Abstract
Purpose
Dry eye (DE) is a common ocular surface disease, particularly among women and the elderly, with chronic symptoms of eye irritation and, in severe cases, blurred vision. Several studies have shown that there is an inflammatory component in DE, although the pathogenesis is not thoroughly understood. Resolvin E1 (RvE1; RX-10001) is an endogenous mediator derived from the omega-3 polyunsaturated fatty acid eicosapentaenoic acid and is involved in inflammation resolution and tissue protection. Here we investigated the role of RvE1 in a DE mouse model.
Methods
Thirteen- to 14-week-old female BALB/C mice were exposed to desiccating conditions. One week after DE exposure, animals were treated topically with drug or vehicle 4 times per day for an additional week. Controls were nontreated animals placed in a normal environment. Schirmer's test was performed before treatment initiation and at days 2 and 4 after treatment. Density of corneal epithelial cells was analyzed in vivo using the Rostock Cornea Module of the Heidelberg Retina Tomograph (HRT-II). Corneas were processed using Western blot analysis and immunofluorescence examination.
Results
Schirmer's test showed a significant decrease in tear production in DE compared with controls. There was no change at 2 and 4 days after treatment with the vehicle, but a significant increase was observed at 2 and 4 days in the RvE1-treated group. The density of the superficial epithelial cells showed a significant decrease after DE compared with controls, which increased after 7 days of RvE1 treatment. Western blot analysis showed that α-smooth muscle actin and cyclooxygenase-2 (COX-2) expression were strongly upregulated after DE and decreased after 7 days of RvE1 treatment. Immunofluorescence confirmed strong positive staining of α-smooth muscle actin and COX-2 in stroma and/or in epithelia after DE, which decreased with RvE1 treatment. The percentage of infiltrating CD4+ T cells and CD11b+ cells decreased after RvE1 treatment when compared with DE.
Conclusion
RvE1 promotes tear production, corneal epithelial integrity, and a decrease in inflammatory inducible COX-2. In the stroma, RvE1 inhibits keratocyte transformation to myofibroblasts and lowers the number of monocytes/macrophages in this DE mouse model. These results suggest that RvE1 and similar resolvin analogs have therapeutic potential in the treatment of DE.
Introduction
Dry eye (DE) syndrome is one of the most prevalent of all chronic inflammatory conditions, including rheumatoid arthritis and psoriasis, with an estimate of up to 25 million people affected in the United States alone, of whom possibly as many as 25% have a severe debilitating form.1,2 The pathogenesis of DE is not thoroughly understood, but aging, hormonal changes during menopause, endocrine disorders, and environmental factors all contribute. Inflammation and cell death are considered major contributors to DE symptoms and loss of corneal integrity.3,4 Therefore, controlling the inflammatory response is an important aspect of preserving the integrity and health of the ocular surface. Treatment options remain limited; some patients respond to the only approved compound, cyclosporine eye drops, but most patients are confined to artificial tears, which only offer temporary relief and do not change the chronic progressive course of the condition.5,6
Resolvins belong to a novel class of lipid-derived endogenous molecules that have potent immune-modulating properties, which regulate the resolution phase of an active immune response. These molecules are derived from the omega-3 polyunsaturated fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), and act locally to stop leukocyte recruitment and promote resolution of the inflammatory response.7 The first described resolvin, resolvin E1 [RvE1; 5(S), 12(R), 18(R)-trihydroxyeicosapentaenoic acid; RX-10001], which is derived from EPA, was isolated in inflammatory exudates during the resolution phase of acute inflammation in mice. Following its identification and synthesis, administration of RvE1 prior to an acute insult was shown to ameliorate the inflammatory response.8 Since then, resolvins have been demonstrated to exert broad regulatory actions that would favor their use as therapeutics in both acute and, more importantly, chronic inflammation. RvE1 is now demonstrated to stimulate the resolution of inflammatory airway responses in the lung by suppressing interleukin (IL)-23, IL-17, and IL-6 production9; increase survival and reduce tissue inflammation in a mouse model of colitis10; and reduce retinal neovascularization in an animal model of oxygen-induced retinopathy.11 The endogenous formation of resolvins may also explain the recently published data from the longitudinal women's health study in which women on a high daily diet of omega-3 polyunsaturated fatty acids had a lower frequency of DE symptoms than those on a more common regimen.12
In addition to dampening antigen-dependent inflammation, RvE1 was recently demonstrated to promote host tissue cell survival during induced stress conditions (either oxidative stress or hypoxia/reoxygenation) by downregulating proapoptotic pathways, including caspases, and activating prosurvival pathways, including the PI3K/Akt pathway, a major regulator of cell survival.13–15 RvE1 activation of the PI3K/Akt pathway may also explain its wound-closing properties, as demonstrated in the human corneal epithelial cell scratch wound assay. In this assay, RvE1 was as efficacious as the reference compound, epidermal growth factor.13 Taking into account their properties of immune modulation, effect on host tissue survival, and stimulation of epithelial cell migration in the repair of a disintegrated epithelial barrier, resolvins make ideal candidates for therapeutic treatment of DE syndrome.
