Abstract
Objective
The protective effect of physical activity (PA) on risk of stroke remains controversial as a result of lack of insight into the sources of heterogeneity between studies. We performed a comprehensive meta-analysis of studies to (1) quantify the association between PA level and risk of stroke outcomes and (2) test the hypothesis that the association of PA level with stroke outcomes will be similar between men and women. The outcome measures are stroke incidence, stroke mortality, or both.
Methods
Cohort studies were identified by searching MEDLINE and EMBASE (from 1986 to 2005) and meta-analysis conducted according to meta-analysis of Observational Studies in Epidemiology (MOOSE) group recommendations. Data were reported as pooled relative risk (RR) and 95% confidence intervals (CI) using random-effects models to assess the association of stroke outcomes with PA level. Heterogeneity was investigated, and sensitivity analysis was performed. Stratified analysis by gender was performed.
Results
Of 992 articles, 13 satisfied all eligibility criteria and were studied. Compared with low PA, moderate PA caused an 11% reduction in risk of stroke outcome (RR = 0.89, 95% CI 0.86-0.93, p < 0.01) and high PA a 19% reduction (RR = 0.81, CI 0.77-0.84, p < 0.01). Among the men, results showed a 12% reduction in risk associated with moderate PA (RR = 0.88, CI 0.82-0.94, p < 0.01) and 19% reduction for high PA (RR = 0.81, CI 0.75-0.87, p < 0.01). Among the women, results showed a 24% reduction in risk for high PA (RR = 0.76, CI 0.64-.89, p < 0.01). There was, however, no significant risk reduction associated with a moderate PA level in women.
Conclusions
Increased PA level appears beneficial in reduction of risk of stroke and related outcomes. However, higher levels of PA may be required in women to achieve as significant a risk reduction as in men. An exercise regimen tailored to women to improve related physiological mechanisms will likely be beneficial.
Introduction
There is ample epidemiological evidence indicating stroke as the third leading cause of long-term disability and death in the United States and other industrialized countries.1–4 The condition can cause severe physical and mental impairment1 resulting in extended hospitalizations, long-term care for patients, and the associated significant productivity and economic losses.2 Although patients suffering from acute ischemic stroke may be candidates for thrombolytic drugs, there are currently no large treatment effects for most cases of stroke.3 Hypertension and cardiac disease are among the primary risk factors for stroke outcomes. Preventive measures, such as physical activity (PA) and nutritious low-salt and low-fat diets (particularly diets low in cholesterol and saturated fats), have been investigated and established to reduce the risk of hypertension and coronary heart disease (CHD).
Fewer studies, however, have investigated the relationship between PA and stroke outcomes,4–21 with conflicting and controversial conclusions. Whereas some of these studies reported an inverse association between PA and stroke outcomes,8,9,10,13 others showed no significant or demonstrated positive associations.11,17,19 Agnarsson et al.,8 for example, showed intense PA to be protective in men ≥40 years of age but not in younger men. To add to the controversy, other studies showed moderate or intense PA to be protective against stroke in men but not in women.16 To the best of our knowledge, there is no meta-analysis that investigated if men and women would require a similar level of PA to achieve significant stroke risk reduction. In this report, (1) we systematically reviewed published studies and performed a comprehensive meta-analysis to quantify the relationship between level of PA and the risk of stroke outcomes in apparently healthy populations and (2) tested the hypothesis that the association of PA level with stroke outcomes would be similar between men and women. Data were extracted from cohort studies published between 1986 and 2005 that examined the exposure to low PA level and the risk of stroke outcomes, defined as first stroke or death due to stroke.
Materials and Methods
Literature search and study design
A meta-analysis was conducted and reported according to guidelines of the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group.22 We identified cohort studies published from January 1986 through September 2005 by searching the MEDLINE (National Library of Medicine database assessed through PubMed) and EMBASE (the Excerpta Medica database) using the medical subject headings and terms: physical activity, exercise, leisure-time activity, stroke, stroke outcomes, and cerebrovascular disorders. We also consulted the studies that reported modifiable (e.g., cigarette smoking, obesity, drug administration) and nonmodifiable (e.g., age, sex, family history of stroke) risk factors of stroke. We then carried out a manual search and read articles in stroke and epidemiology journals.
Two authors (L.D., an epidemiologist/physician; J.K., a biostatistician) independently reviewed each study. To be included in the meta-analysis, studies had to satisfy the following predetermined inclusion criteria: (1) reported original data and published as a full peer-reviewed article, (2) carried out in healthy human participants ≥18 years of age, with no history of cardiovascular disease (CVD) or stroke, (3) published in the English language, (4) primary outcome defined as first stroke or stroke death or both, (5) specifically assessed the relationship between PA and stroke or stroke death in a cohort study, (6) specifically measured the level of PA as low, moderate, or high, and (7) provided relative risk (RR) estimates or sufficient statistical data to allow calculation of such estimates.
