Abstract
Background
Dietary iodine is often restricted before radioactive iodine (RAI) scanning or treatment of well-differentiated thyroid cancer. Our objective was to examine the impact of a low-iodine diet (LID) before RAI treatment or scanning on the following outcomes: (i) the efficacy of thyroid remnant ablation (or residual disease elimination), (ii) urinary iodine measurements, (iii) RAI kinetics, and (iv) long-term thyroid cancer outcomes.
Methods
We performed a systematic review of the English literature. We searched four electronic databases and conducted a hand search. Two reviewers independently screened citations and reviewed full-text articles and reached consensus on included articles. Two reviewers independently abstracted data.
Results
We reviewed 76 abstracts or citations and 26 full-text articles. Eight studies were included in the review. The most commonly studied diets allowed ≤50 μg/day of iodine for 1–2 weeks. In one study, 6-month successful remnant ablation rates were higher in patients following an LID than in controls. However, in another study, there was no significant benefit of an LID. LIDs reduce urinary iodine measurements and appear to increase I-131 uptake or lesional radiation compared to regular diets. No studies have examined long-term recurrence or mortality rates.
Conclusions
Given that LIDs reduce urinary iodine measurements, increase I-131 uptake, and possibly improve efficacy of I-131 treatment, we currently favor the use of a 1–2-week LID before I-131 therapy or scanning. However, more research is needed to clarify the role of this dietary intervention.
Introduction
In the treatment and follow-up of well-differentiated thyroid cancer, a temporary low-iodine diet (LID) is generally recommended before radioactive iodine (RAI) (I-131) treatment or RAI scanning. The rationale for utilization of the LID in such circumstances is to deplete whole-body iodine and optimize RAI uptake in thyroid cells. However, the stringency of the restriction and the duration of restriction around the time of therapy is debatable. Currently, the American Thyroid Association recommends an LID defined by an intake of <50 μg/day for 1–2 weeks before I-131 ablation (1), the British Thyroid Association recommends an LID for 2 weeks before I-131 ablation or therapy (stringency not specified) (2), the European Thyroid Cancer Taskforce recommends an LID for 3 weeks before I-131 administration (stringency not specified) (3), and the American Association of Clinical Endocrinologists recommends consumption of an LID for 2–4 weeks before radioiodine scanning (for follow-up), with no specific recommendations on stringency or diet before I-131 treatment (4). Many patients find an LID unpalatable, boring, and difficult to comply with. We have conducted a systematic review examining the impact of an LID on (1) the efficacy of thyroid remnant ablation (or elimination of any residual disease with I-131 treatment), (ii) urinary iodine measurements, (iii) RAI kinetics (such as RAI uptake or lesional radiation), and (iv) long-term thyroid cancer outcomes (such as mortality or recurrence rate, beyond the initial assessment of ablation efficacy). Our overall aim was to define the optimal diet, in terms of dietary iodine restriction stringency and duration, before RAI treatment (or scanning).
Patients and Methods
Inclusion and exclusion criteria for studies
We restricted this review to published English-language studies examining the use of an LID in preparation for RAI treatment or scanning in patients with well-differentiated nonmedullary thyroid carcinoma who had undergone thyroidectomy. An iodine-restricted diet was required to be compared to no diet or a variation of the diet (including variations in stringency or duration, with or without coadministration of a diuretic). Internal controls (for studies examining outcomes such as urinary iodine measurements before and after initiation of the LID) or external controls (including concurrent or historical controls) were deemed acceptable. Published randomized trials, systematic reviews, cohort studies, cross-sectional studies, and case series were eligible for inclusion, whereas individual case reports, narrative reviews, editorials, and unpublished abstracts were not. The primary outcome of interest was the efficacy of remnant ablation or elimination of any residual cancer (defined by absence of measurable RAI uptake or observation of RAI uptake on a scan) or a stimulated thyroglobulin measurement <2 μg/L, or combination, in the setting of follow-up testing after thyroid hormone withdrawal or recombinant human thyrotropin administration (measured at any point after initial post-therapy scanning). Secondary outcomes included urinary iodine measurements (before and after the diet or in comparison to a control group), RAI kinetics (such as RAI uptake or lesional radiation), and long-term thyroid cancer outcomes (such as disease recurrence or mortality). We also collected information on side effects, if available; however, this was not a requirement for inclusion. The presence of any of the primary or secondary outcomes was a requirement for inclusion in the study. In the case of multiple studies on the same population or overlapping populations, the largest or the most completely reported of the duplicate studies examining the intervention and outcome of interest was included.
