Abstract
There have been a handful of previously published cases of athletes who were still symptomatic from a prior head injury, and then suffered a second injury in which a thin, acute subdural hematoma (SDH) with unilateral hemisphere vascular engorgement was demonstrated on CT scan. In those cases, the cause of the brain swelling/dysautoregulation was ascribed to the presence of the acute SDH rather than to the acceleration/deceleration forces that caused the SDH. We believe that the brain swelling is due to “second-impact dysautoregulation,” rather than due to the effect of the SDH on the underlying hemisphere. To support our hypothesis, we present 10 additional cases of acute hemispheric swelling in association with small SDHs in athletes who received a second head injury while still symptomatic from a previous head injury. The clinical history and the unique neuroimaging features of this entity on CT are described and illustrated in detail. The CT findings included an engorged cerebral hemisphere with initial preservation of grey-white matter differentiation, and abnormal mass effect and midline shift that appeared disproportionately greater than the size of the SDH. In addition, the imaging similarities between our patients and those with non-accidental head trauma (shaken-baby syndrome) will be discussed.
Key words: acute subdural hematoma; cerebral swelling; dysautoregulation; non-accidental head trauma; second-impact syndrome, shaken-baby syndrome; vascular brain engorgement
Introduction
What Saunders and Harbaugh (Saunders and Harbaugh, 1984) called “the second-impact syndrome of catastrophic head injury” was actually first described by Schneider (Schneider 1973). The syndrome occurs when an athlete sustains an initial head injury and then suffers a second head injury before the symptoms associated with the first impact have cleared, (Cantu, 1995; Cantu and Voy, 1995; Schnitker, 1949). Typically, the athlete suffers post-concussion symptoms after the first head injury, which may include headache; labyrinthine dysfunction; visual, motor, or sensory changes; or mental difficulty, especially cognitive and memory problems. Before these symptoms resolve, which may take days or weeks, the athlete returns to competition and receives a second blow to the head. The second blow may be remarkably minor, perhaps involving only a blow to the chest that indirectly injures the athlete's head by imparting accelerative forces to the brain. The affected athlete may appear stunned, but usually does not experience loss of consciousness (LOC) and in the case of football, he often completes the play. Indeed, the individual usually remains on their feet for 15 sec to 1 min or so, but appears dazed, like someone suffering from a grade 1 concussion without LOC. Frequently the affected athlete remains on the playing field or walks off under their own power. What happens in the next few seconds to several minutes, however, sets this syndrome apart from a concussion. During this period the athlete, who is conscious yet stunned, quite precipitously collapses to the ground, semi-comatose with rapidly dilating pupils, loss of eye movement, and respiratory failure. Although the vast majority of the second-impact syndrome (SIS) cases in the literature involve athletes under the age of 18, it can also be seen in college athletes, as our experiences and those of others indicate, (Mori et al., 2006). Herein we demonstrate that in addition to this characteristic clinical scenario, the admission and follow-up imaging findings are also remarkably similar in these patients.
Pathophysiology of Second-Impact Syndrome
The pathophysiology of the SIS is controversial, but it is generally believed to be caused by a loss of autoregulation of the cerebrovasculature (Cantu, 2000; Junger et al., 1997; (Strebel et al., 1997). This dysautoregulation leads to hyperemic brain swelling within the cranium, which in turn increases intracranial pressure (ICP), and causes subsequent inferomedial herniation of the temporal lobes, herniation of the cerebellar tonsils through the foramen magnum, and brainstem compression. Animal research has shown that vascular engorgement of the brain after mild head injury can be difficult, if not impossible, to control (Langfitt et al., 1965; McQuillen et al., 1988). As mentioned above, the usual time period from the second impact to brainstem failure is rapid, taking 2–5 min. Once brain herniation and brainstem compromise occur, ocular involvement and respiratory failure precipitously ensue. Of note, clinical deterioration occurs far more rapidly than is usually seen with an epidural hematoma (Cantu, 1995). CT and MRI can demonstrate the imaging manifestations and complications of SIS. Specifically, they reveal the initial cerebral hyperemic swelling, brain herniation, post-herniation ischemia, and potential intracranial hemorrhage (Le and Gean, 2009). Although MRI can more precisely characterize the injury, CT is the initial imaging study of choice, because it rapidly and accurately demonstrates intracranial hemorrhage and midline shift that may require immediate neurosurgical intervention (Orrison et al., 1994). Furthermore, CT technology more easily handles the life-support equipment that usually accompanies these patients.