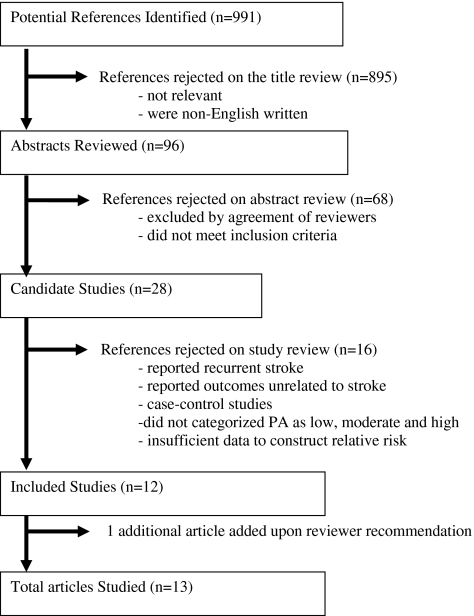
The systematic search (Fig. 1) identified 991 potentially relevant articles published in English. Of these, 895 articles were excluded based on titles and abstracts that did not satisfy some or all of the inclusion criteria. Five were non-English-language articles. Ninety-six abstracts were reviewed independently by the two authors on eligibility forms and evaluated for inclusion or exclusion. Sixty-eight articles were excluded by agreement among the reviewers because these articles did not satisfy all the inclusion criteria. We retrieved and reviewed 28 full text articles that met all the inclusion criteria and identified published epidemiological studies of PA and stroke. We excluded 16 studies from the analysis for the following reasons: 3 articles included recurrent stroke, 9 articles reported outcomes that were unrelated to stroke or stroke death, 2 articles did not stratify the measure of PA, 1 article provided insufficient data to reconstruct RR, and 1 article was a case-control study. Finally, data abstraction was done on 13 articles. Following the abstraction, 1 article was excluded because it reported outcome as mortality but provided insufficient data to reconstruct statistical analysis. Twelve articles10–21 satisfied all the eligibility criteria for inclusion in the meta-analysis; at the recommendation of an anonymous reviewer, an additional article was added for a total of 13 articles studied in this report. The characteristics of the 13 articles studied are presented in Table 1. Study populations included well-characterized cohorts in the United States, Japan, Britain, Norway, and Finland.
FIG. 1.
Summary results of systematic literature search for articles that satisfied the inclusion/exclusion criteria for meta-analysis.
Table 1.
Summary of Abstracted Articles That Satisfied Eligibility Criteria for Meta-Analysis
| |
|
|
|
|
|
Moderate vs. low |
High vs. low |
|
||
|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Gender | Population/Location | Year of follow-up | Total n | Age | RR | 95% CI | RR | 95% CI | Adjusted for confoundersa |
| Abbott et al.10 | Men | Hawaiian men/nonsmoker, U.S. | 22 | 7,530 | 45–68 | N/A | N/A | 0. 65 | 0.49-0.86 | 1,2,3,4 |
| Evenson et al.11 | Men and Women | Communities, U.S. | 7.2 | 14,575 | 45–64 | N/A | N/A | 0.79 | 0.62-1.00 | 1,3,4,5 |
| Folsom et al.9 | Women | Older U.S. women, U.S. | 2 | 41,837 | 55–69 | 0.6 | 0.4-0.9 | 0.8 | 0.5-1.1 | 1,2,5,9 |
| Gillium et al.12 | Men | Adults in U.S. | 12 | 2,368 | 45–74 | N/A | N/A | 0.64 | 0.50-0.82 | 1,2,3,5,6,8,9,13,19 |
| Gillium et al.12 | Women | Adults in U.S. | 12 | 2,713 | 45–74 | N/A | N/A | 0.44 | 0.31-0.64 | 1,2,3,5,6,8,9,13,20 |
| Haheim et al.13 | Men | Scandinavian men, Norway | 12 | 14,403 | 40–49 | 0.65 | 0.43-0.97 | 0.79 | 0.18-3.45 | 1,2,3,7,8,9,10 |
| Hu et al.14 | Women | Nurses, U.S. | 8 | 72,488 | 40–66 | 0.90 | 0.74-1.10 | 0.70 | 0.56-0.88 | 1,2,3,7,8,9,10,16,17,18 |
| Hu et al.15 | Men | 5 geographic areas in Finland | 19 | 21,344 | 25–64 | 0.90 | 0.83-0.97 | 0.84 | 0.75-0.95 | 1,2,3,5,6,8,9,10,11,20 |
| Hu et al.15 | Women | 5 geographic areas in Finland | 19 | 23,514 | 25–64 | 0.90 | 0.83-0.96 | 0.86 | 0.79-0.94 | 1,2,3,5,6,8,9,10,11,20 |
| Kiely et al.16 | Men | Adults in Framingham, MA, U.S. | 32 | 1,897 | 35–69 | 0.90 | 0.62-1.31 | 0.84 | 0.59-1.18 | 1,3,6,8,9,12,13,14,20 |
