Abstract
Treatment with sorafenib prolongs both the median survival and time to progression by nearly 3 months in patients with advanced hepatocellular carcinoma. Although the effects of combining sorafenib therapy with other anticancer treatment modalities have not been clarified, combination treatment is strongly expected to be beneficial. We report the case of a 50-year-old man who exhibited a partial response and portal vein thrombosis (PVT) revascularization after sorafenib combined with hepatic arterial infusion chemotherapy (HAIC). He exhibited a decrease in tumor size and PVT after 2 months of sorafenib monotherapy. However, no additional response was seen during the subsequent 2 months. To achieve a better tumor response, we combined HAIC with sorafenib. Daily cisplatin (7 mg/m2 on days 1-5) and 5-fluorouracil (170 mg/m2 on days 1-5) were infused repeatedly every 4 weeks, and the sorafenib prescription was modified. After four cycles of combined therapy, both the tumor size and PVT were much improved and exhibited partial response.
Keywords: Hepatocellular carcinoma, Portal vein thrombosis, Sorafenib, Hepatic arterial infusion chemotherapy
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third most common cause of cancer-related death worldwide, behind only lung and stomach cancers.1
When patients is diagnosed with HCC, only 30% of patients is indicated to potentially curative treatments, such as surgical therapies (resection and liver transplantation) and locoregional treatment (radiofrequency ablation and percutaneous ethanol injection).2 For unresectable HCC without vascular invasion or extrahepatic spread, transarterial chemoembolization (TACE) is recommended as the first-line, non-curative therapy.3 However, TACE is contraindicated in HCC with portal vein thrombosis (PVT), and no systemic therapy has improved survival in patients with advanced HCC.4,5 Advanced HCC with PVT has an extremely poor prognosis, with a median survival of only 3 months.6-8 Various therapeutic methods, including surgical resection of PVT,9-11 transcatheter chemoembolization to necrotize PVT,12,13 and arterial or systematic infusion of chemotherapeutic agents14,15 have been used to eliminate PVT. However, the results have been disappointing. Radiation is sometimes effective, but the indication is often limited by the extent of the lesion or impaired liver function.16
Sorafenib is an oral multikinase inhibitor with antiangiogenic and antiproliferative effects. In the multicenter, double-blind, randomized, phase III study, the median survival and time to radiologic progression were nearly 3 months longer for advanced HCC patients treated with sorafenib than for those given placebo.17
Sorafenib is the only systemic treatment shown to be effective against advanced HCC, and combining other anticancer treatment modalities with sorafenib is strongly suggested. Recently sorafenib combined with TACE18 and sorafenib combined with radiation19 have been reported. Here, we report a case of successful treatment combing sorafenib with hepatic arterial infusion chemotherapy (HAIC) of PVT in advanced HCC.
CASE REPORT
A 50-year-old man affected by hepatitis B virus (HBV)-related cirrhosis was hospitalized with abdominal distension due to ascites, in May 2008. He had been diagnosed with liver cirrhosis 5 years previously, but had not been followed up. Upon admission, shifting dullness and pretibial pitting edema were observed. Initial laboratory investigations were as follows: white blood cell count 6,900/mm3, hemoglobin 13.3 g/dL, hematocrit 39.7%, platelets 136,000/mm3, prothrombin time 66.9% (international normalized ratio, 1.41), aspartate aminotransferase 124 IU/L, alanine aminotransferase 122 IU/L, albumin 2.5 g/dL, total bilirubin 1.1 mg/dL, alpha-fetoprotein (AFP) 214.68 ng/mL. Hepatitis B surface antigen was positive and antibody was negative, and hepatitis B e antigen and antibody were negative, and HBV DNA was 2.99×106 copies/mL. The initial liver dynamic computed tomography (CT) revealed an 8.5×7.5-cm sized enhancing mass in the right lobe and multiple daughter nodules in the left lobe, with massive thrombosis in the right portal vein that extended to the main portal vein (Fig. 1). The common hepatic artery and para-aortic lymph nodes were also noted. Chest enhancing CT showed metastatic intrathoracic lymphadenopathy. The clinical stage was stage IV, based on UICC TNM classification (6th edition). Performance status was Eastern Cooperative Oncology Group (ECOG) 1 and Child-Pugh score was B8 and MELD was 7.