While the SIS may not always occur with intracranial hemorrhage, a number of cases have been reported recently in which acute hemispheric swelling occurred in association with a thin subdural hematoma (SDH) in athletes who received a second injury while still clinically symptomatic from the first impact (Mori et al., 2006). Those authors presented four cases of their own and four cases from the literature. We present 10 additional cases of what is thought to be SIS in association with a thin acute SDH. Further, we carefully scrutinized the imaging characteristics of these patients.
Methods
Eighteen prior published cases of repetitive head injury while the athlete was still symptomatic from a prior head injury, including eight with a thin, acute SDH, have been reported (Mori et al., 2006). Here we add 10 new cases of our own, all of which were characterized by a small acute SDH, associated with impressive cerebrovascular engorgement (brain swelling) on admission CT (Table 1). Our cases were analyzed with regard to gender, age, sport, duration of symptoms before the second injury, and Glasgow Coma Scale score (GCS) after the second injury. CT and MRI examinations, when available, were assessed for the following features: (1) maximal thickness of the SDH, (3) heterogeneity of the SDH, (4) degree of midline shift, (5) effacement of basal cisterns and cerebral sulci, (5) brainstem distortion, (6) the presence of intra-axial hemorrhage (e.g., contusions or traumatic axonal injury), (7) hemispheric asymmetry (defined as the thickness of the hemisphere measured at the level of the lateral ventricles), (8) preservation of hemispheric gray-white matter differentiation, and (9) post-traumatic ischemic infarction on the follow-up imaging study (when available). All of the studies were reviewed by both a board-certified neuroradiologist with > 25 years of experience in interpreting neurotrauma images at a level 1 trauma center (A.D.G.), and a board-certified neurosurgeon with > 40 years of experience in reviewing CT images (R.C.C.).
Table 1.
Ten Cases of Second-Impact Syndrome and a Small Subdural Hematoma
| Case | Age/gender | Sport | First injury | Ongoing S/S | Delay to second injury (days) | Second injury | Head CT | GCS score/outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 13/male | AF | −LOC | Headache, dizziness | 0 | −LOC/RC | ASDH/UHS | 7/SND |
| 2 | 16/male | AF | −LOC | Headache | 7 | −LOC/RC | ASDH/UHS | 4/SND |
| 3 | 17/male | AF | −LOC | Headache | 14 | −LOC/RC | ASDH/UHS | 3/SND |
| 4 | 19/male | AF | −LOC | Headache | 32 | +LOC/RC | ASDH/UHS | 5/D |
| 5 | 15/male | AF | −LOC | Headache | 7 | −LOC/RC | ASDH/UHS | 4/SND |
| 6 | 15/male | AF | −LOC | Headache | 14 | −LOC/RC | ASDH/UHS | 4/SND |
| 7 | 17/male | AF | −LOC | Bell rung | 0 | −LOC/RC | ASDH/UHS | 4/SND |
| 8 | 16/male | AF | −LOC | Headache | 3 | −LOC/RC | ASDH/UHS | 3/D |
| 9 | 16/male | AF | −LOC | Headache | 28 | −LOC/RC | ASDH/UHS | 3/D |
| 10 | 10/female | AF | −LOC | Headache | 3 | −LOC/RC | ASDH/UHS | 3/D |
AF, American football; −LOC/RC, no loss of consciousness with rapid deterioration to deep coma in minutes; +LOC, loss of consciousness; ASDH/UHS, thin acute subdural hematoma with marked unilateral hemisphere swelling; SND, severe neurological deficit; D, death; S/S, signs and symptoms; GCS, Glasgow Coma Scale; CT, computed tomography.
Results
Case 1
A 13-year-old eighth-grade middle school football player was involved in a helmet-to-helmet collision while attempting to recover a fumble of a kick off. He was not rendered unconscious, and he was able to walk back into the huddle where he complained of a headache and dizziness. Despite these post-concussion symptoms he remained in the game. On videotape, he was observed to miss assignments by lining up inappropriately both in the huddle and in the wrong position for the play. Several minutes after a less severe hit, he precipitously became comatose on the field with fixed, dilated pupils, no gag reflex, and labored respirations. He was intubated and emergently transported to the hospital where his GCS score was 7. An emergent head CT scan revealed a small right convexity acute SDH with severe midline shift and mass effect that appeared disproportionate to the size of the collection. An urgent right frontotemporal-parietal craniotomy was performed and the acute SDH was removed. No active bleeding site was found, but massive swelling was observed. Due to a persistent elevation in ICP, a second surgical procedure (left frontotemporo-parietal decompressive craniectomy) was performed several days later. The child survived with severe cognitive, motor, and sensory deficits.