We now have investigated the therapeutic potential of RvE1 in a murine model of desiccating stress with proven translational value.4 To enhance its penetration through the corneal epithelium, RvE1 was administered as an ester prodrug (RX-10005) using the prodrug principle that was successfully utilized for prostaglandin-derived products in treating glaucoma.16 Independent pharmacokinetic studies also have confirmed the applicability of this approach for resolvins with rapid cleavage into the active parent moiety.17
The data reported here indicate that RvE1 was efficacious when administered to mice with established desiccating stress-induced DE.
Methods
Drug preparation
Stock solution (1 mg/mL) of RX-10005 (the methyl ester prodrug of RvE1/RX-10001) was provided by Resolvyx Pharmaceuticals (Bedford, MA). The vehicle was 1.0% Tween 80 (DPECTRUM, Gardena, CA) in a 100 mM aqueous phosphate buffer (pH 6.8). Before use, the vehicle was filtered through a 0.2-μm syringe filter to ensure sterility. Drugs were diluted to 100 μg/mL (0.01%). Drug preparation was performed twice a day.
Induction of DE and treatment groups
Female BALB/C mice (Charles River, Wilmington, MA), 13–14 weeks of age, were used in the experiments. The animal protocol was approved by the Institutional Animal Care and Use Committee, Louisiana State University Health Sciences Center, New Orleans, and conformed to the standards in the Association for Research in Vision and Ophthalmology statement for the Use of Animals in Ophthalmic and Vision Research.
Experimental DE was induced as previously described by topical treatment of 1% atropine (Bausch & Lomb, Tampa, FL) every other day for 2 weeks. Mice were exposed to a desiccating environment created by placing the animals between 2 fans to obtain a continuous airflow (15 L/min) in a room at 22°C with a relative humidity of 25% for 2 weeks.18
Before starting the experiment, the mice were randomly divided into 4 groups. Group 1 was placed in normal conditions as a control group. Group 2, the DE control, received no drug treatment. Group 3 was DE + vehicle treatment, and group 4 was DE + RvE1 treatment. Vehicle and RvE1 were delivered as a 5 μL drop, 4 times per day, starting at day 8.
Measurement of tear volume (Schrimer's test)
Tear volume, without systemic and topical anesthesia, was assessed with a phenol red-soaked cotton thread (Menicon America, San Mateo, CA) and applied using forceps in the lateral canthus for 15 s. The wetting length of the thread was read by the examiner in a masked fashion under a microscope by using a ruler offered by the manufacturer. Tear volume was measured in all groups on the initial day of treatment but before the first administration of RvE1 and again at 2 and 4 days of treatment.
In vivo confocal microscopy
The Rostock Cornea Module of the Heidelberg Retina Tomograph (HRT-II/RCM; Heidelberg Engineering GmbH, Heidelberg, Germany) was used for corneal surface layer examination. Mice were anesthetized by intramuscular injection of 2 mg/kg body weight of xylazine and 50 mg/kg body weight of ketamine and placed in a modified 50-mL centrifugation tube mounted on a test tube holder, as explained previously.18 Corneas from 3 animals per group were examined. Six confocal microscope images of the superficial and basal epithelia were recorded per sample. The laser source was a diode laser with a wavelength of 670 nm, and the microscope objective was an immersion lens with magnification 60 × and numerical aperture 0.90 (Olympus, Hamburg, Germany). A drop of genteal gel (Novartis, St. Louis, MO) was placed on the tip of the objective lens to maintain immersion contact between the objective lens and the eye. Images were taken covering an area of 400 × 400 μm and transverse optical resolution of ∼1 μm/pixel. Then, images of the superficial epithelium were analyzed qualitatively and quantitatively and compared between the 5 groups. A counting area was defined using the software program after superimposing a 0.64-mm2 box boundary made manually. Each cell was marked using the interactive computer display. By convention, the cells overlapping the edges of the boundary were only counted on the left and lower sides of the box. Finally, the number of marks was counted by the computer, and cellular densities were expressed as cells/mm2. All readings were done by an observer masked to the treatment.
