Abstract
Purpose
The retinal pigment epithelium (RPE) is a major source for endothelin-1 (ET-1), a potent vasoactive peptide, at the outer blood–retinal barrier. Factors that regulate ET-1 synthesis at this site may help identify its normal function and its role in pathologic states accompanying retinal injury. Thrombin is one such factor that might act on the RPE after injury and breakdown of the blood–retinal barrier. The present study was conducted to identify signaling intermediates in thrombin-induced ET-1 synthesis and secretion in primary human RPE (hRPE) and transformed RPE cells (ARPE-19) and a possible pharmacological strategy to block excess release of ET-1.
Methods
Cultured hRPE cells were treated with different concentrations of thrombin and thrombin receptor agonists, and a time course to measure levels of preproET-1 (ppET-1) mRNA and secreted mature ET-1 was performed. Levels of secondary messengers [Ca2+]i and RhoA were measured and pharmacologically inhibited to determine how receptor-mediated thrombin activity lead to changes in ET-1 levels.
Results
Thrombin primarily acts via the protease-activated receptor-1 (PAR-1) subtype in RPE to induce ET-1 synthesis. Thrombin and other receptor agonists increased both [Ca2+]i and active RhoA. PAR-1-dependent rho/Rho kinase activation led to increase in ppET-1 mRNA and mature ET-1 secretion.
Conclusions
Transient intracellular calcium mobilization and protein kinase C activation by thrombin play a minor role, if any, in ET-1 synthesis in RPE. Instead, rho/Rho kinase activation after PAR-1 stimulation strongly increased ppET-1 mRNA and ET-1 secretion in hRPE cells.
Introduction
The retinal pigment epithelium (RPE) forms the outer blood–retinal barrier. This barrier prevents macromolecules and blood-borne substances infiltrating the neural retina from the vascular choroidal side.1 Additionally, the RPE offers metabolic and neurotrophic support to the apically located photoreceptors and the basally located choroid. Growth factors like vascular endothelial growth factor and fibroblast growth factor 2 that are secreted by the RPE2 may play important roles in regulating its microenvironment either in an autocrine and/or paracrine manner. Endothelin-1 (ET-1) is secreted by RPE cells in vitro and is expressed in situ in the RPE and photoreceptor layers and the inner retina.3–8
ET-1, −2, and −3 are potent regulators of blood flow.9–11 ET-1 is one of the most potent vasoconstrictors implicated in several cardiovascular and developmental defects.9,12 It is widely expressed and secreted mainly by endothelial and epithelial cells, which are critical in maintaining blood–organ barriers.13 Elevated levels of ET-1 have been reported in conditions that breach the blood–organ barrier, notably in cerebral ischemia and stroke after subarachnoid hemorrhage,14 pre-eclampsia, and eclampsia15,16 that involve placental ischemia after breakdown of the blood–placental barrier, and in certain carcinomas.17 Little is known about how choroidal and retinal vascular insults influence ET-1 secretion and the role of ET-1 at this region.
Thrombin, a blood-derived serine protease, is rapidly produced at sites of tissue injury, resulting in platelet aggregation and clot formation.18 Additionally, thrombin is a potent inducer of ET-1 synthesis and secretion in porcine aortic endothelial cells.19,20 Thrombin acts via protease-activated receptors (PAR)-1, −3, and −4 subtypes that belong to the G-protein class of receptors. Receptor cleavage at the N-terminal by thrombin results in unmasking sites involved in receptor activation.18,21 These receptors activate multiple classes of G-proteins downstream that include Gq/11, Gi, and G12/13, resulting in transient inositol 1,4,5-triphosphate (IP3)-mediated [Ca2+]i elevation, decrease in intracellular cyclic-AMP (cAMP), or activation of Rho respectively.22–24 Guanine nucleotide exchange factors for Rho (RhoGEFs), including p115 rhoGEF, are direct effectors of Gα12/1325–27 that in turn catalyze GTP loading and activation of Rho. Active Rho and its effector Rho kinase (ROCK) are involved in multiple signaling events that promote cytoskeletal rearrangement,28,29 changes in paracellular permeability,30 cell division and migration,31,32 and in transcription initiation by directly regulating the activity of activating protein-1 (AP-1) family of transcription factors.33,34
We hypothesized that extravasated thrombin after outer blood–retinal barrier breakdown could exert effects on ET-1 synthesis and secretion in the RPE by a thrombin receptor-dependent mechanism that involves activation of Rho and ROCK1/2.
Materials and Methods
Materials
Thrombin, thrombin receptor activating peptide (TRAP6/PAR-1 agonist/SFLLR), hirudin (thrombin inhibitor), Ro 31-8425 (2-[8-(aminomethyl)-6,7,8,9-tetrahydropyrido[1,2-a]indol-3-yl]-3-(1-methyl-1H-indol-3-yl)maleimide, HCl; pan-protein kinase C [PKC] inhibitor), and Y-27632 ((R)-(+)-trans-N-(4-Pyridyl)-4-(1-aminoethyl)-cyclohexanecarboxamide, 2HCl; ROCK inhibitor) were purchased from Calbiochem/EMD Biosciences Inc. (San Diego, CA). U73122 (1-(6-((17β-3-methoxyestra-1,3,5(10)-trien-17-yl)amino)hexyl)-1H-pyrrole-2,5-dione, PLC inhibitor) was purchased from Biomol Research Laboratories Inc. (Plymouth Meeting, PA). The PAR-4 peptide agonist [peptidergic PAR-4 (pPAR-4)/AYPGKF] was custom synthesized with C-terminal amidation at over >95% purity by Bioworld (Dublin, OH). In studies including inhibitors, cells were pretreated with the inhibitor for 20–30 min before the agonist treatment except in case of hirudin where the hirudin–thrombin mixture (3:1) was incubated at room temperature for 1 h before adding it to cells.
Cell culture
Human RPE (hRPE) cells (ARPE-19), a spontaneously transformed cell line, was purchased from American Type Culture Collection (ATCC, Manassas, VA). ARPE-19 cells (passage #s: 20–23) were maintained at 37°C and 5% CO2 in a 1:1 mixture of Dulbecco's modified Eagle's medium (DMEM) and Ham's F-12 medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT), 2 mM l-glutamine, 23 mM NaHCO3, and penicillin and streptomycin (Invitrogen). ARPE-19 cells were seeded at 1.4 × 105 cells/well (6-well plate) and maintained in culture for 4–5 weeks [mature RPE (mRPE)] developing well-defined tight junctions according to previously described methods.35 Primary human fetal RPE cells (passages 1–2) were purchased from ScienCell Research Laboratories (San Diego, CA). Cells were grown in the RPE medium mentioned above except that it contained growth supplements (ScienCell Research Laboratories) and 2% FBS. Additionally, these cells were grown in poly l-lysine (2 μg/mL)-coated flasks or plates.