Case 2
A 16-year-old high school football player developed a headache after making a tackle late in the game. The headache persisted over the next week, but he did not tell his coaches. During the next game, he became dazed after he was tackled with a head hit. Several minutes later he lapsed into a coma on the field with agonal respirations. At the hospital his GCS score was 4. The admission head CT scan showed a small left hemispheric acute SDH with severe ipsilateral hemispheric swelling and midline shift. The patient was urgently taken to surgery where the acute SDH was removed and a severely hyperemic brain was observed. Postoperatively, the brain swelling was treated with mannitol and controlled ventilation. The patient survived with severe cognitive and right-sided hemiplegic deficits.
Case 3
A 17-year-old high school quarterback sustained a concussion in a helmet-to-helmet collision. Despite a headache and notably diminished skills, he continued to play and finished the game. Two weeks later, despite still experiencing a headache, he played the entire first half of the game as the starting quarterback and free safety. In the third quarter after scoring his third touchdown, he told his coach that he felt “weird” and indicated that his vision was “blotchy,” like he was “looking into the sun.” His coach apparently saw no reason for concern and allowed him to remain on the sidelines without seeking medical attention. His fellow players, who were aware that he had a progressively more intense headache, helped him as he lay on the ground seeking relief. The headache progressed to the point where he began crying, and a towel was placed over his head so that friends and family in the stands could not see him crying. Eventually, an electric car was brought to the sidelines and he was driven to the trainer's locker room area, where he lapsed into coma. He was airlifted to the hospital where he arrived with a GCS score of 3. Head CT showed a small acute SDH with massive unilateral hemisphere swelling. Despite urgent neurosurgical evacuation of the acute SDH he died, with severe uncal and tonsillar herniation noted at post-mortem examination.
Case 4
A 19-year-old college football player sustained two separate concussions 4 days apart due to helmet-to-helmet hits. He was held out 2 weeks, but he was still symptomatic with headaches when he played 1 month later. In that game he received another helmet-to-helmet hit, briefly lost consciousness, regained consciousness, attempted to stand up, and then lapsed into a coma. He was decerebrate at the hospital, with a fixed 5-mm right pupil and a fixed 1-mm left pupil. His GCS score was 5. An emergency CT showed a small right SDH with abnormal cerebral hyperemic swelling. The patient was rushed to the operating room and the SDH was removed. The neurosurgeon commented that the brain was so swollen that he had to do a hemicraniectomy. One month later the patient was conscious, but could not follow commands. Though there has been neurological improvement, the patient has been left with devastating neurological cognitive, visual, motor, and sensory deficits.
Case 5
A 15-year-old high school football player developed a headache following a game earlier in the week. On the day of the next game, he told his fellow players that he had the “worst headache of my life.” He played nonetheless, not telling coaches or trainers about his headache. He was involved in several significant hits, including one to the back of his head. He came off the field on his own, but within minutes lapsed into a coma with fixed and dilated pupils. At the hospital his GCS score was 4. Admission head CT showed a small acute SDH with disproportionate cerebral hyperemic swelling. An emergency craniotomy and removal of the SDH was performed. He ultimately made a remarkable recovery and was able to return to school, where his grades were B's and C's, down from A's and B's. He currently has dense right homonymous hemianopia and right hemiparesis.
Case 6
A 15-year-old high school linebacker suffered a concussion in a scrimmage, and a fellow linebacker said that after the hit he was “dazed and confused, out of it.” The trainer documented dizziness and headache, but there was no follow-up of the head injury. In the subsequent days, the patient reported having a headache to his mother, who gave him acetaminophen. In a game 2 weeks later he received a helmet-to-helmet hit and was assisted to the bench. Shortly thereafter he vomited, collapsed, and became unresponsive with decorticate posturing. At the hospital he was comatose with a GCS score of 5. He had a dilated left pupil, inadequate respirations, and he responded only to painful stimulation. The emergent head CT showed a small left SDH, abnormal cerebral hyperemic swelling, complete effacement of the basal cisterns, and brainstem distortion (Fig. 1). An emergency craniotomy with evacuation of the SDH was performed. Postoperative CT and MRI exams revealed multifocal ischemic infarction. He has been able to return to school at a reduced cognitive level, altered personality, and spasticity of the right limbs.