Western blot analysis
After mice were euthanized, corneas (6–8 corneas/sample) were isolated and digested with 0.3% collagenase A at 37°C overnight. Cells were collected after centrifugation and homogenized in 50 mM Tris-HCl (pH 7.5), 1 mM ethylenediaminetetraacetic acid, 1 mM ethyleneglycol-bis-N,N,N′,N′-tetraacetic acid (EGTA), 0.5 mM sodium orthovanadate, 1 mM dithiothreitol, 1% Triton X-100, 5 mM sodium fluoride, 1 mM sodium pyrophosphate, 150 mM NaCl, 10 mM sodium β-glycerophosphate, 1 mM phenylmethylsulfonyl fluoride, 1 μg/mL aprotinin and leupeptin, and 1 μM microcystin (lysis buffer). The homogenate was centrifuged at 12,000 rpm for 15 min, and total protein was determined in the supernatant. All procedures were performed at 4°C. Samples were resolved by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (9%–12% gel) and transferred to polyvinylidene difluoride membranes (Amersham Pharmacia Biotech, Piscataway, NJ). Biotinylated protein molecular weight standards were applied in 1 lane of each gel. The nonspecific proteins were blocked with 5% nonfat milk in tris-buffered saline [20 mM Tris-HCl, 150 mM NaCl (pH 7.6)] plus 0.1% Tween-20 for 1 h and then probed with polyclonal anticyclooxygenase-2 (COX-2) antibody (1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) and monoclonal anti-α-smooth muscle actin (α-SMA, 1:500 dilution; Sigma, St. Louis, MO), as described in the experiments, for 2 h at room temperature or overnight at 4°C. The membranes were washed 6 times with tris-buffered saline plus 0.1% Tween-20 and further incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies. Monoclonal anti-β actin antibody (1:40,000; Sigma) was used to reprobe the gels as a loading control. Protein bands were visualized using chemiluminescence detection reagents (ECL Plus; Amersham Pharmacia Biotech) and exposed to Fujifilm LAS-3000 system. The intensity of the bands was calculated by densitometric analysis (Fujifilm LAS-3000 Molecular Analysis program).
Immunofluorescence staining
Four eyes from different mice for each group were processed for histological analysis. Isolated eyes were embedded in optimal cutting temperature compound (Sakura Finetek USA, Torrance, CA). Serial 6-μm cryostat sections were cut, air-dried, and stored at −80°C until use. The slides were washed in phosphate-buffered saline (PBS), fixed with 2% paraformaldehyde in 0.1 M phosphate buffer for 30 min at 4°C, and permeabilized with 0.3% Triton X-100 solution for 5 min on ice. All the procedures were performed at room temperature. After 3 washes with PBS, the tissues were incubated with 10% normal goat serum in PBS containing 0.1% bovine serum albumin for 30 min to block nonspecific binding. Afterward, the tissues were incubated overnight at 4°C with monoclonal anti-COX-2 (1:200 dilution; BD Transduction Laboratories, Mississauga, Canada), monoclonal anti-α-SMA (1:1,000 dilution; Sigma), FITC anti-CD11b (1:100 dilution; BD Pharmingen), and monoclonal rat anti-CD4 (GK1.5; 1:500 dilution) (sc-13573; Santa Cruz Biotechnology) in PBS containing 1.5% normal goat serum (Santa Cruz Biotechnology). After they were washed in PBS containing 0.1% bovine serum albumin (3 times, 5 min each), the tissues were incubated with the corresponding secondary antibodies for 45 min. Controls without first antibody did not show staining. 4,6-Diamidino-2-phenylindole (Sigma) was used to counterstain the nuclei. The number of CDb11b+ cells was calculated by counting the cells/mm2. CD4+ cell density was calculated by counting the cells/mm2 area of stroma in each image. Six different fields were analyzed for each sample and values from 4 different eyes were the average.
Statistical analysis
Data from all experimental groups were expressed as mean ± standard error of the mean. Statistical analyses were performed using SPSS for Windows version 10.0. One-way ANOVA analysis with post hoc test was performed; a P value of less than 0.05 was considered to indicate statistical significance.
Results
RvE1 improves tear production
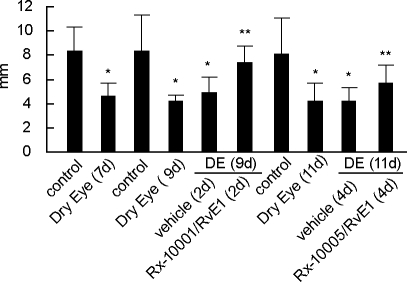
Schirmer's test was used to measure tear production. To confirm that the model could induce a state of reduced tear production, a first measurement was obtained after 7 days of desiccating conditions prior to initiation of treatment. Aqueous tear volume was significantly reduced in DE mice (4.5 ± 0.34 mm), compared with the normal control group (8.3 ± 0.75 mm; P < 0.001). These changes persisted at day 9 (7.57 ± 1.23 vs. 4.17 ± 0.15) and day 11 (8.14 ± 1.15 vs. 4.20 ± 0.47). By day 9, the RvE1 group already showed an improved tear secretion (7.36 ± 0.34), which was significant compared with the vehicle treatment (4.87 ± 0.41; P < 0.001). Compared with the untreated DE group, this amounted to an improvement of 60% over that achieved by vehicle. The difference between the vehicle and active treatments remained significant at day 11 (Fig. 1).
FIG. 1.
Schirmer's test in mice treated with resolvin E1 (RvE1). Tear volume was measured after 7 days in dry eye (DE) conditions and then at 2 and 4 days after treatment initiation. Values correspond to mean ± standard error of the mean (SEM). Number of animals tested: control = 7, vehicle = 9, DE = 9, and RvE1 = 13. *Significant differences versus control; **significant differences versus DE.