ET-1 extraction and measurement by radioimmunoassay
ARPE-19 cells were grown for 3–4 weeks (mRPE) in 6-well culture plates (35 mm diameter/well, ∼ 1.4 × 105 cells/well) in 1:1 DMEM + Ham's F12 culture medium containing 10% FBS. Primary hRPE cells were grown as per ScienCell's instructions. On the day of treatments, mRPE cells were rinsed 3 times with serum-free 1:1 DMEM + Ham's F12 culture media (SF-DMEM/F12) and treated with 1 mL SF-DMEM/F12 containing thrombin (5, 10, 20 nM), SFLLR (pPAR-1, 10, 25, 50 μM), or AYPGKF (pPAR-4, 50 μM). Treatment incubations were for 24 h in most of the experiments or a time course (Fig. 2). Primary hRPE cells were treated with or without thrombin (10 nM) for 8 h. The extraction protocol for ET-1 was performed as previously described.36 Efficiency of ET-1 recovery was 75% ± 3% (n = 3). Measurement of immunoreactive ET-1 was according to manufacturer's instructions in a commercially available radio-immunoassay kit for ET-1 (Bachem Peninsula Laboratories, Belmont, CA).
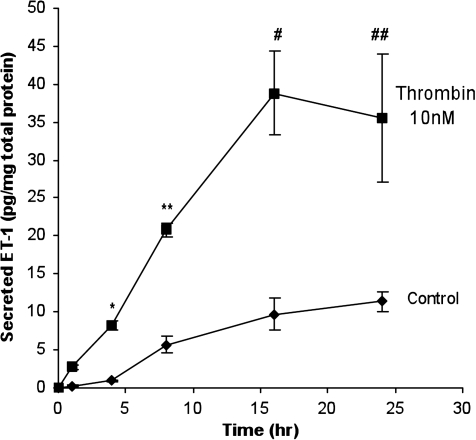
FIG. 2.
Time-dependent increase in ir-ET-1 secretion in mRPE cells after thrombin (10 nM) stimulation. Mature ARPE-19 (mRPE) cells were treated with thrombin (10 nM) for 1, 4, 8, 16, and 24 h. The medium was collected and assayed for ir-ET-1 content as previously described. Thrombin stimulated ir-ET-1 secretion in a time-dependent manner. A significant increase in ir-ET-1 was observed at the end of 4, 8, 16, and 24 h compared to control. Secretion of ir-ET-1 reached a plateau after 16 h. Data are represented as mean ± SEM. Statistical comparisons were performed by t-test. Asterisk (*), double asterisk (**), pound (#), and double pound symbols (##) denote significance versus controls at 4, 8, 16, and 24 h, respectively (P < 0.001) (n = 9).
Intracellular Ca2+ ([Ca2+]i) measurement
Intracellular Ca2+ in mRPE cells was measured at 37°C by the ratiometric technique using fura-2AM (excitation at 340 nm and 380 nm; emission at 500 nm) according to Prasanna et al.37
Rho pull-down assay
The glutathione S-transferase Rhotekin Rho-binding domain (GST-RBD) construct was a generous gift from Dr. Martin Schwartz, University of Virginia Health Science Center, Charlottesville, VA. Expression and purification of the GST-RBD fusion protein, preparation of cell lysates, and the active RhoA pull-down assay were performed as previously described.38 Mature ARPE-19 cells (3–4 weeks in culture) were treated with 10 nM thrombin in serum-free ARPE-19 medium (1:1 DMEM + F12) at indicated periods. This concentration of thrombin was chosen after a concentration–response experiment in ARPE-19 cells (data not shown). Control cells were incubated in the serum-free medium alone for the same period. Immunoblot analysis was performed using the mouse monoclonal anti-RhoA antibody (1 μg/mL in 3% bovine serum albumin) from Santa Cruz Biotechnology (Santa Cruz, CA). All blots were developed using the ECL Advance chemiluminescence kit (Amersham Pharmacia Biotech, Piscataway, NJ), exposed for the same time and developed on a phosphoimager. Densitometric values of active and total RhoA were obtained using Scion Image 4.0.
Real-time reverse transcriptase (RT-PCR) RT-polymerase chain reaction
Total RNA extraction and cDNA synthesis were performed according to Krishnamoorthy et al.39 The primer sequences for preproET-1 (ppET-1) and β-actin were as follows: ppET-1, forward/sense 5′-TATCAGCAGTTAGTGAGAGG-3′ and reverse/antisense 5′-CGAAGGTCTGTCACCAATGTGC-3′ with an expected amplicon/product size of 180 bp; β-actin, forward/sense 5′-TGTGATGGTGGGAATGGGTCAG-3′ and reverse/antisense 5′-TTTGATGTCACGCACGATTTCC-3′ with an expected amplicon/product size of 514 bp. Quantitative real-time polymerase chain reaction (PCR) was performed using the SYBR-green detection system (Applied Biosystems, Foster City, CA) as described by Zhang et al.40 PCR products were confirmed by DNA sequencing. Quantitation of relative ppET-1 transcript levels in ARPE-19 was achieved using the comparative CT method (as described in the PE Biosystems User Bulletin #2: http://docs.appliedbiosystems.com/pebiodocs/04303859.pdf.
Data analysis
Quantitative data are represented as mean ± standard error of the mean. Statistical comparisons between control and multiple treatments were made by analysis of variance and Student-Newman Keuls (SNK) test (P < 0.05 was deemed as significant). In certain experiments a comparison in the mean value between the untreated control versus treated sample was made by Student's t-test (P < 0.05). In [Ca2+]i measurements, comparisons between baseline, peak, and 1 min postpeak (not shown) values were made by one-way repeated measures analysis of variance. Sample size and P-values for each experiment are indicated in the figure legends.