Description of the search for relevant studies
An electronic search was conducted for potentially relevant citations using the following electronic databases (no language restriction) (July 8, 2009): Ovid Medline (beginning 1966), Ovid Medline-in-Process and non-indexed citations, EMBASE Classic, and EMBASE Text words. MESH headings relating to thyroid cancer, diet, and not hyperthyroidism or nuclear accidents were utilized in the electronic search. Additional full-text articles were obtained for review via hand search of cross-referenced clinical practice guidelines (1–4), cross-referenced reviewed studies, and studies suggested by clinical content experts (including coauthors as well as article reviewers from Thyroid). Non-English citations were excluded from this review because of the lack of resources for translation; however, if an English abstract was available, this was used to judge potential relevance.
Selection of studies and data abstraction
All abstracts and citations were reviewed independently by two reviewers. Any abstract or citation deemed potentially relevant by either reviewer was retrieved and reviewed in full-text form for formal review by two independent reviewers. Any citation obtained through the hand search by either reviewer was also reviewed in full-text form by both reviewers. Any discrepancies in assessment of inclusion of potentially relevant full-text articles were resolved by reviewer discussions. A reason for exclusion for excluded full-text articles was provided by each reviewer. We achieved consensus between reviewers for all studies included in the final systematic review. Data were abstracted and methodologic quality of studies was appraised, by two independent abstractors, and then checked by a third party with clinical and methodologic expertise (A.M.S.) for correction of any inaccuracies. Methodologic quality was assessed using the following criteria: study type, type of control group comparison, single or multicenter study, any mention of blinding or independent confirmation of study outcomes, report of loss to follow-up, and any description of statistical methods.
Statistical analyses
We calculated kappa statistics to assess agreement between reviewers for relevance at the citation or abstract review stage (for citations obtained using the electronic search) and for inclusion at the full-text review stage, before achieving consensus. The kappa statistics and 95% CIs were calculated using CIA software (Version 2.0, London, United Kingdom).
Results
Description of the studies
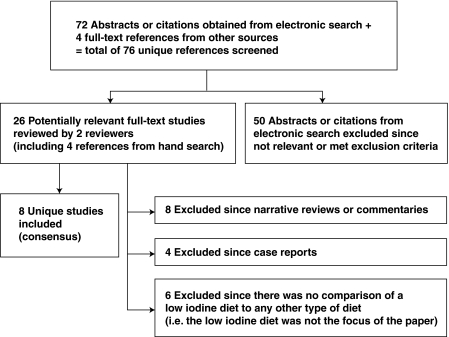
We retrieved 72 potentially relevant citations using our electronic search and an additional 4 citations from other sources (hand search), for a total of 76 reviewed citations (Fig. 1). Agreement between reviewers for relevance of 72 abstracts and citations retrieved by the electronic search was reflected by a kappa statistic of 0.58 (95% CI 0.35, 0.81). There were 26 potentially relevant full-text articles reviewed by two reviewers (including 4 articles from a hand search). The kappa statistic measuring agreement between reviewers for inclusion of full-text articles at the initial review stage was 0.74 (95% CI 0.47, 1.00). After considering the inclusion and exclusion criteria, there was consensus between reviewers to include eight studies in the review (5–12) (Fig. 1). It is important to note that a recent article by Tala Jury et al., examining the relationship between urinary iodine excretion and successful thyroid ablation in thyroid cancer patients, was excluded, since an LID was not specifically prescribed to patients in the study, and patients were simply advised to avoid iodine-containing drugs or supplements for 4 weeks before ablation (13). Thus, the intervention of interest, specifically an LID, was not systematically studied by Tala Jury et al. (13).
FIG. 1.
Process of study selection for the systematic review.