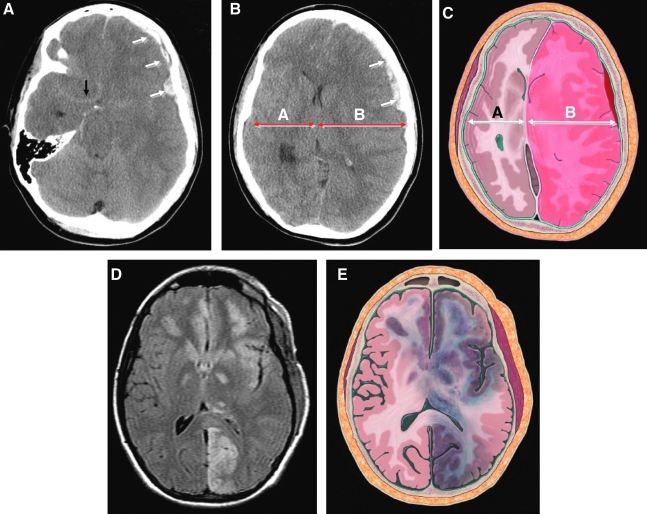
FIG. 1.
(Case 6) Typical imaging findings of dysautoregulation/second-impact syndrome (DSIS). (A and B) Admission non-contrast axial CT images, and (C) artist's rendition demonstrate a small heterogeneous left frontal subdural hematoma (SDH; white arrows), that causes complete effacement of the basal cisterns and brainstem distortion. Note the subtle linear increased density in the region of the circle of Willis (black arrow), consistent with “pseudo-subarachnoid hemorrhage,” resulting from the marked elevation in intracranial pressure. Although there is preservation of the gray-white matter differentiation, there is asymmetric enlargement of the left hemisphere, consistent with hyperemic cerebral swelling (dysautoregulation). Note how side A is smaller than side B, even though the left hemisphere is mildly compressed by the overlying SDH. The extent of mass effect and midline shift is disproportional to the volume of the SDH (compare with Figs. 3 and 4). This 3-day-postoperative FLAIR MR image (D), and artist's rendition (E), demonstrate bilateral multifocal ischemic lesions involving several vascular territories, including the left posterior cerebral artery, thalamus, insular cortex, basal ganglia, and orbitofrontal cortex. Diffusion-weighted MR images were positive for acute ischemic injury, and the gradient-echo sequence excluded hemorrhage in these areas (not shown).
Case 7
A 17-year-old high school football player was involved in several hard tackles and appeared stunned, though he did not leave the game. Later, after a hard hit in which his head was violently snapped back, he got up and returned to the huddle, but before the next play could be run he collapsed into a coma. The GCS score at the hospital was 4 and he had a blown right pupil. He was intubated and treated with mannitol. The emergency head CT showed a small right acute SDH with significant right-sided brain swelling and marked midline shift. A right decompressive craniectomy and evacuation of the SDH was performed. His GCS score on the day following the surgery was 6, but it improved to 12 over the next week. He gradually improved over the next few months to the point where he could care for himself, and he ultimately returned to school at a diminished cognitive level.
Case 8
A 16-year-old high school football player sustained a helmet-to-helmet collision during practice. He was briefly unconscious, but the incident was not reported to medical personnel. Thereafter he complained of a severe headache, but played 2 days later. After making and receiving several tackles, he walked off the field and promptly collapsed into a coma. At the hospital his GCS score was 3. The head CT showed a small acute SDH and cerebral hyperemic swelling. Neurosurgery was consulted but it was felt that his was a non-survivable injury and no surgery occurred. He was declared brain dead the following day.