RvE1 prevents superficial epithelial cell loss
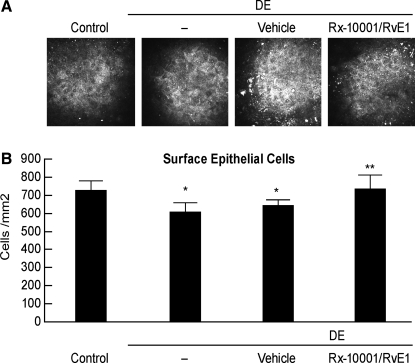
The superficial epithelial cells consistently displayed polygonal and sometimes hexagonal shapes. The cytoplasm and nucleus for each cell was visualized, and the bright nucleus, with its dark perinuclear space, was clearly visible in the center of each cell. No difference in cell shape was found among the various groups. The superficial epithelial cell density of the control group was 726 ± 22 cells/mm2. There was a 17% reduction of superficial epithelial cell density in the DE group (607 ± 20 cells/mm2) compared with the normal control group (P < 0.001; Fig. 2). Treatment with RvE1 for 1 week completely restored epithelial cell density at a level of 774 ± 32 cells/mm2 (P < 0.05 vs. vehicle control). No improvement was observed in the vehicle-treated group, which remained at significantly reduced cell numbers compared with the normal controls (P < 0.05).
FIG. 2.
Superficial epithelial cell density in mice treated with RvE1. (A) Representative images of mice cornea epithelial cell surfaces in different groups after 14 days of DE and 7 days of drug/vehicle treatments starting at day 8. (B) Quantification of epithelial cell density; the values correspond to average ± SEM of 3 animals in each group. Six different areas for each animal were quantified. The experiment was repeated 1 time with similar results. *Significant differences versus controls; **significant differences versus DE.
DE-induced α-SMA expression in the stroma is inhibited by RvE1
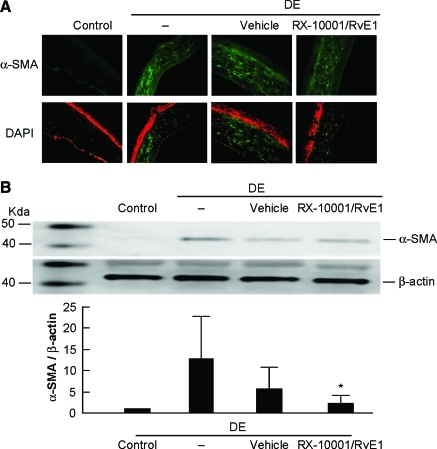
DE conditions promoted the expression of α-SMA, as analyzed by immunostaining and Western blot (Fig. 3). This indicates that stromal keratocytes were transformed into myofibroblasts. The decreased α-SMA staining observed after treatment indicates that RvE1 could prevent this transformation (Fig. 3A). In addition, Western blot analysis detected a 46 kDa band, corresponding to α-SMA, in corneas of DE mice (Fig. 3B). Quantification of blots from 3 different experiments showed that RvE1 significantly decreased the expression of α-SMA induced by DE (P < 0.05). The vehicle group had no significant effect.
FIG. 3.
Expression of α-smooth muscle actin (α-SMA) in mice treated with RvE1. Animals were in DE conditions for 14 days; drug and vehicle were applied for the last 7 days. (A) Representative images of immunostaining with an α-SMA antibody. Nuclei were counterstained with 4,6-diamidino-2-phenylindole (DAPI). (B) Western blot analysis shows a band at 47 kDa corresponding to α-SMA after induction of DE; β-actin shows equal loading of the gel. Values are average ± SEM of 3 blots corresponding to 3 different experiments. *Significant difference versus DE. Color images available online at www.liebertonline.com/jop.
RvE1 decreases DE-induced COX-2 expression
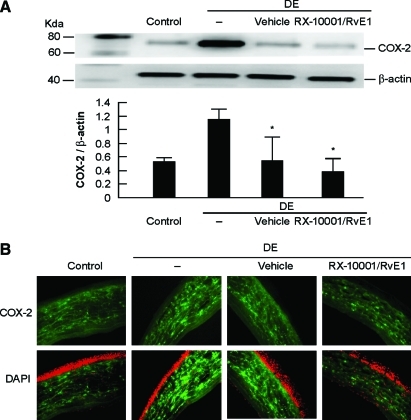
An increase in prostaglandins derived from COX-2 induction can be observed after corneal injury.19,20 Using both Western blot analysis and immunohistochemistry, we first determined that DE also can induce an upregulation of COX-2 expression. Protein extracts from mouse corneas showed a strong 78 kDa band corresponding to COX-2 in the DE group (Fig. 4A). COX-2 expression was significantly decreased in the group treated with RvE1 (P < 0.001). The vehicle group also displayed a decrease in COX-2 expression (P < 0.005).
FIG. 4.