| Kiely et al.16 | Women | Adults in Framingham, MA, U.S. | 32 | 2,299 | 35–68 | 1.21 | 0.89-1.63 | 0.89 | 0.60-1.31 | 1,3,6,8,9,12,13,14,20 |
| Lee and Paffenbarger.17 | Men | Harvard Alumni, U.S. | 13 | 11,130 | 43–88 | 0.65 | 0.47-.91 | 0.81 | 0.72-0.92 | 1,3,10,20 |
| Lee et al.18 | Men | Male physicians, U.S. | 11.1 | 22,071 | 40–84 | 0.93 | 0.77-1.13 | 0.97 | 0.71-1.32 | 2,3,7,8,9,10,16,17,18 |
| Nakayama et al.19 | Men | Adults in Japan | 15.5 | 1,182 | 40+ | 0.72 | 0.14-3.70 | N/A | N/A | 1,3,6,8,10,20 |
| Nakayama et al.19 | Women | Adults in Japan | 15.5 | 1,469 | 40+ | 0.35 | 0.11-1.14 | N/A | N/A | 1,3,6,8,10,21 |
| Paganini-Hill et al.20 | Men | Retirement community, U.S. | 17 | 4,722 | 44–101 | 0.91 | 0.77-1.07 | 0.85 | 0.72-1.01 | 1,2,3,7,17,20 |
| Paganini-Hill et al.20 | Women | Retirement community, U.S. | 17 | 8,532 | 44–101 | 0.88 | 0.77-0.99 | 0.83 | 0.73-0.95 | 1,2,3,7,17,20 |
| Wannamethee and Shaper21 | Men | Middle-aged men, Britain | 9.5 | 1,799 | 40–59 | 0.67 | 0.39-1.16 | 0.66 | 0.23-1.86 | 1,3,9,10,15 |
Adjusted confounders:
Age = 1, diabetes mellitus (DM) = 2, smoking = 3, race = 4, education = 5, systolic blood pressure = 6, hypertension = 7, cholesterol = 8, body mass index (BMI) = 9, alcohol = 10, geography = 11, glucose = 12, coronary heart disease (CHD) = 13, occupation = 14, social class = 15, parental history <60 years = 16, aspirin = 17, menopause = 18, hemoglobin = 19, other(s) = 20.
CI, confidence interval; N/A, not available; RR, relative risk.
Data extraction
Measures of independent variables reported within a single study as associated with different population groups, gender, age groups, or total incident cases/1000 patients as outcome measures were analyzed as separate entities. For example, in the Honolulu Heart Program,10 we used the data units for men, nonsmokers with different age groups (aged 45–54 and 55–68 years) separately. In the National Health and Nutrition Examination Survey (NHANES) I epidemiological follow-up study,12 we analyzed men aged 45–74 years and women aged 45–74 years as separate data units. For the Finnish study,15 the men and women in the five different geographic locations were treated as 2 data units. In the Framingham Study,16 despite the closeness of the age groups, we separated the men and women as 2 data units. Also in the Shibata study in Japan,19 we separated the men and women as 2 data units. Similarly the data retrieved from the Leisure World study20 was treated as 2 data units for men and women. The 13 studies that satisfied the inclusion criteria thus yielded a total of 17 data units.
Study quality assessment
Studies in which PA was used as an estimate for physical fitness were considered eligible only if the assessment contained levels of PA based on the questionnaire administered (12 of 12) or interviewer administered (4 of 12). The outcome assessment of incidence of stroke must have been documented through medical record and death certificate review (12 of 12) or confirmed by radiographic evaluation (4 of 12). Study selection for inclusion also considered adjustment for such confounders as age, body mass index (BMI), smoking, hypertension, CHD, and diabetes mellitus.
Characterization of level of physical activity
Characterization of the level of PA varied among the studies. Many were obtained from self-reported questionnaires. In this meta-analysis, PA level was characterized as low, moderate, or high as reported in the articles or reported in a manner for reclassification.