Results
Thrombin acts via the PAR-1 subtype in RPE cells to mediate ET-1 secretion
Breakdown of the blood–retinal barrier may result in thrombin-mediated effects on surrounding cells. In this study we examined whether thrombin could induce ET-1 secretion in primary hRPE and ARPE-19 cells and whether this action required the PAR-1 or PAR-4 subtypes. The PAR-2 is trypsin sensitive, whereas PAR-3 signaling remains unclear and in certain cases reported to signal autonomously.41
Thrombin significantly increased ET-1 secretion that was ∼2- to 4-fold above untreated control at all time points examined (Figs. 1 and 2). On the basis of these initial experiments we chose a submaximal concentration of 10 nM thrombin for all experiments that followed because at this concentration we consistently achieved a significant response over control (untreated) in terms of measurable ET-1 secretion. In primary hRPE cells, thrombin (10 nM after 8 h incubation) increased ET-1 secretion about 2-fold (Fig. 1B).
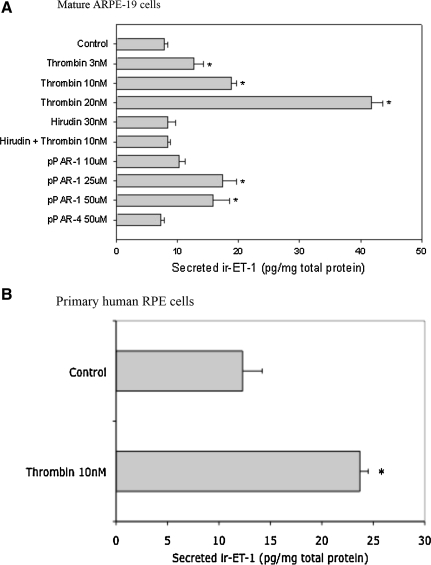
FIG. 1.
Secreted endothelin-1 (ET-1) in mature ARPE-19 (A) and primary human retinal pigment epithelium (hRPE) (B) cells measured by radio-immunoassay (RIA). Cells were treated with the indicated agonists and/or inhibitors for 24 h in serum-free Dulbecco's modified Eagle's medium/F-12 medium. Immunoreactive ET-1 (ir-ET-1) released in the medium was extracted and measured by RIA. In mature RPE (mRPE) cells, thrombin significantly increased ir-ET-1 secretion versus control, an effect that was concentration dependent. A similar effect was observed in primary hRPE cells. Hirudin (30 nM) when preincubated with thrombin (10 nM) inhibited ET-1 secretion as opposed to thrombin (10 nM) alone. Peptidergic protease-activated receptor-1 (pPAR-1), but not pPAR-4 (both at 50 μM), significantly increased ET-1 secretion, suggesting that thrombin-mediated effects on ET-1 secretion involves the PAR-1. Data are represented as mean ± standard error of the mean (SEM). Statistical comparisons were performed using analysis of variance and Student-Newman Keuls (SNK) test. *Significance versus control (P < 0.05) (n = at least 6 per treatment).
To determine if thrombin-mediated effects were either PAR-1 or PAR-4 dependent, we tested 2 synthetic peptides that mimicked the tethered ligands of PAR-1 (pPAR-1/SFLLR) and PAR-4 (pPAR-4/AYPGKF). The pPAR-1 but not pPAR-4 ligand significantly increased ET-1 secretion in ARPE-19 cells (Fig. 1), and this suggests that thrombin-mediated effects on ET-1 primarily act through the PAR-1 subtype. Hirudin (antithrombin) binds to the catalytic subunit of thrombin42 and blocks its protease activity. Hirudin alone had no effect on ET-1 secretion but neutralized the thrombin-effect when preincubated with thrombin before treatment (Fig. 1), suggesting that observed effects on ET-1 were thrombin specific.
PAR-1 and PAR-4-mediated [Ca2+]i mobilization in ARPE-19 cells is PLC dependent
PAR are known to couple Gq/11 family of heterotrimeric G-proteins that can activate an IP3-dependent increase in intracellular calcium ([Ca2+]i).23 To determine if thrombin, pPAR-1, or pPAR-4 ligands increase [Ca2+]i, we examined their effects on ARPE-19 cells in real time. Thrombin-mediated increase in [Ca2+]i was concentration dependent, and a typical biphasic profile for Ca2+ mobilization was seen at all concentrations (Fig. 3; Tables 1 and 2). Both pPAR-1 and pPAR-4 ligands increased [Ca2+]i, indicating the presence of distinct functional receptor subtypes in ARPE-19 cells. Thrombin was more potent in elevating [Ca2+]i compared to pPAR-1 or −4 (Tables 1 and 2). Both hirudin (antithrombin or thrombin-neutralizer) and U73122 inhibited this rise in [Ca2+]i, suggesting a PLC-dependent mechanism. The ROCK1/2 inhibitor Y27632 had no effect on thrombin-induced rise in [Ca2+]i (Tables 1 and 2).
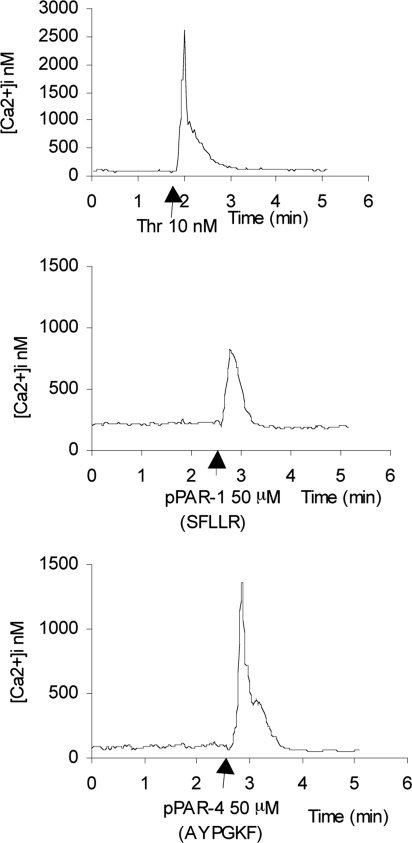
FIG. 3.
Intracellular [Ca2+]i measurements in mRPE cells. Representative [Ca2+]i trends in response to thrombin (10 nM), pPAR-1 (50 μM), and pPAR-4 (50 μM) in mRPE cells. Thrombin-mediated rise in mean [Ca2+]i mobilization was concentration dependent and was attenuated by thrombin neutralization agent Hirudin or the PLC inhibitor U73122 (see Tables 1 and 2). Thrombin was more potent in mobilizing [Ca2+]i in mRPE cells than pPAR-1 or pPAR-4 (compare y-axes scales and Tables 1 and 2). Arrows represent time of addition of compound to cells in buffer.
Table 1.