Expression of cyclooxygenase-2 (COX-2) in mice treated with RvE1. Corneas were obtained after 14 days of DE conditions and treatment with drug and vehicle for the last 7 days. (A) Western blot analysis shows a 78 kDa band corresponding to COX-2. Values correspond to average ± SEM of 3 different experiments. (B) Immunostaining with a COX-2 antibody. The nuclei were counterstained with 4,6-diamidino-2-phenylindole. *Significant differences versus DE. Color images available online at www.liebertonline.com/jop.
Immunohistochemistry further confirmed the observations seen with Western blot. The corneas from DE animals exhibited a very strong immunoreactivity for COX-2 (Fig. 4B). COX-2 immunoreactivity was weaker in corneal stroma of RvE1-treated animals, when compared with those in the DE group.
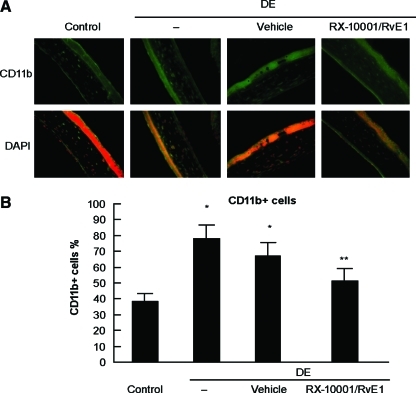
RvE1 decreases CD11b+ and CD4+ expression in corneal stroma
We performed immunohistochemistry with antibodies against CD11b+ and CD4+ to examine the effect of RvE1 on the corneal infiltration of dentritic cells/macrophages and T cells induced by DE.21,22 Corneas from mice treated with RvE1 and vehicle were harvested, and the density of CD11b+ cells in the corneal stroma was determined (Fig. 5). In the control group, there were 154 ± 20 CD11b+ cells/mm2. DE conditions produced an increase in the number of CD11b+ cells, with 429 ± 20 positive cells/mm2 (P < 0.0001). The density of the infiltrating CD11b+ cells was decreased to 211 ± 20 cells/mm2 after treatment with RX-10001 (P < 0.001, compared with DE). There was no statistical difference between vehicle at 397 ± 59 cells/mm2 and DE.
FIG. 5.
Effect of RvE1 on CD11b+ expression after DE conditions. Mice were in DE conditions for 14 days and treated with drug or vehicle for the last 7 days. (A) Representative images of corneal sections in the different groups. (B) Density of CD11b+ cells. Values correspond to average ± SEM of 4 different eyes/group, analyzed as explained in the Methods section. *Significant differences versus control; **significant differences versus DE and vehicle. Color images available online at www.liebertonline.com/jop.
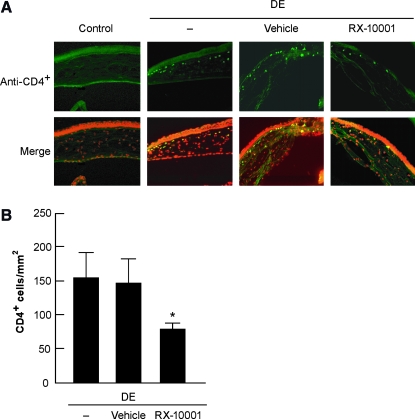
In the normal controls, no CD4+ cells were detected in the clear cornea (Fig. 6). CD4+ cell infiltration was observed in the anterior stroma near the limbal area at a density of 156 ± 37 cells/mm2 in DE conditions, which decreased to 79 ± 8 cells/mm2 stromal area (P < 0.05) after treatment with RvE1. No significant difference was observed between vehicle at 146 ± 35 cells/mm2 stromal area and DE.
FIG. 6.
Effect of RvE1 on CD4+ expression after DE conditions. Mice were in DE conditions for 14 days and treated with drug or vehicle for the last 7 days. (A) Representative images of corneal sections in the different groups. No CD4+ cells were found in the normal corneas, whereas DE induced CD4+ cell infiltration into cornea. These cells were mostly seen in the anterior stroma close to the limbal area. (B) Density of CD4+ cells. Values correspond to average ± SEM of 4 different eyes/group. *p < 0.05. Color images available online at www.liebertonline.com/jop.
Discussion
The current study demonstrates a beneficial effect of topical application of RvE1 (RX-10001) in treating ocular signs and reversing inflammatory changes during DE conditions, when administered as its ester prodrug. The drug restored tear secretion during DE conditions, with a significant effect noted within 2 days of treatment. This was accompanied by an improved corneal integrity manifested by increased density of superficial epithelial cells, when compared with untreated and vehicle-treated eyes. Improvement of corneal integrity is an important clinical goal, particularly for patients with severe DE. Finally, several indicators of inflammation were also downregulated, which may at least partly explain the improved tear secretion and the improved corneal integrity.