Statistical analysis
Data were extracted from the original studies, double-checked, and entered into an EXCEL database. RR estimate and 95% CI of stroke outcomes associated with moderate or high PA compared with low PA were obtained from the published articles. Otherwise, they were calculated directly from the data given in the article where possible. Summary (pooled) RRs were generated using random-effects meta-analysis23–26 to account for within-study and across-study variation to exposure. Summary and individual studies RRs were represented as point estimates with 95% CI on a forest plot.23–26 Heterogeneity of the true effect RR was tested using Cochrane's Q-statistics.23,24
Assessment of publication bias and sensitivity analysis
Publication bias was assessed by construction and inspection of funnel plots—that is, plot of logarithm of the RR against the logarithm of the standard error of the RR—and by formal testing of funnel plot asymmetry with the Egger test and Beggs test.23 Sensitivity analysis23,25 to determine the individual effect of each study on the pooled RR estimates was performed by recalculating the RR estimate while omitting each study one at a time.
Subgroup analysis
Data were then stratified by gender, and separate pooled RR estimates were obtained for men and women. All analyses were performed using the statistical software Stata version 9.0 (Stata Corp, College Station, TX).
Results
Qualitative analysis
Table 1 presents a summary of the 13 articles that were used to investigate the relationship between level of PA in healthy individuals and the stroke outcomes, defined as first stroke or death due to stroke, and satisfied all the inclusion criteria for meta-analysis. Table 1 also details the statistical data and the adjusted confounders of the individual studies.
Quantitative analysis
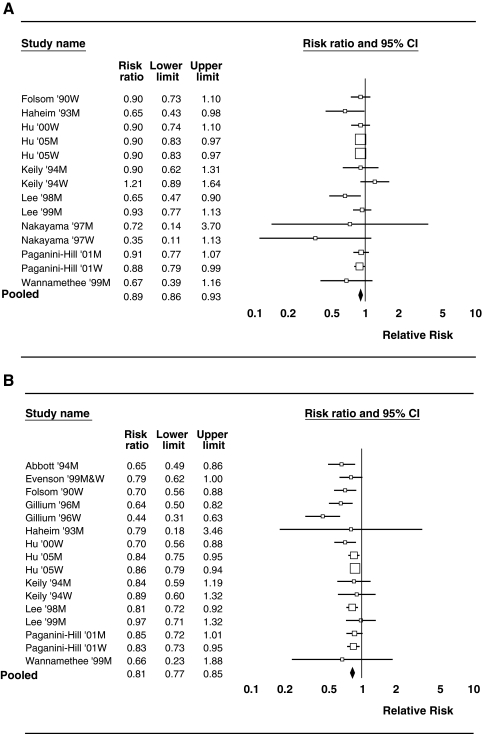
Table 2 presents the summary risk estimates for the total data and by gender stratification. For the total data, the pooled statistical estimate showed an overall significant risk reduction of stroke outcome with moderate to high PA compared with low PA. In particular, compared with low PA, moderate PA resulted in an 11% reduction in risk of stroke outcome (RR = 0.89, CI 0.85-0.94, p < 0.01), and high PA resulted in a 21% reduction in risk (RR = 0.79, CI 0.74-0.85, p < 0.01). Figure 2 shows forest plots that depict the RR for comparing low with moderate PA (Fig. 2A) and comparing low with high PA (Fig. 2B). Test for heterogeneity of the true RR was insignificant (Cochrane's Q = 13.728, df = 12, p = 0.318). This suggests that all studies share a common true effect size. A visual inspection of the funnel plots (not provided) showed that all the studies were within the confidence limits, confirming symmetry and supporting evidence of lack of publication bias. Moreover, Egger test for asymmetry was not significant (t = −1.54, standard error [SE] = 0.4492, p = 0.151), confirming no evidence of publication bias. Sensitivity analysis to determine the individual effect of each study on the pooled RR estimates showed no significant influence of any particular study on the estimates for comparing moderate PA or high PA with low PA.
Table 2.
Pooled Relative Risk Estimate of Incidence of Stroke for Different Physical Activity Levels
| |
Moderate PA vs. low PA |
High PA vs. low PA |
||
|---|---|---|---|---|
| Data units | RR (95% CI) | p | RR (95% CI) | p |
| Combined | 0.89 (0.85-0.94) | <0.01 | 0.79 (0.74-0.85) | <0.01 |
| Men | 0.88 (0.82-0.94) | <0.01 | 0.81 (0.75-0.87) | <0.01 |
| Women | 0.99 (0.88-1.07) | ns | 0.76 (0.64-0.89) | <0.01 |
ns, not significant; PA, physical activity.
FIG. 2.
(A) Forest plot of relative risk of stroke outcomes comparing moderate physical activity with low physical activity using the random-effects model. M, men; W, women. (B) Forest plot of relative risk of stroke outcomes comparing high physical activity with low physical activity using the random-effects model.