Concentration-Dependent Elevation of [Ca2+]i
| Treatment | [Ca2+]i nM, mean ± SEM | Number of cells (n) |
|---|---|---|
| Baseline | 70 ± 10 | 11 |
| Thrombin 5 nM | 2,049 ± 184a | 12 |
| Baseline | 64 ± 5 | 79 |
| Thrombin 10 nM | 2,141 ± 277a | 79 |
| Baseline | 95 ± 8 | 19 |
| Thrombin 20 nM | 6,164 ± 1,620a | 19 |
| Baseline | 219 ± 10 | 16 |
| pPAR-1 (SFLLR) 50 μM | 817 ± 52a | 16 |
| Baseline | 87 ± 9 | 18 |
| pPAR-4 (AYPGKF) 50 μM | 1,194 ± 177a | 18 |
Summary of thrombin, pPAR-1, and pPAR-4-mediated [Ca2+]i mobilization in mature retinal pigment epithelium cells measured by fura-2AM imaging.
Statistical significance between baseline, peak, and 1 min postpeak (not shown) mean values, performed by one-way repeated measures ANOVA and SNK method for multiple pair-wise comparison (P < 0.001).
Abbreviations: ANOVA, analysis of variance; pPAR, peptidergic protease-activated receptor; SEM, standard error of the mean; SNK, Student-Newman Keuls test.
Table 2.
Inhibition of Thrombin-Mediated Elevation in [Ca2+]i
| Treatment | [Ca2+]i nM, mean ± SEM | Number of cells (n) |
|---|---|---|
| Baseline | 64 ± 5 | 79 |
| Thrombin 10 nM | 2,141 ± 277a | 79 |
| Baseline | 124 ± 12 | 17 |
| Hirudin + thrombin (10 nM each) | 165 ± 16 | 17 |
| Baseline | 64 ± 5 | 16 |
| Hirudin + thrombin (25 nM each) | 109 ± 5 | 16 |
| Baseline/U73122 10 μM | 53 ± 5 | 16 |
| U73122 + thrombin 10 nM | 53 ± 4 | 16 |
| Baseline | 94 ± 66 | 29 |
| Thrombin (10 nM) | 1,120 ± 652a | 29 |
| Baseline/Y27632 10 μM | 50 ± 39 | 38 |
| Y27632 + thrombin | 1,027 ± 550a | 38 |
Hirudin (antithrombin) was preincubated with thrombin for 1 h at room temperature at equimolar or 3-fold higher concentration. In U73122 studies, cells were preincubated with 10 μM U73122 for 20 min before thrombin addition.
Statistical significance between baseline, peak, and 1 min postpeak (not shown) mean values, performed by one-way repeated measures ANOVA and SNK method for multiple pair-wise comparison (P < 0.001).
Activation of RhoA and ROCK1/2 and its role in inducing ppET-1 transcription
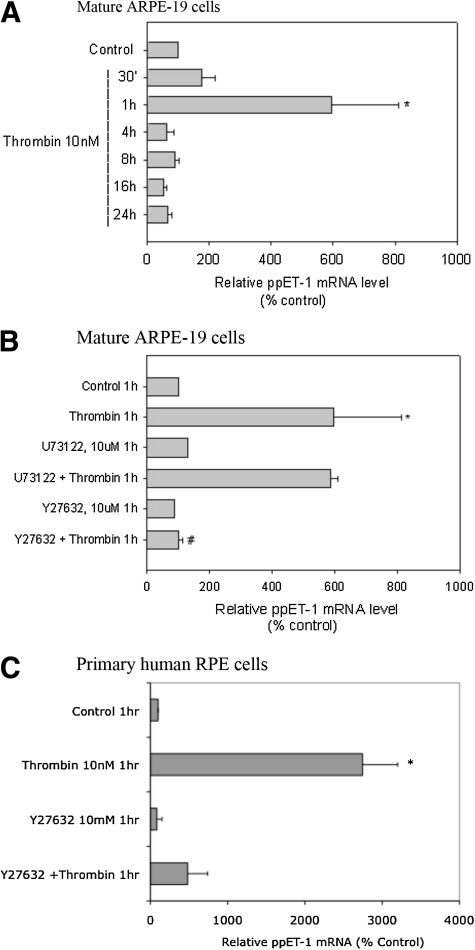
We tested if thrombin increased active RhoA and whether the downstream effector ROCK1/2 was an intermediate in inducing ET-1 in RPE. Elevated active RhoA levels were evident within 5 min of thrombin treatment and persisted for 30 min (Fig. 4A, B). Thrombin also increased ppET-1 mRNA levels transiently in both mature ARPE-19 cells as well as primary hRPE cells (Fig. 5A–C). Since rho and [Ca2+]i elevations were observed well before increase in ET-1 mRNA, we sought to determine if elevation in ppET-1 mRNA was dependent on either cascade or both. Rho-associated kinase (ROCK1/2) is a direct rho effector. Inhibition of the rho/ROCK1/2 pathway by Y27632, a selective ROCK1/2 inhibitor, prevented thrombin-induced rise in ppET-1 mRNA, whereas U73122, a PLC inhibitor, had no effect. A similar result on ppET-1 mRNA levels was observed in primary hRPE cells (Fig. 5C). This finding implicated the rho/ROCK1/2 pathway as being critical for thrombin increase in ET-1 in RPE cells independent of the IP3/Ca2+ pathway.
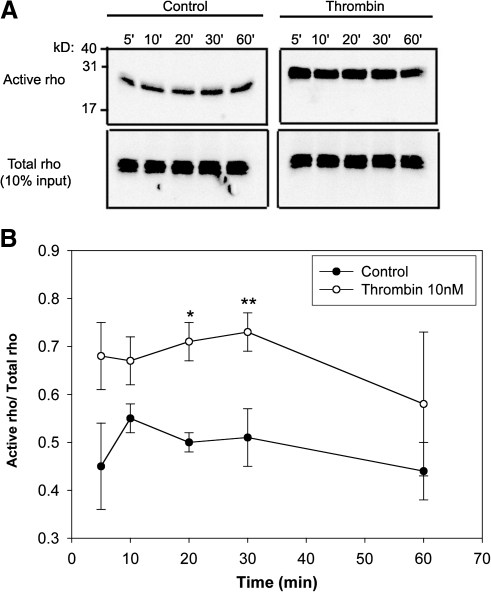
FIG. 4.
RhoA pull-down assay in ARPE-19 cell lysates after thrombin treatment. Thrombin-activated RhoA in ARPE-19 cells at 5, 10, and 30 min compared to unstimulated cells (A, top panel). Total RhoA in cell lysates remain unchanged (A, lower panel). (B) The scanning densitometric data plotted as a ratio of active RhoA/total RhoA at the indicated periods. Data are represented as mean SEM. Statistical comparisons were performed by t-test. Asterisk (*) and double asterisk (**) denote significance versus control at 20 and 30 minutes respectively.