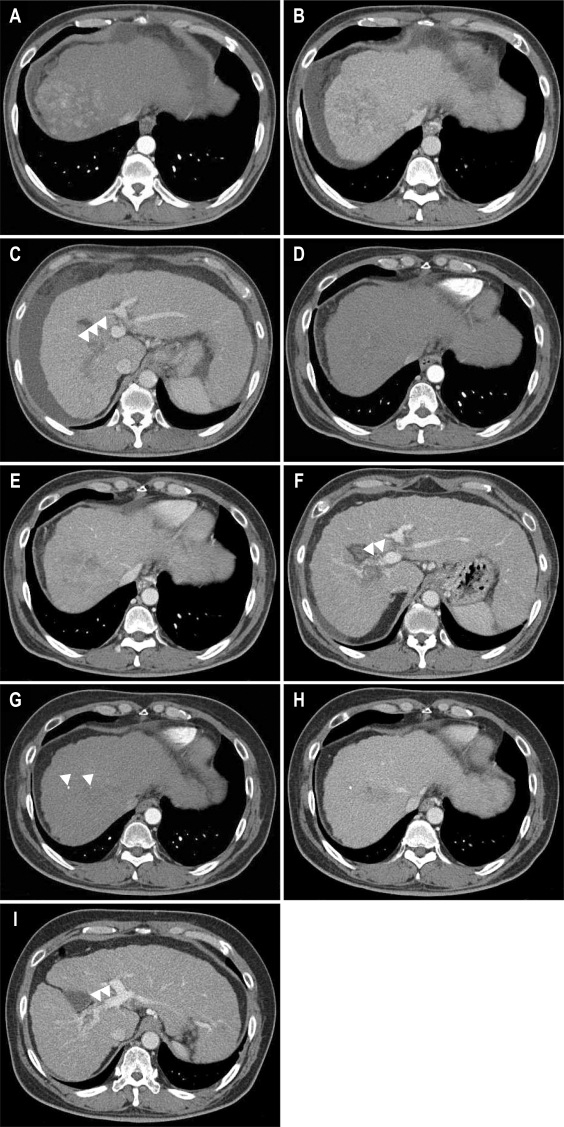
Fig. 1.
Liver dynamic computed tomography images obtained before and after treatment. (A-C) Before treatment. (A) An arterial phase, 8.5×7.5-cm-sized, well-enhanced mass is noted in the hepatic dome. (B) Portal phase, showing mass washout. (C) Portal phase, with the right main portal vein appearing enlarged with massive portal vein thrombosis (arrows). (D-F) Two months after sorafenib monotherapy. (D) Arterial phase, showing that arterial enhancement in the hepatic mass is markedly decreased. (E) Portal phase, showing continued presence of the hypoattenuated mass. (F) Portal phase, showing revascularization of the portal vein thrombosis (arrows). (G-I) Eight months after sorafenib treatment combined with hepatic arterial infusion chemotherapy and transcatheter arterial chemoembolization. (G) Arterial phase, showing no definite arterial enhancement with a tiny amount of lipiodol uptake (arrows). (H) Portal phase, showing a smaller hypoattenuated mass. (I) Portal phase, showing revascularization of the portal vein thrombosis (arrows).
For this case of advanced HCC with PVT and extrahepatic metastasis, we considered systemic chemotherapy and prescribed sorafenib (Nexavar®; 400 mg b.i.d). He also received lamivudine 100 mg/day for HBV-related cirrhosis. After 2 months, the longest diameter of the main tumor had decreased from 8.5 cm to 6.3 cm, and the right main PVT was considerably diminished (Fig. 1). However, no additional response was seen during the subsequent 2 months of sorafenib monotherapy. After 4 months of the initial treatment, tumor response was stable disease (SD) according to the Response Evaluation Criteria in Solid Tumors (RECIST).20 To achieve better tumor response, we combined HAIC with sorafenib.
Daily cisplatin (7 mg/m2 on Days 1-5) and 5-fluorouracil (170 mg/m2 on Days 1-5) were infused repeatedly every 4 weeks via an implantable port system. After 3 cycles of HAIC therapy, liver dynamic CT revealed that both the tumor size and PVT were much improved. Finally, we could perform the first TACE after PVT revascularization. As the common hepatic arteriogram revealed tumor staining in the right lobe, we infused 20 mg of adriamycin and 4 mL of lipiodol into the hepatic branch of segment 7 and 8 and performed gelform embolization. One month after TACE, liver dynamic CT revealed that slight lipiodol uptake was noted at liver dome and right lobe (Fig. 1). Because the lipiodol uptake of HCC in TACE was not satisfactory, we performed a fourth HAIC of cisplatin and 5 FU, as before. By the end of this treatment session, the largest diameter of the main tumor had decreased to 3.3 cm and the right main PVT was diminished (Fig. 1), and the tumor response exhibited partial response (PR), according to the RECIST.20 The AFP was also dropped to 27.49 ng/mL and the intrathoracic lymph nodes were slightly diminished, compared with the initial size.