The hyperosmolar corneal environment in patients with DE commonly leads to a stress response that triggers release of proinflammatory mediators.21,23,24 In this murine model of DE, a mitogen-activated protein kinase response is evident within a few days of desiccating stress, and an inflammatory response with corneal epithelial release of various mediators, such as IL-1α/β, tumor necrosis factor α, IL-6, and KC (murine equivalent of human IL-8), is well documented.4 Additionally, COX-2 expression is upregulated in cornea after injury and during inflammation.19,20 This may also involve platelet activating factor-induced COX-2 activation in corneal epithelia.25,26 Platelet activating factor-induced COX-2 upregulation is believed to be involved in the activation of matrix metalloproteinases MMP-1 and MMP-9, which are responsible for collagenolytic damage and ultimately for ulcer formation.27,28 Increased expression of COX-2 and MMP-9 has been documented in DE.4 Thus, testing potential drugs in the currently used model should give valuable insights to their potential clinical use.
Our data show that RvE1 decreased COX-2 expression in this DE model. The mechanism of this inhibition is not known, but both COX-2 and MMP-9 contain consensus binding sites for the transcription factor nuclear factor kappa B, which, when activated, increase the gene promoter activation of COX-2 and MMP-9.29,30 A role for RvE1 in regulating nuclear factor kappa B expression in response to tumor necrosis factor α has been previously suggested.31 Pain during inflammation is commonly linked to the presence of COX-2 products, and control of COX-2 regulation may indicate that RvE1 could ameliorate common symptoms of DE syndrome, which in severe DE patients may also be painful.3
The increased expression of CD4+ and CD11b+ after DE exposure indicates migration of proinflammatory T cells and dendritic cells/macrophages into the corneal stroma. Previous work in a similar model of murine DE suggests that both macrophage and T-cell infiltration contribute to corneal inflammation22 and that inflammation will change the dendritic cell phenotype expression.32 The now observed marked attenuation of the influx of T cells and macrophage/dendritic cells is in agreement with observations in different models that show RvE1-mediated prevention of leukocyte influx following a proinflammatory insult.7,9,10,33,34 Resolvin-mediated regulation of leukocyte migration is now extended to the DE mouse model, which, in contrast to previous immune activation models used for exploring the effects of RvE1, is more a stress-activation–dependent model. It should be noted that corneal T-cell infiltration following desiccating stress has been mainly studied in C57BL/6 mice, which show predominantly Th-1 infiltration, whereas a Th-2 response is predominant in balb/c mice, which were used in these experiments.35
The increase in α-SMA expression in the corneal stroma following DE exposure is a novel finding and indicates a rapid activation and conversion of keratocytes into profibrotic myofibroblasts. The prevention of keratocytes into myofibroblasts further supports a role for resolvins in regulating wound healing.36
In the last few years, the concept of lipids involved in inflammation resolution has been expanded; it is now commonly accepted that not only do proinflammatory products from the omega-6-derived arachidonic acid, namely prostaglandins and leukotrienes, participate in the inflammatory process, but also the products, including resolvins and protectins (derived from omega-3 fatty acids), possess significant regulatory actions. The omega-3 fatty acids are known to exert beneficial actions,37 and their recently identified endogenous oxidation products, of which RvE1 is one, are shown to be potent regulators of the resolution phase of inflammation,8 which may explain some of the benefits attributed to omega-3 intake. Both EPA and DHA stored in cell membranes can be released and, through different lipooxygenases, be metabolized to potent resolution-contributing mediators. DHA-derived resolution and tissue protective products (D series resolvins or neuroprotectins) have been isolated from brain tissue and the corneal epithelium and are released from retinal pigment epithelial cell incubations.38–40 The formation of resolvins and protectins may explain the findings in animal models and human observations that topical treatment or diets enriched with omega-3 fatty acids can decrease biological signs and symptoms of DE.12,41 Additionally, the reported effects by RvE1 and neuroprotectin D1 on reepithelialization and lateral cell migration following corneal wounding, and prevention of corneal neovascularization by RvE1, may further add to a potential clinical treatment effect of resolvins in corneal disease.13,33,36
In conclusion, our results demonstrate that the endogenous resolvin, RvE1 (RX-10001), decreases the inflammatory response, increases tear volume, and maintains corneal epithelial integrity in a DE mouse model. This study expands upon previous observations of resolvin-mediated control of the inflammatory response in models of human disease, and now includes also regulation of a stress-induced tissue response.
Footnotes
This work was presented in part at the 2008 Association for Research in Vision and Ophthalmology Annual Meeting.
Acknowledgments
This work was supported by the National Institutes of Health, National Eye Institute grant EY004928 and by a grant from Resolvyx Pharmaceuticals, Bedford, MA.
Author Disclosure Statement
C.E.S. and P.G. are employed by Resolvyx Pharmaceuticals, in which H.E.P.B. is a consultant. N.L. and J.H. have no competing financial interests.