Data were stratified by gender, and separate pooled RR estimates were obtained for men and women. Results of the risk estimates are shown in Table 2. Among the men, the results showed a 12% reduction in risk of stroke outcome (RR = 0.88, CI 0.82-0.94, p < 0.01) for moderate PA and a 19% reduction for high PA (RR = 0.81, CI 0.75-0.87, p < 0.01) compared with low PA. Among the women, results showed a 24% reduction in risk for high PA (RR = 0.76, CI 0.64-0.89, p < 0.01) compared with low PA. There was, however, no significant difference in risk of stroke outcome for a moderate PA level compared with low PA level in women. Figures 2A and 2B, respectively, show the forest plots for comparing high PA and moderate PA with low PA in men and women. Sensitivity analysis, when stratified by gender, showed significant influence by a few individual studies on the pooled RR estimate. In particular, for the case of comparing high PA with low PA, there was a significant reducing influence on the RR estimates observed by omitting the studies of Hu et al.15 in both male and female subgroup analysis, whereas the studies of Gillum et al.12 and Hu et al.14 increased the risk estimates. In addition, for comparing moderate PA with low PA, omitting the study of Kiely et al.16 reduced the RR estimate, whereas omitting that of Lee et al.17 increased the risk estimate in the women subgroup analysis. Omission of these studies, however, did not change the resulting significance in RR reduction of stroke due to high or moderate PA compared with low PA.
Discussion and Conclusions
In previous studies, the investigators have assessed the RR of stroke associated with exposure to PA, but the reported results were mixed.8–21,27–36 The present study was performed to systematically evaluate published data on the relationship of different levels of PA and the risk of stroke outcomes in apparently healthy individuals with no history of CVD. In addition, this study examined if the same level of PA is required to achieve the same level of risk reduction between genders. The results from this study suggest that, overall, increased PA level has the potential to reduce stroke incidence and related outcomes. In subgroup analysis, however, results showed women required a higher level of PA than men to achieve significant risk reduction of stroke outcomes.
There is no compelling evidence for the mechanisms underlying the favorable effects of PA on the reduction of the risk of stroke outcomes. Nonetheless, several studies suggest the beneficial physiological effects of PA on the cardiovascular system. A negative association between PA and stroke mortality may be related to deceleration of the atherosclerotic process, structural modification of the arteries, amelioration of endothelial dysfunction, enhancement of myocardial electric stability, and attenuation of blood hypercoagulability and inflammatory mechanisms. There is also evidence of reductions in plasma fibrinogen and platelet activity, as well as elevations in plasma tissue plasminogen activator activity and high-density lipoprotein cholesterol (HDL-C) levels, lowering blood pressure, maintaining normal glucose tolerance and improving insulin sensitivity.37–44 The protective effect of PA may also be mediated by controlling risk factors for stroke, such as hypertension, diabetes mellitus, and body weight.40–44 Because of the complexity of the atherosclerotic process and the multitude of physiological, clinical, and psychosocial variables that may alter or influence the effects of PA on the cerebrovascular systems, our ability to predict outcomes in stroke patients remains imperfect. The role of other risk factors, such as age, smoking, CHD, hypertension, and the optimum intensity of PA (frequency and duration), in modifying the risk of stroke should be reevaluated to reach unquestionable conclusions.
The discrepancy in risk reduction between genders is uncertain from this study but may be explained in part as follows. Methodologically, it is commonly noted that men are more likely than women to engage in strenuous exercise regimens.46 What is considered a high level of PA in women could be considered moderate in men. Moreover, some studies45,47 have suggested that there are differences between genders in benefits associated with PA that may result from the dissimilarity and variations in hormonal conditions.
The present study has limitations. One limitation is that few studies were available to investigate the relation of level of PA to specific subtypes of stroke. Moreover, the meta-analysis was limited to English-language articles, thus excluding potentially valuable results that may be in other languages. Another limitation is the self-reporting and varied definitions of classification of low, moderate, and high PA among the different studies, making it impossible to be entirely specific about the level of PA required to prevent stroke outcomes. The number and types of confounding variables also differed from study to study (Table 1). There was a wide range of age groups among the study participants in each individual study. In addition, there were variable follow-up periods among the studies, ranging from 7 to 32 years (Table 1). The shorter follow-up periods may be inadequate to observe the risk of stroke outcomes because of a lack of exposure to PA. Nonetheless, the report of Blair et al.46 suggests that there is good consensus across studies, with most showing an inverse dose-response gradient across both PA and physical fitness categories for morbidity from CHD, stroke, CVD, or all-cause mortality.