The characteristics of the included studies (5–12) are described in Table 1. There were two studies from The Netherlands (5,11), one from Japan (12), and the remaining five from the United States (6–10). In about half of the included studies, patients were reported to have either distant metastatic thyroid cancer or residual disease (5,7–9). In the remaining studies the disease status of participants was as follows: study by Lakshmanan et al.—all patients athyreotic and had negative I-131 scans (6); Park et al.—no report of disease status; Pluijmen et al.—some patients had extrathyroidal extension of thyroid cancer or positive lymph node metastases at surgery before RAI treatment, but there were no participants with distant metastatic disease (11); Tomoda et al.—no report of disease status (12). Urinary iodine outcomes were assessed in all of the included studies (5–12), whereas only two studies assessed ablation rates after first-time RAI treatment (9,11), and three studies assessed RAI kinetics (5,7,11). None of the studies assessed long-term recurrence or mortality rates after initial follow-up RAI scan. Only two studies reported any assessment of potential side effects of the LID (7,8) (Table 1). In terms of methodologic quality, six of the studies provided some longitudinal (cohort) data (5–8,10,11), all studies were performed in respective single institutions (5–12), and the majority of studies utilized either internal controls (in the case of comparisons before and after institution of an LID) or historical controls from the same institution (Table 2). Only the study by Pluijmen et al. (11) reported the use of blinding and independent confirmation by two nuclear physicians of RAI outcome scans. Losses to follow-up were generally not explicitly reported in the included studies (Table 2). Most studies reported some form of statistical methodology for comparisons (6,7,9–12). It is important to note that in the study by Pluijmen et al., the authors excluded from their analysis an unknown number of patients who had been prescribed the LID but whose urinary iodine excretion remained above 49.4 μg/day (11). Thus, the comparison by Pluijmen et al. did not represent an intention-to-treat analysis and appeared to exclude noncompliant patients or those who had preceding iodine exposure from the intervention group.
Table 1.
Characteristics of the Included Studies
| |
|
|
Outcomes examined |
|
|||
|---|---|---|---|---|---|---|---|
| Author and year (reference) | Country | Thyroid cancer patients in study | Ablation rates | Urinary iodine measurements | RAI kinetics | Recurrence or mortality | Side effects |
| Goslings 1975 (5) | The Netherlands | 15 patients with metastatic thyroid cancer (7 LID, 8 LID + ethacrynic acid) | No | Yes | Yes | No | Not assessed |
| Lakshmanan et al. 1988 (6) | United States | 5 athyreotic patients with a history of thyroid cancer who had negative RAI scans | No | Yes | No | No | Not assessed |
| Maruca et al. 1984 (7) | United States | 3 patients with metastatic thyroid cancer (1 bone, 2 neck) | No | Yes | Yes | No | Yes, present (nausea, diarrhea, attributed to sodium chloride 12 g/day) |
| Maxon et al. 1983 (8) | United States | 40 thyroid cancer patients, some of whom had prior treatment or residual disease (19 LID and 21 regular diet) | No | Yes | No | No | Yes, present (diet “boring,” no other adverse effects) |
| Morris 2001 (9) | United States | 94 thyroid cancer patients undergoing initial I-131 ablative therapy after thyroidectomy, including patients with metastatic lesions to lymph nodes or lungs (44 LID and 50 regular diet) | Yes | Yes | No | No | Not assessed |
| Park et al. 2004 (10) | United States | 58 thyroid cancer patients undergoing RAI scanning (excludes overlap among groups) 21 rhTSH+ 1 week LID, 24 rhTSH+ 2 week LID, 28 hypothyroid + 2 week LID (overlap 15 patients from rhTSH groups) |
No | Yes | No | No | Not assessed |
| Pluijmen et al. 2003 (11) | The Netherlands | 120 patients with papillary or follicular thyroid carcinoma, treated with RAI after total thyroidectomy (including some patients with extrathyroidal extension of disease or lymph node metastases, but no distant metastases) | Yes | Yes | Yes | No | Not assessed |
| Tomoda et al. 2005 (12) | Japan | 252 thyroid cancer patients who had a thyroidectomy and were undergoing RAI scans | No | Yes | No | No | Not assessed |
LID, low-iodine diet; RAI, radioactive iodine; rhTSH, recombinant human thyrotropin.
Table 2.