Case 9
A 16-year-old high school football player was “hit hard during scrimmage” and subsequently complained of headaches. He was seen by a physician 4 days later and was advised not to play. A CT scan was obtained at that time but was incorrectly read as negative. Later, a tiny SDH was found to be present. The patient sought a “second-opinion physician” who cleared him to play. He then participated in non-contact practice complaining only of minimal visual difficulty but no headache. During a game 4 weeks later he was “hit from all sides,” and immediately felt tingling and dizzy. He was removed from the game but the team physician failed to examine him. Twelve plays later, he re-entered the game, but then voluntarily walked off the field four plays later feeling nauseated. He became unresponsive within minutes and was life-flighted with a GCS score of 3. The emergent CT exam revealed a holohemispheric heterogeneous left SDH with marked mass effect and midline shift that was disproportionate to the size of the collection (Fig. 2). Emergency evacuation of the SDH and a decompressive hemicraniectomy was performed. Postoperative CT exams revealed post-herniation ischemic infarction involving the anterior and posterior cerebral artery territories. He died several days postoperatively.
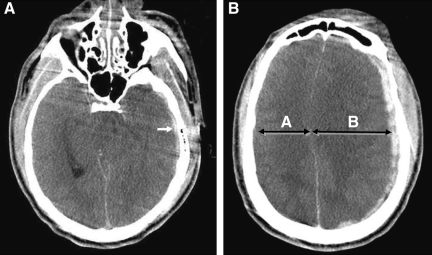
FIG. 2.
(Case 9). Typical imaging findings of dysautoregulation/second-impact syndrome (DSIS). (A) Admission non-contrast axial CT image demonstrates a small mildly heterogeneous holohemispheric left temporal subdural hematoma (SDH; arrow), with complete effacement of the perimesencephalic cisterns and convexity sulci, and severe brainstem distortion. (B) An image at the level of the centrum semiovale shows a 9-mm SDH with asymmetrical enlargement of the left hemisphere, and relative preservation of gray-white matter differentiation, consistent with hyperemic cerebral swelling (dysautoregulation). Note how side A is smaller than side B, despite the mild compression from the overlying SDH. The extent of mass effect and midline shift is disproportional to the volume of the SDH (compare with Figs. 3 and 4).
Case 10
A 10-year-old girl was playing in the pee-wee football league. She was hit during a scrimmage and voluntarily went to the sideline complaining of dizziness and a headache. During practice 3 days later, she started to cry and said that her head hurt. She took off her helmet and began to vomit. Within minutes she collapsed, lost consciousness, and her pupils became fixed and dilated. Her admission GCS score was 3. An emergent CT scan demonstrated a small heterogeneous SDH and hyperemic swelling of the subjacent hemisphere. An emergency decompressive hemicraniectomy was performed and the SDH was removed. Marked cerebral swelling was noted at surgery. A follow-up CT study demonstrated severe hypoxic-ischemic injury and external herniation. She died of refractory intracranial hypertension 3 days later.
Discussion
According to the records at the National Center for Catastrophic Sports Injury Research, (Mueller and Cantu, 2009), an acute SDH is the most common cause of death due to head injury in sports. In our own research involving American football, we found that 38% of athletes receiving such an injury were playing while still symptomatic from a prior head injury sustained during that season (Boden et al., 2000). We also found that a number of those athletes experienced dramatic and immediate brain swelling consistent with dysautoregulation and SIS (Boden et al., 2000; Mueller and Cantu, 2009). A recent publication reviewed 18 patients with repetitive head injury who were still symptomatic from a prior head injury (Mori et al., 2006). All of the athletes showed immediate SIS on emergent head CT imaging. The authors also reviewed eight cases of repetitive head injury with a thin acute SDH in combination with “more impressive” brain swelling. The authors were likely correct in their belief that SIS occurs because of the loss of autoregulation of cerebral blood flow, with resultant vascular engorgement, increased ICP, and eventual brain herniation. We believe that they may be incorrect in ascribing the cause of the loss of autoregulation and brain swelling to the thin SDH. Rather, we believe that the cause of the brain swelling in these repetitive head injury cases is the acceleration/deceleration forces that caused both the SDH and SIS. The acceleration forces required to produce an SDH are greater than those required to produce a concussion or SIS (Cantu, 2000). Thus, in those athletes receiving a second head injury while still symptomatic from the first impact, and with forces being severe enough to produce a thin SDH, the forces are more than severe enough to produce the SIS. We believe, therefore, that it is the acceleration forces, not the SDH, that produces the vascular engorgement (i.e., SIS), with resultant increased ICP and brain herniation.
Vascular engorgement can definitely be seen following a single injury that results in an SDH. However, in those cases the forces are usually far greater than with SIS, and there is usually instantaneous and prolonged coma secondary to direct reticular activating tract injury, not secondary to brain herniation. The lucid interval, or brief LOC followed by several minutes of being lucid that is characteristic of SIS, is not observed in those cases.