Despite the limitations of the present study, the observed data can be viewed as hypotheses generating and supporting further study. Would the same level of PA be required to achieve the same level of risk reduction between men and women? Future studies will, however, require more rigorous design. The degree of controlling confounding variables differed among the studies included in the meta-analysis. Most studies adjusted for some risk factors besides age and hypertension. We recommend that future cohort observational studies should quantify measures of PA and standardize outcome assessments. Where it is feasible, a subgroup analysis among specific populations should be considered as well as the nutritional habits of the participants and use of any medications. The use of medications (prescribed or over-the-counter) was not mentioned in any of the studies, although it is common knowledge that some elderly populations take some medication that could be a confounding factor in the meta-analysis. Furthermore, there are comparatively fewer data on nonexercise PA, or lifestyle, which includes other forms of PA that occur in the context of commuting to work, household activities, and other instances involving walking, cycling, and climbing stairs, which may occur throughout the day in an unstructured setting with variable duration and frequency.48 The identification, measurement, and analysis of nonexercise components of PA on the risk of stroke outcome among men and women would be clinically informative.
Although it is unclear how PA plays a protective role against stroke, results of this study suggest long-term benefits. Future research to examine the impact of PA on the pathophysiological mechanism of stroke will be desirable. Some studies37–41 have suggested that impact of PA on reducing the risk of stroke mortality could be explained by deceleration of the atherosclerotic process, amelioration of endothelial dysfunction, structural modification of the arteries, enhancement of myocardial electric stability, or attenuation of hypercoagulability.
The results of this meta-analysis suggest that increased PA has a beneficial effect in reduction of risk of stroke incidence and stroke mortality. Our results also suggest that higher levels of PA may be required in women than in men to achieve similar levels of significant stroke reduction. These observations are hypothesis generating and support further studies. People at risk of stroke should, therefore, endeavor to engage in some form of PA. Exercise regimens tailored to women to help increase and improve physiological function and properties may be beneficial.
Acknowledgments
This study was partially supported by the Multidisciplinary Clinical Research Career Development Program and Johns Hopkins University Clinical Research Scholars Program Award to L.D., NIH/NCRR grant K12RR023266; Howard University NIH/NCRR grant MO1 RR 10284; funds in the Howard University Department of Neurology; AAASPS grant 525-116, and funds from PROFESS Study on Stroke Patients. We thanks AeRang Kim, M.D., Lori Jordan, M.D., Jon Sevransky, M.D., Mona Duggal, M.P.H., Shilpa Bhardwaj, M.P.H., and Tsilidis Konstantinos, M.P.H for various support in the retrieval of many studies and an anonymous reviewer for useful comments and suggestions. Some of these findings were presented at the American Neurological Association 132nd Annual Meeting, October 7–10, 2007, Washington, D.C. (Abstract Number: 950220).
Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.Williams GR. Incidence and characteristics of total stroke in the United States. BMC Neurol. 2001;1:2. doi: 10.1186/1471-2377-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams JE.Ayala CS.Croft JB, et al. National Center for Chronic Disease Prevention and Health Promotion, CDC MMWR 200251429–433.12056498 [Google Scholar]
- 3.Goldstein LB. Adams R. Becker K, et al. Primary prevention of ischemic stroke. Circulation. 2001;103:163–182. doi: 10.1161/01.cir.103.1.163. [DOI] [PubMed] [Google Scholar]
- 4.NIH Consensus Development Panel on Physical Activity and Cardiovascular Health. Physical activity and cardiovascular health. JAMA. 1996;276:241–246. [PubMed] [Google Scholar]
- 5.Lee DO. Folsom R. Blair SN. Physical activity and stroke risk: A meta-analysis. Stroke. 2003;34:2475–2481. doi: 10.1161/01.STR.0000091843.02517.9D. [DOI] [PubMed] [Google Scholar]
- 6.Wendel-Vos GCW. Schuit AJ. Feskens EJM. Boshuizen HC. Verschuren WMM. Physical activity and stroke: A meta-analysis of observational data. Int J Epidemiol. 2004;33:787–798. doi: 10.1093/ije/dyh168. [DOI] [PubMed] [Google Scholar]
