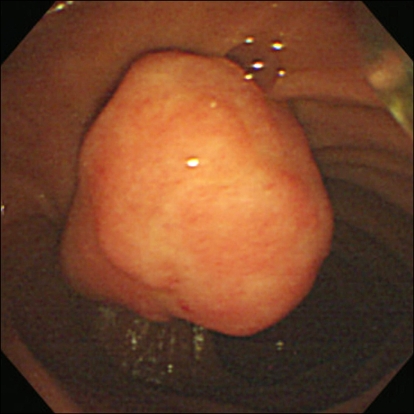
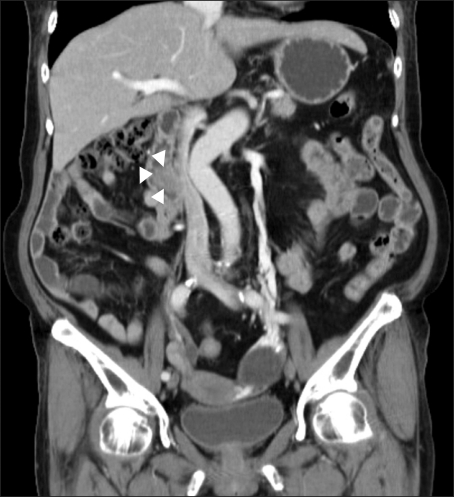
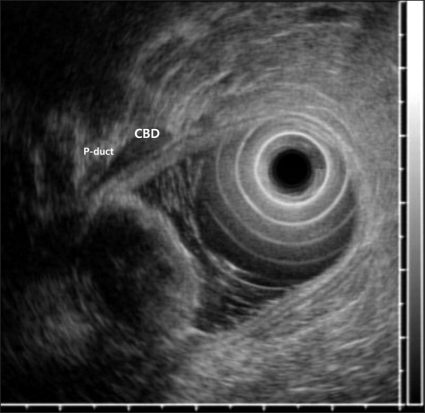
A 65-year-old woman with an unremarkable medical history was investigated for epigastric discomfort. Initial laboratory test results were as follows: white blood cell count, 8.2×109/L (normal, 4.5-10.5×109/L); hemoglobin, 14.7 g/dL; platelet count, 320×109/L (normal, 150-350×109/L); AST, 56 IU/L (normal, 5-38 IU/L); ALT, 146 IU/L (normal, 4-43 IU/L); alkaline phosphatase, 968 IU/L (normal, 102-333 IU/L), total bilirubin, 0.5 mg/dL (normal, 0.2-1.2 mg/dL), amylase, 54 U/L (normal, 45-108 U/L) The results of viral study such as hepatitis A, B, or C were negative. An endoscopy showed a 2.5×2 cm sized, oval shaped lesion on the ampulla of Vater (Fig. 1). Endoscopic biopsy using forcep was obtained, but the pathology only showed chronic inflammation. There was a 2.3×1.7 cm, heterogeneous contrast enhancing mass in the ampulla of Vater area, resulting in mild EHBO without paraduodenal tumor invasion, lymphadenopathy or pancreatic duct dilatation on abdominal computed tomography (CT) (Fig. 2). A EUS showed a well delineated oval-shaped mass with predominant hypoechogenecity with common bile duct dilatation without ductal infiltration (Fig. 3). With the side-view scope, endoscopic papillectomy was performed without complication (Fig. 4).
Fig. 1.
Side endoscopy view showing a 2.5×2-cm oval-shaped elevated mucosal lesion at the ampulla of Vater.
Fig. 2.
Abdominal computed tomography showing a 2.3× 1.7-cm, heterogeneous, contrast-enhanced mass in the ampulla of Vater area (white arrow head) with a mild extrahepatic biliary obstruction.
Fig. 3.
Endoscopic ultrasonography showed a well-delineated, oval-shaped mass with predominant hypoechogenicity and common-bile-duct dilatation.