After the last session of sorafenib combined with HAIC, the patient underwent sorafenib monotherapy, with no change in HCC for 5 months. However, after 5 months of sorafenib monotherapy, HCC aggravated and AFP elevated. We performed TACE. His treatment course is shown in Fig. 2. We have treated this patients for 15 months with sorafenib and combined treatment. After HCC aggravation, we stopped sorafenib.
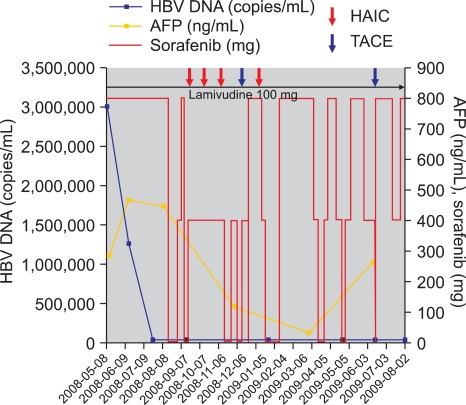
Fig. 2.
Treatment course of sorafenib and combined treatment. HBV, hepatitis B virus; AFP, α-Fetoprotein; HAIC, hepatic arterial infusion chemotherapy; TACE, transarterial chemoembolization.
DISCUSSION
This patient experienced a significant regression of advanced HCC with PVT after sorafenib treatment combined with HAIC. Moreover, PVT revascularization was achieved after sorafenib monotherapy and following HAIC, making the TACE approach possible. This case is the first report of a partial response and PVT revascularization after sorafenib therapy combined with HAIC and TACE.
Although sorafenib is the only proven systemic chemotherapy for HCC, the Sorafenib Hepatocellular Carcinoma Assessment Randomised Protocol (SHARP) trial resulted in no complete responses and a partial response rate of only 2%. However, 71% of the patients exhibited stable disease, and patients who had received sorafenib treatment had a median survival benefit, of nearly a 3-months compared with those who had received placebo.17 Thus, sorafenib combined with other antitumor treatment strategies has been strongly required to increase tumor response and survival rate.
In the case, we are reporting, three important points stand out. First, a 2-month course of sorafenib monotherapy resulted in considerable PVT revascularization. Novi et al.21 have reported HCC improvement and PVT revascularization after 15 weeks of sorafenib monotherapy. For the mechanism for the PVT revascularization of sorafenib, Li et al.22 have suggested that vascular endothelial growth factor may play a pivotal role both in HCC angiogenesis and in PVT onset and evolution, so sorafenib could exert a beneficial effect on PVT by the inhibition of the vascular endothelial growth factor receptor pathway. Second, sorafenib combined with HAIC and TACE subsequent to sorafenib monotherapy, resulted in further tumor response. We suppose that the antitumor effects of HAIC and TACE added to the effect of targeted sorafenib therapy and doubled the tumor response. Third, the toxicity of combined treatment is a substantial problem. During sorafenib treatment combined with HAIC, we modified the sorafenib dose to reduce toxicity and side effects. In the first combined session, sorafenib was stopped 5 days prior to beginning HAIC. After HAIC, sorafenib was resumed at half-dose (200 mg b.i.d.). In the second session, the patient tolerated a half-dose of sorafenib throughout the course of combined treatment. However, during the third session, the patient showed general weakness on third day of HAIC combined with a half-dose of sorafenib. The sorafenib was stopped, and the half-dose was resumed 1 week after treatment. In the fourth session, sorafenib was stopped for a period of 1 week before until 1 week after HAIC to prevent general weakness. The dosage of sorafenib was also modified during TACE, with sorafenib stopped from 1 day before until 1 day after TACE and then resumed at full dose. The patient was more tolerant of sorafenib combined with TACE than sorafenib combined with HAIC. Caution should be observed in combining sorafenib with HAIC because of the toxicity, and the dosage and period of combined treatment should be modified as necessary. Careful patient selection is also essential.
In this case, we stopped sorafenib after HCC aggravation. Although sorafenib resistance has not been elucidated yet, sorafenib resistance and sorafenib chemosensitivity before treatment are most important factors and have to be investigated in sorafenib therapy.