- 7.Oczkowski W. Complexity of the relationship between physical activity and stroke: A meta-analysis. Clin J Sport Med. 2005;15:399–405. doi: 10.1097/01.jsm.0000179228.78532.45. [DOI] [PubMed] [Google Scholar]
- 8.Agnarsson U. Gudmundur T. Sigvalason H. Sigfusson N. Effects of leisure-time physical activity and ventilator function on risk for stroke in men: The Reykjavik Study. Ann Intern Med. 1999;130:987–990. doi: 10.7326/0003-4819-130-12-199906150-00006. [DOI] [PubMed] [Google Scholar]
- 9.Folsom AR. Prineas RJ. Kaye SA. Munger RG. Incidence of hypertension and stroke in relation to body fat distribution and other risk factors in older women. Stroke. 1990;21:701–706. doi: 10.1161/01.str.21.5.701. [DOI] [PubMed] [Google Scholar]
- 10.Abbott RD. Rodriguez BL. Burchfiel CM. Curb JD. Physical activity in older middle-aged men and reduced risk of stroke: The Honolulu Heart Program. Am J Epidemiol. 1994;139:881–893. doi: 10.1093/oxfordjournals.aje.a117094. [DOI] [PubMed] [Google Scholar]
- 11.Evenson KR. Rosamond WD. Cai J, et al. Physical activity, ischemic stroke risk: The Atherosclerosis Risk in Communities Study. Stroke. 1999;30:1333–1339. doi: 10.1161/01.str.30.7.1333. [DOI] [PubMed] [Google Scholar]
- 12.Gillum RF. Mussolino ME. Ingram DD. National Center for Health Statistics, Centers for Disease Control and Prevention. Physical activity and stroke incidence in women and men: The NHANES I epidemiologic follow-up study. Am J Epidemiol. 1996;143:860–869. doi: 10.1093/oxfordjournals.aje.a008829. [DOI] [PubMed] [Google Scholar]
- 13.Haheim LL. Holme I. Hjermann I. Leren P. Risk factors of stroke incidence and mortality. A 12-year follow-up of the Oslo Study. Stroke. 1993;24:1484–1489. doi: 10.1161/01.str.24.10.1484. [DOI] [PubMed] [Google Scholar]
- 14.Hu FB. Stampfer MJ. Colditz GA, et al. Physical activity and risk of stroke in women. JAMA. 2000;283:2961–2967. doi: 10.1001/jama.283.22.2961. [DOI] [PubMed] [Google Scholar]
- 15.Hu G. Sarti C. Jousilahti P. Silventoinen K. Barengo NC. Tuomilehto J. Leisure time, occupational, and commuting physical activity and the risk of stroke. Stroke. 2005;36:1994–1999. doi: 10.1161/01.STR.0000177868.89946.0c. [DOI] [PubMed] [Google Scholar]
- 16.Kiely DK. Wolf PA. Cupples LA. Beiser AS. Kannel WB. Physical activity and stroke risk: The Framingham Study. Am J Epidemiol. 1994;140:608–620. doi: 10.1093/oxfordjournals.aje.a117298. [DOI] [PubMed] [Google Scholar]
- 17.Lee IM. Paffenbarger RS. Physical activity and stroke incidence. The Harvard Alumni Health Study. Stroke. 1998;29:2049–2054. doi: 10.1161/01.str.29.10.2049. [DOI] [PubMed] [Google Scholar]
- 18.Lee IM. Hennekens CH. Berger K. Buring JE. Manson JE. Exercise and risk of stroke in male physicians. Stroke. 1999;30:1–6. doi: 10.1161/01.str.30.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Nakayama T. Date C. Yokoyama T, et al. A 15.5-year follow-up study of stroke in a Japanese provincial city. The Shibata Study. Stroke. 1997;28:45–52. doi: 10.1161/01.str.28.1.45. [DOI] [PubMed] [Google Scholar]
- 20.Paganini-Hill A. Perez Barreto M. Stroke risk in older men and women: Aspirin, estrogen, exercise, vitamins, and other factors. J Gend Specif Med. 2001;4:18–28. [PubMed] [Google Scholar]
- 21.Wannamethee G. Shaper AG. Physical activity and stroke in British middle aged men. BMJ. 1992;304:597–601. doi: 10.1136/bmj.304.6827.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stroup DF. Berlin JA. Morton SC, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 23.Houwelingen HC. Arends LR. Stijnen T. Advanced methods in meta-analysis: Multivariate approach and meta-regression. Stat Med. 2002;21:589–624. doi: 10.1002/sim.1040. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JPT. Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 25.Egger M. Davey Smith G. Schneider M. Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Begg CB. Berlin JA. Publication bias: A problem in interpreting medical data. JR Statist Soc A. 1988;151:419–463. [Google Scholar]
- 27.U.S. Department of Health and Human Services. Physical activity and health. A report of the Surgeon General: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion. 1996:85–172. [Google Scholar]
- 28.Caspersen CJ. Powell KE. Christensen GM. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–131. [PMC free article] [PubMed] [Google Scholar]
- 29.Booth ML. Owen N. Bauman A, et al. Relationship between 14-day recall measure of leisure time physical activity and a submaximal test of physical work capacity in Australian adults. Res Q Exerc Sport. 1996;67:221–227. doi: 10.1080/02701367.1996.10607948. [DOI] [PubMed] [Google Scholar]