FIG. 5.
Measurement of preproET-1 (ppET-1) mRNA by quantitative polymerase chain reaction (PCR) in mature ARPE-19 and primary hRPE cells. Quantitative RT-PCR was performed using the SYBR-green PCR core reagents. Quantitation of ppET-1 transcripts was done by the comparative CT method (see the Materials and Methods section) with β-actin cDNA as the external control. Thrombin-induced ppET-1 mRNA reached a maximal level at 1 h and returned to basal values at all time points tested thereafter (A). Thrombin-induced rise in ppET-1 mRNA was completely inhibited by Y27632, a ROCK1/2 inhibitor, but not the PLC inhibitor-U73122 (B). Similar effects were observed in primary hRPE cells (C). Data are represented as mean ± SEM. Statistical comparisons were performed by t-test. Asterisks (*) denote significance versus control, and pound (#) denotes significance versus thrombin, 1 h (P < 0.05).
ROCK1/2 but not PKC mediates PAR-1-dependent ET-1 secretion in RPE
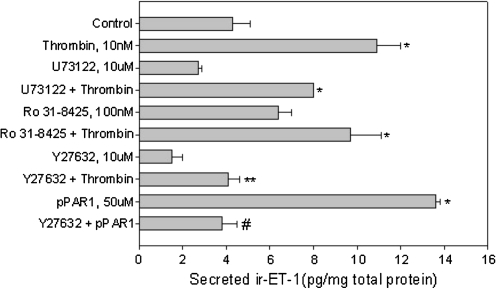
To examine if prolonged inhibition of ROCK1/2, PLC, or PKC had an effect on ET-1 secretion, we preincubated RPE cells with pharmacological inhibitors as indicated followed by adding thrombin in the presence of the inhibitor for 24 h. Previous studies on regulated secretion of ET-1 have suggested the involvement of agonist-induced rise in [Ca2+]i with subsequent PKC activation in inducing ET-1.19 In ARPE-19 cells we found that the pan-PKC inhibitor, Ro 31-8425, had no effect on thrombin-mediated ET-1 secretion (Fig. 6). The PLC inhibitor U73122, despite completely inhibiting [Ca2+]i mobilization, had little effect on ET-1 secretion. The ROCK1/2 inhibitor Y27632, on the other hand, completely blocked ET-1 secretion induced by thrombin as well as the peptide agonist pPAR-1.
FIG. 6.
Secreted ET-1 in mature ARPE-19 (mRPE) measured by RIA. Cells were treated with the indicated agonists (thrombin or pPAR-1) or were pretreated with the indicated inhibitors for 20–30 min followed by the agonist for 24 h in serum-free Dulbecco's modified Eagle's medium/F-12 medium. ir-ET-1 released in the medium was extracted and measured by RIA. Thrombin-mediated increase in ET-1 secretion was partially blocked by the PLC inhibitor, U73122, and completely inhibited by ROCK1/2 inhibitor, Y27632. The pan-PKC inhibitor (Ro 31-8425) had no effect on thrombin-induced ET-1 release. pPAR-1 (SFLLR)-mediated ET-1 secretion was also completely inhibited by Y27632. Data are represented as mean ± SEM. Statistical comparisons were performed using analysis of variance and student newman keuls (SNK) test. Asterisks (*) denote significance versus control, double asterisk (**) denotes significance versus thrombin 10 nM, and pound (#) denotes significance versus pPAR-1 50 μM (P < 0.05) (n = at least 6 per treatment).
Discussion
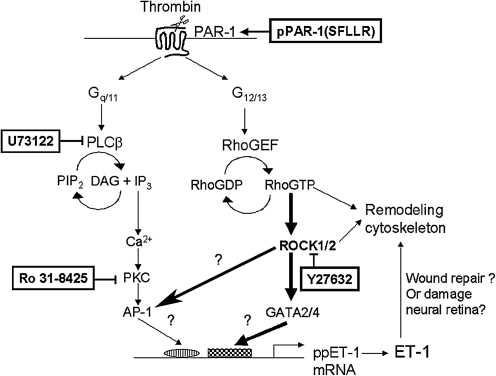
Coordinated activation of the coagulation cascade involves the rapid generation and release of thrombin at sites of tissue injury. In addition to its role in clotting and hemostasis, action of released thrombin on the surrounding tissues can result in inflammation. Retinal injuries that result in breakdown of the RPE/ blood–retinal barrier can result in thrombin-mediated effects at the RPE. We sought to determine if thrombin exerts effects on the RPE by increasing ET-1 expression and secretion and the mechanism of action of thrombin in mediating this effect. ET-1 is secreted constitutively by the RPE and induced by cholinergics and proinflammatory cytokines like tumor necrosis factor-alpha that increase its secretion in RPE cells.8 ET-1 is a proangiogenic and a proinflammatory cytokine43 that can be induced by other cytokines, growth factors, mechanical stretch, and hypoxia.19 In this study, we report that thrombin primarily activates the PAR-1 subtype to induce ET-1 synthesis in RPE. PAR-1 are known to couple with pertussis-toxin insensitive G12/13 G-proteins, and dissociation of the Gα subunit can activate RhoGEF, a guanine-nucleotide exchange factor for rho. RhoGEFs include lbc, lfc, lsc, and p115,44,45 of which p115 was shown to bind Gα12 and Gα13 and mediate Rho activation.25,26 Activated Gα13 induces ppET-1 transcription in c-jun N-terminal kinase-dependent manner.46 Additionally, rho GTPases have been implicated in regulating ET-1 synthesis by increasing endothelin converting enzyme (ECE-1) mRNA.47 Active Rho/ROCK have also been shown to initiate transcription by directly regulating the activity of AP-1 family of transcription factors.33,34
The signaling cross-talk between Rho and calcium-dependent pathways is not well established. Activated RhoA was recently shown to interact with IP3 receptors as well as TRP channel-1 in endothelial cells,48 suggesting that RhoA may regulate store operated calcium entry or receptor-activated calcium entry.48,49 We found that phospholipase C-dependent IP3/Ca2+ pathway was not involved in ET-1 secretion. Additionally, inhibiting the downstream effector of Rho, that is, ROCK1/2, failed to prevent thrombin-mediated rise in intracellular calcium, yet completely inhibited ppET-1 mRNA and secretion. Synthesis and secretion of ET-1 is thought to be PKC dependent.19 Thrombin-mediated activation of PKC-α was shown to associate and phosphorylate the Rho-guanine nucleotide dissociation inhibitor, allowing activation of Rho and increased paracellular permeability in endothelial cells. This suggests that PKC may influence rho activation. In ARPE-19 cells, however, we found that the pan-PKC inhibitor Ro 31-8425 failed to prevent thrombin-mediated ET-1 secretion. Figure 7 summarizes the signaling features that may be involved in thrombin-induced ET-1 synthesis and secretion in RPE.