In conclusion, sorafenib combined with intra-arterial chemoinfusion and TACE showed significant tumor regression and PVT-revascularization. Further randomized and controlled study to identify the efficacy and safety of combined treatment is warranted.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 5.Lopez PM, Villanueva A, Llovet JM. Systematic review: evidence-based management of hepatocellular carcinoma--an updated analysis of randomized controlled trials. Aliment Pharmacol Ther. 2006;23:1535–1547. doi: 10.1111/j.1365-2036.2006.02932.x. [DOI] [PubMed] [Google Scholar]
- 6.Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol. 2006;12:7561–7567. doi: 10.3748/wjg.v12.i47.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okuda K, Ohtsuki T, Obata H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 8.Yeung YP, Lo CM, Liu CL, Wong BC, Fan ST, Wong J. Natural history of untreated nonsurgical hepatocellular carcinoma. Am J Gastroenterol. 2005;100:1995–2004. doi: 10.1111/j.1572-0241.2005.00229.x. [DOI] [PubMed] [Google Scholar]
- 9.Asahara T, Itamoto T, Katayama K, et al. Hepatic resection with tumor thrombectomy for hepatocellular carcinoma with tumor thrombi in the major vasculatures. Hepatogastroenterology. 1999;46:1862–1869. [PubMed] [Google Scholar]
- 10.Tanaka A, Morimoto T, Yamaoka Y. Implications of surgical treatment for advanced hepatocellular carcinoma with tumor thrombi in the portal vein. Hepatogastroenterology. 1996;43:637–643. [PubMed] [Google Scholar]
- 11.Yamaoka Y, Kumada K, Ino K, et al. Liver resection for hepatocellular carcinoma (HCC) with direct removal of tumor thrombi in the main portal vein. World J Surg. 1992;16:1172–1177. doi: 10.1007/BF02067093. [DOI] [PubMed] [Google Scholar]
- 12.Furuse J, Iwasaki M, Yoshino M, et al. Hepatocellular carcinoma with portal vein tumor thrombus: embolization of arterioportal shunts. Radiology. 1997;204:787–790. doi: 10.1148/radiology.204.3.9280260. [DOI] [PubMed] [Google Scholar]
- 13.Yen FS, Wu JC, Kuo BI, Chiang JH, Chen TZ, Lee SD. Transcatheter arterial embolization for hepatocellular carcinoma with portal vein thrombosis. J Gastroenterol Hepatol. 1995;10:237–240. doi: 10.1111/j.1440-1746.1995.tb01086.x. [DOI] [PubMed] [Google Scholar]
- 14.Ando E, Yamashita F, Tanaka M, Tanikawa K. A novel chemotherapy for advanced hepatocellular carcinoma with tumor thrombosis of the main trunk of the portal vein. Cancer. 1997;79:1890–1896. doi: 10.1002/(sici)1097-0142(19970515)79:10<1890::aid-cncr8>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 15.Iwamiya T, Sawada S, Ohta Y. Repeated arterial infusion chemotherapy for inoperable hepatocellular carcinoma using an implantable drug delivery system. Cancer Chemother Pharmacol. 1994;33(Suppl):S134–S138. doi: 10.1007/BF00686685. [DOI] [PubMed] [Google Scholar]
- 16.Chen SC, Lian SL, Chang WY. The effect of external radiotherapy in treatment of portal vein invasion in hepatocellular carcinoma. Cancer Chemother Pharmacol. 1994;33(Suppl):S124–S127. doi: 10.1007/BF00686683. [DOI] [PubMed] [Google Scholar]
- 17.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann K, Glimm H, Radeleff B, et al. Prospective, randomized, double-blind, multi-center, Phase III clinical study on transarterial chemoembolization (TACE) combined with sorafenib versus TACE plus placebo in patients with hepatocellular cancer before liver transplantation - HeiLivCa [ISRCTN24081794] BMC Cancer. 2008;8:349. doi: 10.1186/1471-2407-8-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plastaras JP, Kim SH, Liu YY, et al. Cell cycle dependent and schedule-dependent antitumor effects of sorafenib combined with radiation. Cancer Res. 2007;67:9443–9454. doi: 10.1158/0008-5472.CAN-07-1473. [DOI] [PubMed] [Google Scholar]
- 20.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 21.Novi M, Lauritano EC, Piscaglia AC, et al. Portal vein tumor thrombosis revascularization during sorafenib treatment for hepatocellular carcinoma. Am J Gastroenterol. 2009;104:1852–1854. doi: 10.1038/ajg.2009.140. [DOI] [PubMed] [Google Scholar]
- 22.Li Q, Xu B, Fu L, Hao XS. Correlation of four vascular specific growth factors with carcinogenesis and portal vein tumor thrombus formation in human hepatocellular carcinoma. J Exp Clin Cancer Res. 2006;25:403–409. [PubMed] [Google Scholar]