In many acute SDHs, the volume of the extra-axial collection is proportional to the extent of mass effect and midline shift (Fig. 3). In this situation, there is no hyperemic swelling, and no hemispheric asymmetry. In the chronic SDH, depending on the compliance of the underlying brain parenchyma, the collection compresses a non-hyperemic hemisphere (Fig. 4). In this setting, the volume of the hemisphere beneath the SDH is smaller than the contralateral hemisphere.
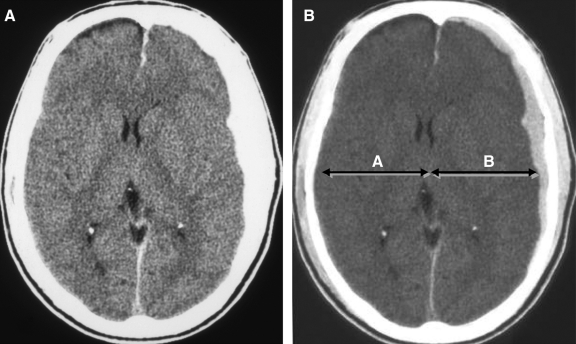
FIG. 3.
Typical acute subdural hematoma (SDH) without dysautoregulation/second-impact syndrome (DSIS) in a 17-year-old male following a motor vehicle accident (compare with Figs. 1 and 2). Admission non-contrast axial CT images at narrow (A) and wide (B) window widths demonstrate a small homogeneous left frontal SDH with minimal midline shift. Note how the volumes of the cerebral hemispheres are relatively symmetrical, and that the extent of mass effect and midline shift are proportional to the volume of the SDH.
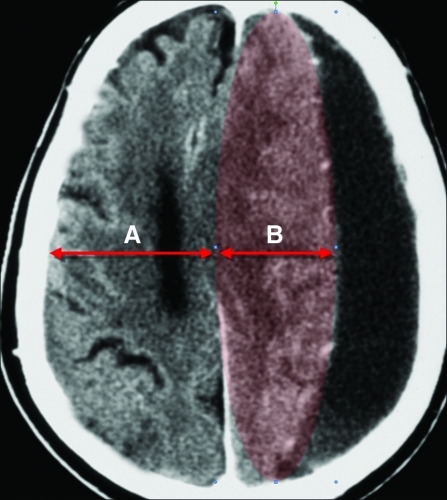
FIG. 4.
Typical chronic subdural hematoma (SDH) without dysautoregulation/second-impact syndrome (DSIS) in a 65-year-old male (compare with Figs. 1 and 2). In contrast to DSIS patients, note how the volume of the left hemisphere (highlighted in red) is smaller than the right hemisphere. This is likely due to the absence of cerebral hyperemic swelling (i.e., intact autoregulation), combined with compression by the overlying SDH.
In the 18 cases reported by Mori (Mori et al., 2006), of male adolescents and young adults who returned to play before symptoms of a prior head injury had resolved, there were 8 patients who had a thin acute SDH in association with unilateral acute hemispheric swelling on brain CT (Mori et al., 2006). In 6 of the 8 cases, there was no LOC with the initial head injury, and all 8 cases had persistent symptoms. After the second head insult, there was no immediate LOC, but within minutes the athlete precipitously lapsed into coma with signs of brain herniation (i.e., the typical SIS scenario).
Similarly, 9 of the 10 patients in our study were young males playing American football, and the 10th patient was a 10-year-old girl playing pee-wee football. There was no LOC with the initial concussion, and they all had persistent post-concussion symptoms. Following the second impact, there was no immediate LOC in 9 patients, and for only seconds in 1 patient, but within minutes of the subsequent hit, all of the athletes rapidly lapsed into a coma with blown pupil, respiratory arrest, and signs of brain herniation; again, the typical SIS scenario.
Nine of our 10 patients were under the age of 18, whereas in Mori's study group 6 out of 8 patients were over the age of 18, including 2 in American football, 2 in boxing, and 1 each from karate and skiing (Mori et al., 2006). This clearly raises the question of whether the recent Zurich guidelines recommending that athletes under the age of 18 should not return to the same contest after a concussion should also be extended to those over the age of 18. While the period between the first injury and the second injury ranged from 0–32 days, the fact that in two instances it was during the same contest supports the concept of not returning an athlete with a concussion to the same contest. Our data also emphasize that many mild concussions, especially in American football, are missed when the athlete does not report symptoms to medical personnel. In the work of Delaney, they found this underreporting rate to be as high as 70% (Delaney et al., 2002).