Assessment of Methodologic Quality of Included Studies
| Author and year (reference) | Study type | Single or multicenter | Type of control comparison | Report of blinding or independent confirmation study outcomes | Explicit reporting of loss to follow-up | Any description statistical methods |
|---|---|---|---|---|---|---|
| Goslings 1975 (5) | Cohort | Single | (i) Internal, pre–post comparison (ii) Comparison to contemporaneous controls (LID vs. LID + ethacrynic acid) |
No | No | No |
| Lakshmanan et al. 1988 (6) | Cohort | Single | Internal, pre–post comparison | No | No, but all patients seem accounted for at end of study | Yes |
| Maruca et al. 1984 (7) | Cohort | Single | Internal, pre–post comparison | No | No, but all patients seem accounted for at end of study | Yes |
| Maxon et al. 1983 (8) | Cohort | Single | Comparison to contemporaneous controls | No | No | No |
| Morris 2001 (9) | Cross sectional | Single | (i) Comparison to historical control data (LID 1997–1999, regular diet 1990 to early 1996) (ii) Comparison to contemporaneous “normal controls” for urinary iodine measurements |
No | No, but cross-sectional comparison | Yes |
| Park et al. 2004 (10) | Cohort(also, cross-sectional comparison with NHANES III data) | Single | (i) Comparison to contemporaneous controls (rhTSH+ 1 week LID compared to 2 week LID, compared to hypothyroidism with 2-week LID, with some of the hypothyroid data being obtained in same patients retrospectively) (ii) Also, comparison to population-based control data for the northeastern United States |
No | No | Yes |
| Pluijmen et al. 2003 (11) | Cohort | Single | Comparison to historical control data (LID group treated from 1992 to 1998 and control group treated from 1986 to 1991) | Yes. The whole-body scans were evaluated by 2 nuclear medicine physicians, who were unaware of LID adherence. | No | Yes |
| Tomoda et al. 2005 (12) | Cross sectional | Single | Comparison to historical control data (1-week LID group studied between May 2004 and August 2004, 1-week less restrictive diet treated between January 2001 and March 2004) | No | No, but cross-sectional comparison | Yes |
The goal dietary intake of iodine in LIDs was ≤50 μg in half of studies (6–8,10), whereas it was not reported in three studies (9–12), and a typographic error was suspected in the report of the oldest study (Goslings, 20–30 g [5]) (Table 3). LIDs ranged in duration from 4 days to 4 weeks, with the majority of studies describing diet durations ranging from 1 to 2 weeks (5,8–10,12) (Table 3). Timing of cessation of the LID was generally at RAI administration (8,9,11,12) or scanning (5,6,10,12) (Table 3). In terms of method of counseling patients, physician counseling was provided in the study by Park et al. (10). Written instructions on the diet were provided to patients in six studies (6,8–12), and a dietician was made available to patients in four studies (6–8,11). Most studies reported using thyroid hormone withdrawal (hypothyroidism) in preparation for RAI treatment or scanning in most (6–12). Thyrotropin stimulation using recombinant human thyrotropin was utilized in only one study (10). Moreover, the authors explicitly reported that no participants had received iodinated contrast materials in the 6 months before any of the diagnostic scans in only one included study (10). The explicit assessment of exposure to vitamins or iodinated contrast before RAI treatment or scanning was not reported in the rest of the studies in this review.
Table 3.
Description of Dietary Iodine Restriction and Related Procedure
| Author and year (reference) | Goal daily intake of iodine | Diet duration (when stopped) | Assessment of recent iodine contrast exposure or vitamin use | LID counseling from physician | Written instructions for patients | Dietician available to patients | Diuretic use | Method of thyrotropin stimulation |
|---|---|---|---|---|---|---|---|---|
| Goslings 1975 (5) | 20–30 ga | 4 days for urinary measurements, another 4 days for RAI uptake measurements (total 8 days) (stopped at RAI scan) | Not reported | Not reported | Not reported | Not reported | Yes | Not reported |
| Lakshmanan et al. 1988 (6) | About 50 μg | 4 weeks (stopped at RAI scan) | Not reported | No | Yes | Yes | Not reported | Hypothyroidism |
| Maruca et al. 1984 (7) | <25 μg | 5 days (unclear when stopped) | Not reported | Not reported | No | Yes | Yes | Hypothyroidism |
| Maxon et al. 1983 (8) | 45–50 μg | 1 week (stopped at RAI administration) | Not reported | Not reported | Yes | Yes | Not reported | Hypothyroidism |
| Morris 2001 (9) | Not reported | 10–14 days (stopped at RAI administration) | Not reported | No (nuclear medicine technician) | Yes | No | Not reported | Hypothyroidism |
| Park et al. 2004 (10) | ≤50 μg | 7 days, 14 days, in respective groups (stopped at RAI scan) | Yes | Yes | Yes | No | Not reported | rhTSH, hypothyroidism, respectively |
| Pluijmen et al. 2003 (11) | Not reported | 4 days (fish and seafood prohibited for 7 days) (stopped at RAI administration) | Not reported | Not reported | Yes | Yes | Not reported | Hypothyroidism |
| Tomoda et al. 2005 (12) | Not reported | 1 week, 2 weeks in respective groups (stopped before RAI studies and therapy) | Not reported | Not reported | Yes | No | Not reported | Hypothyroidism |
We suspect a typographic error in the original study, and suspect the iodine intake is 20–30 μg/day, rather than 20–30 g listed in the study; however, given that the study is published 35 years ago, we were not able to confirm this.