References
- 1.Smith J.A. Albeitz J. Begley C., et al. The epidemiology of dry eye disease: Report of the Epidemiology Subcommittee of the International Dry Eye Workshop. Ocul. Surf. 2007;5:93–107. doi: 10.1016/s1542-0124(12)70082-4. [DOI] [PubMed] [Google Scholar]
- 2.Schaumberg D.A. Sullivan D.A. Dana M.R. Epidemiology of dry eye syndrome. Adv. Exp. Med. Biol. 2002;506(Pt B):989–998. doi: 10.1007/978-1-4615-0717-8_140. [DOI] [PubMed] [Google Scholar]
- 3.Stern M.E. Beuerman R.W. Fox R.I., et al. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998;17:584–589. doi: 10.1097/00003226-199811000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Luo L. Li D.Q. Doshi A., et al. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest. Ophthalmol. Vis. Sci. 2004;45:4293–4301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- 5.Perry H.D. Solomon R. Donnenfeld E.D., et al. Evaluation of topical cyclosporine for the treatment of dry eye disease. Arch. Ophthalmol. 2008;126:1046–1050. doi: 10.1001/archopht.126.8.1046. [DOI] [PubMed] [Google Scholar]
- 6.Dastjerdi M.H. Hamrah P. Dana R. High-frequency topical cyclosporine 0.05% in the treatment of severe dry eye refractory to twice-daily regimen. Cornea. 2009;28:1091–1096. doi: 10.1097/ICO.0b013e3181a16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serhan C.N. Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br. J. Pharmacol. 2008;153(Suppl 1):S200–S215. doi: 10.1038/sj.bjp.0707489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serhan C.N. Clish C.B. Brannon J., et al. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J. Exp. Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haworth O. Cernadas M. Yang R., et al. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat. Immunol. 2008;9:873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arita M. Yoshida M. Hong S., et al. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc. Natl. Acad. Sci. U.S.A. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connor K.M. SanGiovanni J.P. Lofqvist C., et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat. Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miljanović B. Trivedi K.A. Dana M.R., et al. Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. Am. J. Clin. Nutr. 2005;82:887–893. doi: 10.1093/ajcn/82.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang H. Wang Z. Capó-Aponte J.E. Zhang F. Pan Z. Reinach P.S. Epidermal growth factor receptor transactivation by the cannabinoid receptor (CB1) and transient receptor potential vanilloid 1 (TRPV1) induces differential responses in corneal epithelial cells. Exp. Eye Res. 2010 doi: 10.1016/j. exer.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keyes K.T. Yc Y. Lin Y., et al. Resolvin E1 protect the rat heart against reperfusion injury. Am. J. Physiol. Heart. Circ. Physiol. 2010;299:153–164. doi: 10.1152/ajpheart.01057.2009. [DOI] [PubMed] [Google Scholar]
- 15.Reinoso M.A. Mukherjee P.K. Gjorstrup P., et al. Oxidative stress-induced apoptosis and pro-inflammatory cox-2 expression are down-regulated by resolvins in retinal pigment-epithelial (ARPE-19) cells. Assoc. Res. Vis. Ophthalmol. 2008 E-abstract, 2438. [Google Scholar]
- 16.Sjoquist B. Basu S. Byding P. et al. The pharmacokinetics of a new antiglaucoma drug, latanoprost, in the rabbit. Drug Metab. Dispos. 1998;6:745–754. [PubMed] [Google Scholar]
- 17.Schwartz C.E. Savinainen A. Gjorstrup P., et al. Resolvin analogs with pharmacokinetic properties suitable for topical administration to treat ocular diseases. Assoc. Res. Vis. Ophthalmol. 2008 E-abstract, 3179/D912. [Google Scholar]
- 18.Esquenazi S. He J. Li N., et al. Comparative in vivo high-resolution confocal microscopy of corneal epithelium, sub-basal nerves and stromal cells in mice with and without dry eye after photorefractive keratectomy. Clin. Exp. Ophthalmol. 2007;35:545–549. doi: 10.1111/j.1442-9071.2007.01543.x. [DOI] [PubMed] [Google Scholar]
- 19.Bazan H.E. Birkle D.L. Beuerman R.W., et al. Inflammation-induced stimulation of the synthesis of prostaglandins and lipoxygenase-reaction products in rabbit cornea. Curr. Eye Res. 1985;4:175–179. doi: 10.3109/02713688509000847. [DOI] [PubMed] [Google Scholar]
- 20.Bazan H.E. Corneal injury alters eicosanoid formation in the rabbit anterior segment in vivo. Invest. Ophthalmol. Vis. Sci. 1987;28:314–319. [PubMed] [Google Scholar]
- 21.Dana M.R. Hamrah P. Role of immunity and inflammation in corneal and ocular surface disease associated with dry eye. Adv. Exp. Med. Biol. 2002;506(Pt B):729–738. doi: 10.1007/978-1-4615-0717-8_103. [DOI] [PubMed] [Google Scholar]