The above characteristic clinical scenario is also associated with a characteristic imaging scenario. In all of our patients: (1) the maximal thickness of the SDH was < 0.5 cm, (2) the SDH was heterogeneous, (3) the basal (perimesencephalic) cisterns and cerebral sulci were completely effaced, (4) the brainstem was morphologically distorted due to uncal and diencephalic herniation, (5) there was no evidence of intra-axial hemorrhage (e.g., contusions or white matter shearing injury), (6) the gray-white matter differentiation was preserved within the cerebral hemispheres on the admission CT, (7) there was “hemispheric asymmetry” (defined as the thickness of the hemisphere measured at the level of the mid-lateral ventricles), and (8) multifocal post-traumatic ischemic infarction was noted on the follow-up imaging study (if the patient survived).
It is tempting to speculate about the imaging similarities between our cohort of adolescent football players and the typical victims of non-accidental trauma (NAT), and their clinical implications. In both instances, the mechanisms responsible for the brain injury are controversial, but they appear to have several things in common. First, both types of victims have likely suffered repetitive head injury. Second, it has been shown that there is an increased incidence of hypoxic-ischemic injury (HIE) in infants and children who are victims of NAT in comparison to accidental head trauma (Ichord et al., 2007). Our cases also show a clear predilection for HIE, with all of our surviving patients showing imaging evidence of multifocal bilateral ischemic injury in the absence of white matter shearing lesions and intracerebral hemorrhage. Third, victims of NAT, like our patients, frequently have a small SDH that seems to be more of an “innocent bystander” than the cause of the massive elevation in ICP. Fourth, the unusual vulnerability to dysautoregulation seen in NAT also appears to be occurring in our patients. The engorged cerebrovasculature, defined in our study as an increase in volume of the cerebral hemisphere with preservation of the gray-white matter differentiation on CT, was noted in all of our athletes. Complete effacement of the convexity and basal cisterns was noted in all patients. The dysautoregulation contributed more to the elevation in ICP than the SDH. Fifth, it is known that the outcome among survivors of NAT is poor, with the majority of victims suffering permanent morbidity (Duhaime et al., 1998). This is also the case with survivors of SIS. There is also animal study evidence of the increased vulnerability of the younger brain to repeated mild traumatic brain injury. (Raghupathi et al., 2004).
Conclusion
Ten additional cases of acute hemispheric swelling in association with a small acute SDH in athletes receiving a second head injury while still symptomatic from a prior head injury were described. Parallels between victims of NAT and our cohort of patients are theoretically presented. The pathophysiology of the swelling is thought to be due to the dysautoregulation/second-impact syndrome with rapid cerebrovascular congestion leading to increased ICP and subsequent brain herniation, all occurring within minutes of the second head injury. The impressive imaging findings are inconsistent with the often mild nature of the second impact, but they are consistent with the catastrophic clinical scenario of SIS. The most impressive acute CT finding in these patients is the engorged hemisphere, which appears as hemispheric enlargement despite mild compression by the overlying SDH. There is initial preservation of gray-white matter differentiation, but there is abnormal mass effect and midline shift (i.e., the imaging definition of “cerebral hyperemia”). The basal cisterns and cerebral sulci are completely effaced and the brainstem is distorted. A heterogenous thin SDH (<1 cm) may be seen, but its contribution to the overall mass effect is less than that of the swollen hyperemic hemisphere. None of these patients had concomitant intra-axial injury (e.g., contusion or traumatic axonal injury). If the patient survives the initial episode of intracranial hypertension, multifocal bilateral non-hemorrhagic ischemic infarction ensues.
Author Disclosure Statement
No competing financial interests exist.