- 30.American College of Sports Medicine. Position stand: The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness in healthy adults. Med Sci Sports Exerc. 1990;22:265–274. [PubMed] [Google Scholar]
- 31.Ainsworth BE. Haskell W. Whitt MC, et al. Compendium of physical activities: An update of activity codes and MET values. Med Sci Sports Exerc. 2000;32(Suppl):498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 32.Hu G. Tuomilehto J. Silventoinen K. Barengo N. Jousilahti P. Joint effects of physical activity, body mass index, waist circumference and waist-to-hip ratio with the risk of cardiovascular disease among middle-aged Finnish men and women. Eur Heart J. 2004;25:2212–2219. doi: 10.1016/j.ehj.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 33.Kimura N. Toshima H. Nakayama Y. Mizuguchi T. Fukami T. Population survey on cerebrovascular and cardiovascular diseases. The ten years experience in the farming village of Tanushimaru and the fishing village of Ushibuka. Jpn Heart J. 1972;13:118–127. [PubMed] [Google Scholar]
- 34.Noda H. Iso H. Toyoshima H, et al. Walking and sports participation and mortality from coronary heart disease and stroke. J Am Coll Cardiol. 2005;46:1761–1767. doi: 10.1016/j.jacc.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 35.Paffenbarger RS., Jr Brand RJ. Sholtz RI. Jung DL. Energy expenditure, cigarette smoking, and blood pressure level as related to death from specific diseases. Am J Epidemiol. 1978;108:12–18. [PubMed] [Google Scholar]
- 36.Wannamethee SG. Shaper AG. Walker M. Ebrahim S. Lifestyle and 15-year survival free of heart attack, stroke, and diabetes in middle-aged British men. Arch Intern Med. 1998;158:2433–2440. doi: 10.1001/archinte.158.22.2433. [DOI] [PubMed] [Google Scholar]
- 37.Kargman DE. Tuck C. Berglund LF, et al. Elevated high density lipoprotein levels are more important in atherosclerotic ischemic stroke subtypes: The Northern Manhattan Stroke Study. Ann Neurol. 1998;44:442–453. [Google Scholar]
- 38.Nagayama M. Shinohara Y. Nagayama T. Lipoprotein(a) and ischemic cerebrovascular disease in young adults. Stroke. 1994;25:74–78. doi: 10.1161/01.str.25.1.74. [DOI] [PubMed] [Google Scholar]
- 39.Peng DQ. Zhao SP. Wang JL. Lipoprotein(a) and apolipoprotein E4 as independent risk factors for ischemic stroke. J Cardiovasc Risk. 1999;6:1–6. doi: 10.1177/204748739900600101. [DOI] [PubMed] [Google Scholar]
- 40.O'Leary DH. Polak JF. Kronmal RA, et al. Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study: The CHS Collaborative Research Group. Stroke. 1992;23:1752–1760. doi: 10.1161/01.str.23.12.1752. [DOI] [PubMed] [Google Scholar]
- 41.Fine-Edelstein JS. Wolf PA. O'Leary DH, et al. Precursors of extracranial carotid atherosclerosis in the Framingham Study. Neurology. 1994;44:1046–1050. doi: 10.1212/wnl.44.6.1046. [DOI] [PubMed] [Google Scholar]
- 42.Muller JE. Tofler GH. Stone PH. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation. 1989;79:733–743. doi: 10.1161/01.cir.79.4.733. [DOI] [PubMed] [Google Scholar]
- 43.Muller JE. Circadian variation in cardiovascular events. Am J Hypertens. 1999;12:35S–42S. doi: 10.1016/s0895-7061(98)00278-7. [DOI] [PubMed] [Google Scholar]
- 44.Green DJ. Walsh JH. Maiorana A, et al. Exercise-induced improvement in endothelial dysfunction is not mediated by changes in CV risk factors: Pooled analysis of diverse patient populations. Am J Physiol Heart Circ Physiol. 2003;285:H2679–H2687. doi: 10.1152/ajpheart.00519.2003. [DOI] [PubMed] [Google Scholar]
- 45.Inoue M. Yamamoto S. Kurahashi N. Iwasaki M. Sasazuki S. Tsugane S. Daily total physical activity level and total cancer risk in men and women: Results from a large-scale population-based cohort study in Japan. Am J Epidemiol. 2008;168:391–403. doi: 10.1093/aje/kwn146. [DOI] [PubMed] [Google Scholar]
- 46.Blair SN. Cheng Y. Holder JS. Is physical activity or physical fitness more important in defining health benefits? Med Sci Sports Exerc. 2001;33(Suppl):S379–399. doi: 10.1097/00005768-200106001-00007. [DOI] [PubMed] [Google Scholar]
- 47.Shephard RJ. Shek PN. Associations between physical activity and susceptibility to cancer: Possible mechanisms. Sports Med. 1998;26:293–315. doi: 10.2165/00007256-199826050-00002. [DOI] [PubMed] [Google Scholar]
- 48.Matthews CE. Jurj AL. Shu X, et al. Influence of exercise, walking, cycling, and overall nonexercise physical activity on mortality in Chinese women. Am J Epidemiol. 2007;165:1343–1350. doi: 10.1093/aje/kwm088. [DOI] [PubMed] [Google Scholar]