FIG. 7.
Thrombin-induced ET-1 secretion in RPE is rho/ROCK1/2 dependent. Thrombin-mediated PAR-1 activation can simultaneously activate the Gq/11 and G12/13-dependent pathways, which in turn activates PLC-dependent IP3/DAG (Inositol 1,4,5-triphosphate/diacylglycerol) production and Rho activation, respectively. IP3-dependent [Ca2+]i elevation along with DAG can activate protein kinase C (PKC) that may influence ET-1 synthesis in some cells. We demonstrate that the predominant effect of thrombin on ET-1 production in ARPE-19 cells was via the Rho/ROCK1/2-dependent pathway. ROCK1/2 may increase ppET-1 mRNA synthesis by activating the GATA family of transcription factors (i.e., GATA-2/4) or by regulating the AP-1 family of transcription factors known to activate ppET-1 transcription. The physiological function of ET-1 secreted by the RPE is presently unknown. Considering some of the known actions of ET-1, it may mediate tissue repair by acting on its receptors in an autocrine manner or cause further damage to the neural retina by acting on its receptors in the inner retina.
Our results suggest that Rho/ROCK1/2 is an important intermediate in the thrombin-ET-1 interaction pathway and a possible pharmacological strategy to attenuate this effect using Y27632. ET-1 thus secreted may mediate wound repair indirectly by vasoconstriction of nearby blood vessels and/or by autocrine actions that promote RPE cell migration, similar to actions of ET-1 observed in other cells.50,51 Conversely, excessive ET-1 secretion may mediate further damage by increasing nitric oxide production by activating the ETB receptor52 or by inducing production of vascular endothelial growth factor and matrix metalloproteinases,17,53,54 factors when accompanied by blood–retinal barrier breakdown may play a role in retinal and choroidal neovascularization.55 Thus, ET-1 released by the RPE may be important in pathologic conditions that involve blood–retinal barrier breakdown and inflammation as reported in conditions, including uveitis,56 retinitis pigmentosa,57 proliferative vitreoretinopathy, and diabetic retinopathy.58 The role of constitutive ET-1 production in the RPE is of outstanding interest since its physiologic function at the region of the blood–retinal barrier is presently unknown.
Acknowledgments
We thank Dr. Martin Schwartz (U. Virginia HSC) for the GST-RBD construct; Drs. Jerry Simeka, Xiangle Sun, and Xinyu Zhang for their help with the real-time RT-PCR analysis; and Christina Johnson for technical support. This work was funded by a grant from the National Eye Institute (NEI, NIH) (EY: 11979) and the Texas Higher Education Coordinating Board Advanced Technology Program to T.Y.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Marmor M. New York: Oxford University Press; 1998. Structure, Function, and Disease of the Retinal Pigment Epithelium; pp. 3–9. [Google Scholar]
- 2.Campochiaro P.A. Growth factors in the retinal pigment epithelium and retina. In: Marmor M.F., editor; Wolfensberger T.J., editor. The Retinal Pigment Epithelium. New York: Oxford University Press; 1998. pp. 459–477. [Google Scholar]
- 3.MacCumber M.W. Jampel H.D. Snyder S.H. Ocular effects of the endothelins. Abundant peptides in the eye. Arch. Ophthalmol. 1991;109:705–709. doi: 10.1001/archopht.1991.01080050121041. [DOI] [PubMed] [Google Scholar]
- 4.Wollensak G. Schaefer H.E. Ihling C. An immunohistochemical study of endothelin-1 in the human eye. Curr. Eye Res. 1998;17:541–545. doi: 10.1076/ceyr.17.5.541.5187. [DOI] [PubMed] [Google Scholar]
- 5.Ripodas A. de Juan J.A. Roldan-Pallares M., et al. Localisation of endothelin-1 mRNA expression and immunoreactivity in the retina and optic nerve from human and porcine eye. Evidence for endothelin-1 expression in astrocytes. Brain Res. 2001;912:137–143. doi: 10.1016/s0006-8993(01)02731-7. [DOI] [PubMed] [Google Scholar]
- 6.Roldan-Pallares M. Rollin R. Martinez-Montero J.C. Fernandez-Cruz A. Bravo-Llata C. Fernandez-Durango R. Immunoreactive endothelin-1 in the vitreous humor and epiretinal membranes of patients with proliferative diabetic retinopathy. Retina. 2007;27:222–235. doi: 10.1097/01.iae.0000231376.76601.40. [DOI] [PubMed] [Google Scholar]
- 7.Narayan S. Brun A.M. Yorio T. Endothelin-1 distribution and basolateral secretion in the retinal pigment epithelium. Exp. Eye Res. 2004;79:11–19. doi: 10.1016/j.exer.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Narayan S. Prasanna G. Krishnamoorthy R.R. Zhang X. Yorio T. Endothelin-1 synthesis and secretion in human retinal pigment epithelial cells (ARPE-19): differential regulation by cholinergics and TNF-alpha. Invest. Ophthalmol. Vis. Sci. 2003;44:4885–4894. doi: 10.1167/iovs.03-0387. [DOI] [PubMed] [Google Scholar]
- 9.Kedzierski R.M. Yanagisawa M. Endothelin system: the double-edged sword in health and disease. Annu. Rev. Pharmacol. Toxicol. 2001;41:851–876. doi: 10.1146/annurev.pharmtox.41.1.851. [DOI] [PubMed] [Google Scholar]
- 10.Barton M. Yanagisawa M. Endothelin: 20 years from discovery to therapy. Can. J. Physiol. Pharmacol. 2008;86:485–498. doi: 10.1139/Y08-059. [DOI] [PubMed] [Google Scholar]
- 11.Prasanna G. Narayan S. Krishnamoorthy R.R. Yorio T. Eyeing endothelins: a cellular perspective. Mol. Cell. Biochem. 2003;253:71–88. doi: 10.1023/a:1026005418874. [DOI] [PubMed] [Google Scholar]
- 12.Russell F.D. Molenaar P. The human heart endothelin system: ET-1 synthesis, storage, release and effect. Trends Pharmacol. Sci. 2000;21:353–359. doi: 10.1016/s0165-6147(00)01524-8. [DOI] [PubMed] [Google Scholar]