References
- Boden B. Tacchetti R. Cantu R. Knowles S. Mueller F. Catastrophic head injuries in high school and college football players. Am J Sports Medicine. 2000;35:1–7. doi: 10.1177/0363546507299239. [DOI] [PubMed] [Google Scholar]
- Cantu R.C. Voy R. Second impact syndrome a risk in any contact sport. Phys. Sportsmed. 1995;23:27–34. doi: 10.1080/00913847.1995.11947799. [DOI] [PubMed] [Google Scholar]
- Cantu R.C. Neurological Athletic Head and Spine Injuries. WB Saunders; Philadelphia: 2000. p. 135. [Google Scholar]
- Cantu R.C. Second impact syndrome: Immediate management. Phys. Sportsmed. 1995;20:55–58. doi: 10.1080/00913847.1995.11947799. [DOI] [PubMed] [Google Scholar]
- Delaney J.S. Lacroix V.J. Leclerc S.L. Johnston K.M. Concussions among university football and soccer players. Clin. J. Sportsmed. 2002;12:331–338. doi: 10.1097/00042752-200211000-00003. [DOI] [PubMed] [Google Scholar]
- Duhaime A.C. Christian C.W. Rorke L.B. Zimmermen R.A. Nonaccidental head injury in infants—the “shaken baby syndrome.”. N. Engl. J. Med. 1998;338:1822–1828. doi: 10.1056/NEJM199806183382507. [DOI] [PubMed] [Google Scholar]
- Ichord R.N. Naim M. Pollock A.N. Nance M.L. Margulies S.S. Christian C.W. J. Neurotrauma. 2007;24:106–118. doi: 10.1089/neu.2006.0087. [DOI] [PubMed] [Google Scholar]
- Junger E.C. Newell D.W. Grant G.A. Avellino A.M. Ghatham S. Dauville C.M. Lam A.M. Aaslid R. Winn H.R. Cerebral autoregulation following minor head injury. J. Neurosurg. 1997;86:425–532. doi: 10.3171/jns.1997.86.3.0425. [DOI] [PubMed] [Google Scholar]
- Langfitt T.W. Weinstein J.D. Kassell N.F. Cerebral vasomotor paralysis produced by intracranial hypertension. Neurology. 1965;15:622–630. doi: 10.1212/wnl.15.7.622. [DOI] [PubMed] [Google Scholar]
- Le T.H. Gean A.D. Neuroimaging of traumatic brain injury. Mt. Sinai J. Med. 2009;76:145–162. doi: 10.1002/msj.20102. [DOI] [PubMed] [Google Scholar]
- McQuillen J.B. McQuillen E.N. Morrow P. Trauma, sports, and malignant cerebral edema. Am. J. Forensic Med. Pathol. 1988;9:12–16. doi: 10.1097/00000433-198803000-00004. [DOI] [PubMed] [Google Scholar]
- Mori T. Katayama Y. Katayama T. Acute hemispheric swelling associated with thin subdural hematomas: pathophysiology of repetitive head injury in sports. Acta Neurochir. 2006;96:40–43. doi: 10.1007/3-211-30714-1_10. [DOI] [PubMed] [Google Scholar]
- Mueller F.O. Cantu R.C. Chapel Hill; NC: 2009. National Center for Catastrophic Sports Injury Research 27th Annual Report. Fall 1982 to Spring 2009. [Google Scholar]
- Orrison W.W. Gentry L.R. Stimac G.K. Tarrel R.M. Espinosa M.C. Cobb L.C. Blinded comparison of cranial CT and MR in closed head injury evaluation. Am. J. Neuroradiol. 1994;15:351–356. [PMC free article] [PubMed] [Google Scholar]
- Raghupathi R. Mehr M.F. Heflaer M.A. Margulies S.S. Traumatic axonal injury is exacerbated following repetitive closed head injury in the neonatal pig. J. Neurotrauma. 2004;21:307–316. doi: 10.1089/089771504322972095. [DOI] [PubMed] [Google Scholar]
- Saunders R.L. Harbaugh R.E. Second impact in catastrophic contact-sports head trauma. JAMA. 1984;252:538–539. [PubMed] [Google Scholar]
- Schneider R.C. Head and Neck Injuries in Football. Williams & Wilkins; Baltimore: 1973. [Google Scholar]
- Schnitker M.T. A syndrome of cerebral concussion in children. J. Pediatr. 1949;35:557–560. doi: 10.1016/s0022-3476(49)80138-7. [DOI] [PubMed] [Google Scholar]
- Strebel S. Lam A.M. Matta B.F. Newell D.W. Impaired cerebral autoregulation after mild brain injury. Surg. Neurol. 1997;47:128–131. doi: 10.1016/s0090-3019(96)00459-4. [DOI] [PubMed] [Google Scholar]