- 22.Niederkorn J.Y. Stern M.E. Plugfelder S.C., et al. Dessiccating stress induces T cell-mediated Sjogren's syndrome-like lacrimal keratoconjuctivitis. J. Immun. 2006;176:3950–3957. doi: 10.4049/jimmunol.176.7.3950. [DOI] [PubMed] [Google Scholar]
- 23.Gulati A. Sacchetti M. Bonini S., et al. Chemokine receptor CCR5 expression in conjunctival epithelium of patients with dry eye syndrome. Arch. Ophthalmol. 2006;124:710–716. doi: 10.1001/archopht.124.5.710. [DOI] [PubMed] [Google Scholar]
- 24.Solomon A. Dursun D. Liu Z., et al. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest. Ophthalmol. Vis. Sci. 2001;42:2283–2292. [PubMed] [Google Scholar]
- 25.Bazan H.E. Tao Y. DeCoster M.A., et al. Platelet-activating factor induces cyclooxygenase-2 gene expression in corneal epithelium. Requirement of calcium in the signal transduction pathway. Invest. Ophthalmol. Vis. Sci. 1997;38:2492–2501. [PubMed] [Google Scholar]
- 26.Esquenazi S. He J. Li N., et al. A novel platelet activating factor (PAF) receptor antagonist reduces cell infiltration and expression of inflammatory mediators in mice exposed to desiccating conditions after PRK. Clin. Dev. Immunol. 2009;2009:138513. doi: 10.1155/2009/138513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ottino P. Bazan H.E. Corneal stimulation of MMP-1, -9 and uPA by platelet-activating factor is mediated by cyclooxygenase-2 metabolites. Curr. Eye Res. 2001;23:77–85. doi: 10.1076/ceyr.23.2.77.5471. [DOI] [PubMed] [Google Scholar]
- 28.Bazan H.E. Tao Y. PAF antagonists as possible inhibitors of corneal epithelial defects and ulceration. J. Ocul. Pharmacol. Ther. 1997;13:277–285. doi: 10.1089/jop.1997.13.277. [DOI] [PubMed] [Google Scholar]
- 29.Lukiw W.J. Ottlecz A. Lambrou G., et al. Coordinate activation of HIF-1 and NF-kappaB DNA binding and COX-2 and VEGF expression in retinal cells by hypoxia. Invest. Ophthalmol. Vis. Sci. 2003;44:4163–4170. doi: 10.1167/iovs.02-0655. [DOI] [PubMed] [Google Scholar]
- 30.Taheri F. Bazan H.E. Platelet-activating factor overturns the transcriptional repressor disposition of Sp1 in the expression of MMP-9 in human corneal epithelial cells. Invest. Ophthalmol. Vis. Sci. 2007;48:1931–1941. doi: 10.1167/iovs.06-1008. [DOI] [PubMed] [Google Scholar]
- 31.Arita M. Clish C.B. Serhan C.N. The contributions of aspirin and microbial oxygenase to the biosynthesis of anti-inflammatory resolvins: novel oxygenase products from omega-3 polyunsaturated fatty acids. Biochem. Biophys. Res. Commun. 2005;338:149–157. doi: 10.1016/j.bbrc.2005.07.181. [DOI] [PubMed] [Google Scholar]
- 32.Hamrah P. Liu Y. Zhang Q., et al. Alterations in corneal stromal dendritic cell phenotype and distribution in inflammation. Arch. Opthalmol. 2003;121:1132–1140. doi: 10.1001/archopht.121.8.1132. [DOI] [PubMed] [Google Scholar]
- 33.Jin Y. Arita M. Zhang Q., et al. Anti-angiogenesis effect of the novel anti-inflammatory and pro-resolving lipid mediators. Invest. Ophthalmol. Vis. Sci. 2009;50:4743–4752. doi: 10.1167/iovs.08-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vassiliou E.K. Kesler O.M. Tadros J.H., et al. Bone marrow-derived dendritic cells generated in the presence of resolvin E1 induce apoptosis of activated CD4+ T cells. J. Immunol. 2008;181:4534–4544. doi: 10.4049/jimmunol.181.7.4534. [DOI] [PubMed] [Google Scholar]
- 35.Corrales R.M. Villareal A. Farley W., et al. Strain-related cytokine profiles on the murine ocular surface in response to desiccating stress. Cornea. 2007;6:579–584. doi: 10.1097/ICO.0b013e318033a729. [DOI] [PubMed] [Google Scholar]
- 36.Gronert K. Maheshwari N. Khan N., et al. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J. Biol. Chem. 2005;280:15267–15278. doi: 10.1074/jbc.M410638200. [DOI] [PubMed] [Google Scholar]
- 37.Simopoulos A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002;56:365–379. doi: 10.1016/s0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- 38.Lukiw W.J. Cui J.G. Marcheselli V.L., et al. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J. Clin. Invest. 2005;115:2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukherjee P.K. Marcheselli V.L. Serhan C.N., et al. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cortina M. He J. Li N., et al. Neuroprotectin D1 synthesis and corneal nerve regeneration after experimental surgery and treatment with PEDF plus DHA. Invest. Ophthalmol. Vis. Sci. 2010;51:804–810. doi: 10.1167/iovs.09-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Viau S. Maire M.A. Pasquis B., et al. Efficacy of a 2-month dietary supplementation with polyunsaturated fatty acids in dry eye induced by scopolamine in a rat model. Graefes Arch. Clin. Exp. Ophthalmol. 2009;247:1039–1050. doi: 10.1007/s00417-009-1080-z. [DOI] [PubMed] [Google Scholar]