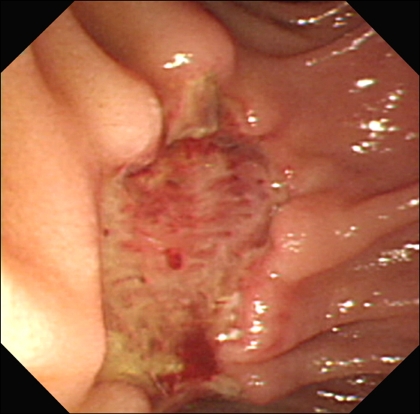
Fig. 4.
Endoscopy revealed a 2.5×3.0-cm-deep ulcer at the ampulla of Vater area after endoscopic papillectomy.
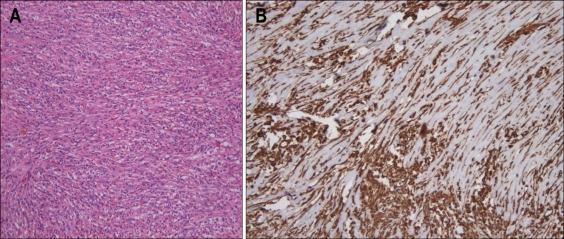
Histopathological examination revealed the completely removed mass of a typical cellular spindle cell proliferation and a prominent inflammatory cell infiltrates without mitotic activity (Fig. 5A), and the margin of which was free. Immunohistochemical staining was positive for vimentin (Fig. 5B) and smooth muscle-specific actin, whereas was negative for S-100 protein. The final pathological diagnosis was the IMT of the ampulla of Vater. In six days after endoscopic papillectomy, the patient got well and discharged. In one year after endoscopic papillectomy, the patient was asymptomatic and underwent endoscopy with no evidence of recurrence.
Fig. 5.
(A) Histological examination demonstrated cytologically uniform spindle-cell proliferation with a prominent inflammatory cell infiltrate (H&E stain, ×200). (B) The spindle cells showed a strong positivity for vimentin (×200).
DISCUSSION
IMTs are rare, but well circumscribed benign tumor-like masses with similar morphological characteristics. Histologically, IMTs are characterized by a benign myofibroblastic proliferation with a vague storiform pattern and a varying degree of inflammatory infilterates but may be characterized by rapid growth and local invasiveness. Moreover, IMTs have clinical importance because the imaging characteristics and locations vary widely and often mimic malignant neoplasm, such as sarcomas, lymphomas or metastases.7 It occur throughout the body such as the central nervous system, major salivary glands, kidney, liver, omentum, ovary, larynx, bladder, breast, lymph nodes, skin, soft tissues, orbit and gastrointestinal tract with the most common site being the lung.8 In the gastrointestinal tract, the documented sites include esophagus, small intestine, colon, appendix, rectum, pancreases, spleen, liver, or a Meckel's diverticulum.9-11 Especially in bilopancreati tract, most IMTs have been reported in pancreas12,13 but the biliary IMT is even rarer.14 Because pancreatic IMT was commonly mistaken for a pancreatic cancer clinically and radiologically, surgical resection was usually known as effective treatment.12,13 A presentation of painless EHBO around the ampulla of Vater is an insidious clinical finding as it is frequently associated with malignancy, typically a pancreatic head tumor or cholangiocarcinoma. Therefore we performed multiple diagnostic work-up to clarify the cause of EHBO and the characteristics of tumor. The diagnosis of IMT is based on the histopathological appearances and is confirmed by immunohistochemical techniques. However endoscopic biopsy may not always be conclusive as our case. Although surgical excision was historically the mainstay of diagnosis and treatment,5 we decided that the lesion had to be removed completely by endoscopic papillectomy because it was located in the endoluminal area without intraductal invasion. The patient got well again and discharged.