- 13.Yanagisawa M. Masaki T. Endothelin, a novel endothelium-derived peptide. Pharmacological activities, regulation and possible roles in cardiovascular control. Biochem. Pharmacol. 1989;38:1877–1883. doi: 10.1016/0006-2952(89)90484-x. [DOI] [PubMed] [Google Scholar]
- 14.Salom J.B. Torregrosa G. Alborch E. Endothelins and the cerebral circulation. Cerebrovasc. Brain Metab. Rev. 1995;7:131–152. [PubMed] [Google Scholar]
- 15.Taylor R.N. Varma M. Teng N.N. Roberts J.M. Women with preeclampsia have higher plasma endothelin levels than women with normal pregnancies. J. Clin. Endocrinol. Metab. 1990;71:1675–1677. doi: 10.1210/jcem-71-6-1675. [DOI] [PubMed] [Google Scholar]
- 16.Granger J.P. Alexander B.T. Llinas M.T. Bennett W.A. Khalil R.A. Pathophysiology of hypertension during preeclampsia linking placental ischemia with endothelial dysfunction. Hypertension. 2001;38:718–722. doi: 10.1161/01.hyp.38.3.718. [DOI] [PubMed] [Google Scholar]
- 17.Nelson J. Bagnato A. Battistini B. Nisen P. The endothelin axis: emerging role in cancer. Nat. Rev. Cancer. 2003;3:110–116. doi: 10.1038/nrc990. [DOI] [PubMed] [Google Scholar]
- 18.Coughlin S.R. Sol Sherry lecture in thrombosis: how thrombin “Talks” to cells molecular mechanisms and roles in vivo. Arterioscler. Thromb. Vasc. Biol. 1998;18:514–518. doi: 10.1161/01.atv.18.4.514. [DOI] [PubMed] [Google Scholar]
- 19.Tasaka K. Kitazumi K. The control of endothelin-1 secretion. Gen. Pharmacol. 1994;25:1059–1069. doi: 10.1016/0306-3623(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 20.Lepailleur-Enouf D. Valdenaire O. Philippe M. Jandrot-Perrus M. Michel J.B. Thrombin induces endothelin expression in arterial smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H1606–H1612. doi: 10.1152/ajpheart.2000.278.5.H1606. [DOI] [PubMed] [Google Scholar]
- 21.Coughlin S.R. How the protease thrombin talks to cells. Proc. Natl. Acad. Sci. U. S. A. 1999;96:11023–11027. doi: 10.1073/pnas.96.20.11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barr A.J. Brass L.F. Manning D.R. Reconstitution of receptors and GTP-binding regulatory proteins (G proteins) in Sf9 cells. A direct evaluation of selectivity in receptor G protein coupling. J. Biol. Chem. 1997;272:2223–2229. doi: 10.1074/jbc.272.4.2223. [DOI] [PubMed] [Google Scholar]
- 23.Hung D.T. Wong Y.H. Vu T.K. Coughlin S.R. The cloned platelet thrombin receptor couples to at least two distinct effectors to stimulate phosphoinositide hydrolysis and inhibit adenylyl cyclase. J. Biol. Chem. 1992;267:20831–20834. [PubMed] [Google Scholar]
- 24.Offermanns S. Laugwitz K.L. Spicher K. Schultz G. G proteins of the G12 family are activated via thromboxane A2 and thrombin receptors in human platelets. Proc. Natl. Acad. Sci. U. S. A. 1994;91:504–508. doi: 10.1073/pnas.91.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hart M.J. Jiang X. Kozasa T., et al. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science. 1998;280:2112–2114. doi: 10.1126/science.280.5372.2112. [DOI] [PubMed] [Google Scholar]
- 26.Kozasa T. Jiang X. Hart M.J., et al. p115 RhoGEF, a GTPase activating protein for Galpha12 and Galpha13. Science. 1998;280:2109–2111. doi: 10.1126/science.280.5372.2109. [DOI] [PubMed] [Google Scholar]
- 27.Hall A. G proteins and small GTPases: distant relatives keep in touch. Science. 1998;280:2074–2075. doi: 10.1126/science.280.5372.2074. [DOI] [PubMed] [Google Scholar]
- 28.Klages B. Brandt U. Simon M.I. Schultz G. Offermanns S. Activation of G12/G13 results in shape change and Rho/Rho-kinase-mediated myosin light chain phosphorylation in mouse platelets. J. Cell Biol. 1999;144:745–754. doi: 10.1083/jcb.144.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norman J.C. Price L.S. Ridley A.J. Hall A. Koffer A. Actin filament organization in activated mast cells is regulated by heterotrimeric and small GTP-binding proteins. J. Cell Biol. 1994;126:1005–1015. doi: 10.1083/jcb.126.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benais-Pont G. Punn A. Flores-Maldonado C., et al. Identification of a tight junction-associated guanine nucleotide exchange factor that activates Rho and regulates paracellular permeability. J. Cell Biol. 2003;160:729–740. doi: 10.1083/jcb.200211047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Etienne-Manneville S. Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 32.Hall A. Rho GTPases and the control of cell behaviour. Biochem. Soc. Trans. 2005;33:891–895. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- 33.Chang J.H. Pratt J.C. Sawasdikosol S. Kapeller R. Burakoff S.J. The small GTP-binding protein Rho potentiates AP-1 transcription in T cells. Mol. Cell. Biol. 1998;18:4986–4993. doi: 10.1128/mcb.18.9.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marinissen M.J. Chiariello M. Tanos T. Bernard O. Narumiya S. Gutkind J.S. The small GTP-binding protein RhoA regulates c-jun by a ROCK-JNK signaling axis. Mol. Cell. 2004;14:29–41. doi: 10.1016/s1097-2765(04)00153-4. [DOI] [PubMed] [Google Scholar]
- 35.Dunn K.C. Aotaki-Keen A.E. Putkey F.R. Hjelmeland L.M. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp. Eye Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- 36.Prasanna G. Dibas A. Tao W. White K. Yorio T. Regulation of endothelin-1 in human non-pigmented ciliary epithelial cells by tumor necrosis factor-alpha. Exp. Eye Res. 1998;66:9–18. doi: 10.1006/exer.1997.0407. [DOI] [PubMed] [Google Scholar]