In conclusion, we report the case of IMT with EHBO originated from the ampulla of Vater. Accordingly, the ampulla of Vater is the one of organs at which IMT can develop. The present case demonstrates the careful therapeutic approach using EUS and endoscopic papillectomy may be the one of treatment option for limited case of IMT.
References
- 1.Coffin CM, Dehner LP, Meis-Kindblom JM. Inflammatory myofibroblastic tumor, inflammatory fibrosarcoma, and related lesions: an historical review with differential diagnostic considerations. Semin Diagn Pathol. 1998;15:102–110. [PubMed] [Google Scholar]
- 2.Czauderna P, Schaarschmidt K, Komasara L, et al. Abdominal inflammatory masses mimicking neoplasia in children-experience of two centers. Pediatr Surg Int. 2005;21:346–350. doi: 10.1007/s00383-005-1410-0. [DOI] [PubMed] [Google Scholar]
- 3.Karnak I, Senocak ME, Ciftci AO, et al. Inflammatory myofibroblastic tumor in children: diagnosis and treatment. J Pediatr Surg. 2001;36:908–912. doi: 10.1053/jpsu.2001.23970. [DOI] [PubMed] [Google Scholar]
- 4.Scott L, Blair G, Taylor G, Dimmick J, Fraser G. Inflammatory pseudotumors in children. J Pediatr Surg. 1988;23:755–758. doi: 10.1016/s0022-3468(88)80419-6. [DOI] [PubMed] [Google Scholar]
- 5.Ramachandra S, Hollowood K, Bisceglia M, Fletcher CD. Inflammatory pseudotumour of soft tissues: a clinicopathological and immunohistochemical analysis of 18 cases. Histopathology. 1995;27:313–323. doi: 10.1111/j.1365-2559.1995.tb01521.x. [DOI] [PubMed] [Google Scholar]
- 6.Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19:859–872. doi: 10.1097/00000478-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Kim SJ, Kim WS, Cheon JE, et al. Inflammatory myofibroblastic tumors of the abdomen as mimickers of malignancy: imaging features in nine children. AJR Am J Roentgenol. 2009;193:1419–1424. doi: 10.2214/AJR.09.2433. [DOI] [PubMed] [Google Scholar]
- 8.Fukuya T, Honda H, Matsumata T, et al. Diagnosis of inflammatory pseudotumor of the liver: value of CT. AJR Am J Roentgenol. 1994;163:1087–1091. doi: 10.2214/ajr.163.5.7976880. [DOI] [PubMed] [Google Scholar]
- 9.Shah SM, Sussman D, Jorda M, Ribeiro A. EUS with EMR of an inflammatory myofibroblastic tumor of the stomach. Gastrointest Endosc. 2008;67:561–563. doi: 10.1016/j.gie.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 10.Kurihara K, Mizuseki K, Ichikawa M, Okada K, Miyata Y. Esophageal inflammatory pseudotumor mimicking malignancy. Intern Med. 2001;40:18–22. doi: 10.2169/internalmedicine.40.18. [DOI] [PubMed] [Google Scholar]
- 11.Hedenbro JL, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc. 1991;5:20–23. doi: 10.1007/BF00591381. [DOI] [PubMed] [Google Scholar]
- 12.Sim A, Lee MW, Nguyen GK. Inflammatory myofibroblastic tumour of the pancreas. Can J Surg. 2008;51:E23–E24. [PMC free article] [PubMed] [Google Scholar]
- 13.Pungpapong S, Geiger XJ, Raimondo M. Inflammatory myofibroblastic tumor presenting as a pancreatic mass: a case report and review of the literature. JOP. 2004;5:360–367. [PubMed] [Google Scholar]
- 14.Ashcroft MW, Ng CS, Frost RA, Freeman AH. Biliary inflammatory pseudotumour: report of two cases and review of the literature. Clin Radiol. 2009;64:449–455. doi: 10.1016/j.crad.2008.12.004. [DOI] [PubMed] [Google Scholar]