- 37.Prasanna G. Dibas A.I. Yorio T. Cholinergic and adrenergic modulation of the Ca2+response to endothelin-1 in human ciliary muscle cells. Invest. Ophthalmol. Vis. Sci. 2000;41:1142–1148. [PubMed] [Google Scholar]
- 38.Ren X.D. Schwartz M.A. Determination of GTP loading on Rho. Methods Enzymol. 2000;325:264–272. doi: 10.1016/s0076-6879(00)25448-7. [DOI] [PubMed] [Google Scholar]
- 39.Krishnamoorthy R. Agarwal N. Chaitin M.H. Upregulation of CD44 expression in the retina during the rds degeneration. Brain Res. Mol. Brain Res. 2000;77:125–130. doi: 10.1016/s0169-328x(00)00035-8. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X. Krishnamoorthy R.R. Prasanna G. Narayan S. Clark A. Yorio T. Dexamethasone regulates endothelin-1 and endothelin receptors in human non-pigmented ciliary epithelial (HNPE) cells. Exp. Eye Res. 2003;76:261–272. doi: 10.1016/s0014-4835(02)00323-8. [DOI] [PubMed] [Google Scholar]
- 41.Ostrowska E. Reiser G. The protease-activated receptor-3 (PAR-3) can signal autonomously to induce interleukin-8 release. Cell. Mol. Life Sci. 2008;65:970–981. doi: 10.1007/s00018-008-7555-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coughlin S.R. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 43.Bek E.L. McMillen M.A. Endothelins are angiogenic. J. Cardiovasc. Pharmacol. 2000;36:S135–S139. doi: 10.1097/00005344-200036051-00043. [DOI] [PubMed] [Google Scholar]
- 44.Glaven J.A. Whitehead I.P. Nomanbhoy T. Kay R. Cerione R.A. Lfc and Lsc oncoproteins represent two new guanine nucleotide exchange factors for the Rho GTP-binding protein. J. Biol. Chem. 1996;271:27374–27381. doi: 10.1074/jbc.271.44.27374. [DOI] [PubMed] [Google Scholar]
- 45.Hart M.J. Sharma S. elMasry N., et al. Identification of a novel guanine nucleotide exchange factor for the Rho GTPase. J. Biol. Chem. 1996;271:25452–25458. doi: 10.1074/jbc.271.41.25452. [DOI] [PubMed] [Google Scholar]
- 46.Yamakaw K. Kitamura K. Nonoguchi H. Takasu N. Miller R.T. Tomita K. Galpha13 induces preproET-1 gene expression via JNK. Hypertens. Res. 2002;25:427–432. doi: 10.1291/hypres.25.427. [DOI] [PubMed] [Google Scholar]
- 47.Eto M. Barandier C. Rathgeb L., et al. Thrombin suppresses endothelial nitric oxide synthase and upregulates endothelin-converting enzyme-1 expression by distinct pathways: role of Rho/ROCK and mitogen-activated protein kinase. Circ. Res. 2001;89:583–590. doi: 10.1161/hh1901.097084. [DOI] [PubMed] [Google Scholar]
- 48.Mehta D. Ahmmed G.U. Paria B.C., et al. RhoA interaction with inositol 1,4,5-trisphosphate receptor and transient receptor potential channel-1 regulates Ca2+ entry. Role in signaling increased endothelial permeability. J. Biol. Chem. 2003;278:33492–33500. doi: 10.1074/jbc.M302401200. [DOI] [PubMed] [Google Scholar]
- 49.Ghisdal P. Vandenberg G. Morel N. Rho-dependent kinase is involved in agonist-activated calcium entry in rat arteries. J. Physiol. 2003;551((Pt 3)):855–867. doi: 10.1113/jphysiol.2003.047050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shao R. Rockey D.C. Effects of endothelins on hepatic stellate cell synthesis of endothelin-1 during hepatic wound healing. J. Cell. Physiol. 2002;191:342–350. doi: 10.1002/jcp.10110. [DOI] [PubMed] [Google Scholar]
- 51.Kernochan L.E. Tran B.N. Tangkijvanich P. Melton A.C. Tam S.P. Yee H.F., Jr. Endothelin-1 stimulates human colonic myofibroblast contraction and migration. Gut. 2002;50:65–70. doi: 10.1136/gut.50.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsukahara H. Ende H. Magazine H.I. Bahou W.F. Goligorsky M.S. Molecular and functional characterization of the non-isopeptide-selective ETB receptor in endothelial cells. Receptor coupling to nitric oxide synthase. J. Biol. Chem. 1994;269:21778–21785. [PubMed] [Google Scholar]
- 53.Pedram A. Razandi M. Hu R.M. Levin E.R. Vasoactive peptides modulate vascular endothelial cell growth factor production and endothelial cell proliferation and invasion. J. Biol. Chem. 1997;272:17097–17103. doi: 10.1074/jbc.272.27.17097. [DOI] [PubMed] [Google Scholar]
- 54.He S. Prasanna G. Yorio T. Endothelin-1-mediated signaling in the expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in astrocytes. Invest. Ophthalmol. Vis. Sci. 2007;48:3737–3745. doi: 10.1167/iovs.06-1138. [DOI] [PubMed] [Google Scholar]
- 55.Campochiaro P.A. Ocular neovascularisation and excessive vascular permeability. Expert Opin. Biol. Ther. 2004;4:1395–1402. doi: 10.1517/14712598.4.9.1395. [DOI] [PubMed] [Google Scholar]
- 56.Luna J.D. Chan C.C. Derevjanik N.L., et al. Blood-retinal barrier (BRB) breakdown in experimental autoimmune uveoretinitis: comparison with vascular endothelial growth factor, tumor necrosis factor alpha, and interleukin-1beta-mediated breakdown. J. Neurosci. Res. 1997;49:268–280. doi: 10.1002/(sici)1097-4547(19970801)49:3<268::aid-jnr2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 57.Vinores S.A. Kuchle M. Derevjanik N.L., et al. Blood-retinal barrier breakdown in retinitis pigmentosa: light and electron microscopic immunolocalization. Histol. Histopathol. 1995;10:913–923. [PubMed] [Google Scholar]
- 58.Hiscott P. Sheridan C.M. The retinal pigment epithelium, epiretinal membrane formation, and proliferative vitreoretinopathy. In: Marmor M.F., editor; Wolfensberger T.J., editor. The Retinal Pigment Epithelium. New York: Oxford University Press; 1998. pp. 478–491. [Google Scholar]