The impact of an LID on urinary iodine measurements
Urinary iodine outcomes were assessed in all of the included studies (5–12). Since variable measurement units for urinary iodine excretion were utilized among studies examining urinary iodine outcomes (some measurements adjusted for urinary creatinine and others not), we were unable to pool these data. We have summarized data on urinary iodine outcomes below, according to the type of comparison that was made.
Urinary iodine was measured before and after institution of an LID, lasting between 4 days and 4 weeks in four studies (5–7,9). In two of these studies, patients also received concurrent diuretics such as ethacrynic acid (5) or hydrochlorothiazide (7). In all three studies in which a statistical comparison was made, urinary iodine measurements were significantly reduced after institution of the LID (duration ranging from 5 days to 4 weeks) compared to before the diet (6,7,9). In the fourth study, in patients not on diuretics, mean urinary iodine measurements were 121.6 μg/day (standard deviation [SD] 36.9) before the diet and 30.0 μg/day (SD 9.7) after 4 days on the diet (5). The addition of ethacrynic acid to an LID did not significantly change urinary iodine measurements relative to LID alone in the one study examining this issue (5).
There were two studies comparing urinary iodine measurements in thyroid cancer patients treated with the LID compared to a regular diet (8,11). In the study by Maxon et al., the comparison was between two groups of patients treated in concurrent time periods (8), whereas in the study by Pluijmen et al., historical controls were used from different time periods (with controls being treated in years earlier than those treated with the LID) (11). In the studies by Maxon et al. (8) and Pluijmen et al. (11), urinary iodine measurements were significantly lower in thyroid cancer patients treated with the LID than in those not treated with the LID. Urinary iodine measurements on the LID were evaluated compared to normal controls (such as healthy volunteers or population-based data) in two studies (9,10); however, neither provided a statistical comparison for these data.
A comparison was made between a 1- and 2-week LIDs in two studies examining urinary iodine outcomes (10,12). In the study by Park et al., thyroid cancer patients were pretreated with recombinant human thyrotropin before RAI scanning and they were directed to follow an LID for either 1 or 2 weeks, respectively (10). Park et al. reported that mean urinary iodine measurements were significantly lower in recombinant human thyrotropin-treated individuals who followed a 2-week LID (mean 84.69, SD 44.04 μg/g creatinine in 24 patients) compared to a 1-week LID (mean 199.87, SD 169.53 μg/g creatinine in 21 patients) (p < 0.03) (10). Park et al. also reported that urinary iodine measurements were <100 μg/g creatinine in 71% of patients treated with a 2-week diet compared to 41% in those treated with a 1-week diet (no statistical comparison performed) (10). Tomoda et al. reported that in patients who underwent thyroid hormone withdrawal before RAI scanning, patients prescribed a 2-week LID also had lower urinary iodine measurements (median 66 μg/g creatinine, range 7–134 in 17 patients) compared to those prescribed a 1-week LID (median 130 μg/g creatinine, range 23–218 in 15 patients) (p < 0.01) (12). In this study, urinary iodine measurements were <100 μg/g creatinine in 70% of patients treated with a 2-week diet compared to 26% in those treated with a 1-week diet (no statistical comparison performed) (12).
Park et al. also examined the impact of thyroid hormone withdrawal compared to recombinant human thyrotropin administration on urinary iodine excretion after a 2-week LID (10). In this study, levothyroxine treatment was continued throughout the study preparation and I-131 scan in patients treated with recombinant human thyrotropin (10). Urinary iodine measurements were significantly lower in hypothyroid patients (mean 59.59, SD 75.95 μg/g creatinine in 28 patients) than in patients pretreated with recombinant human thyrotropin (mean 84.69, SD 44.04 μg/g creatinine in 24 patients), using a 2-week LID (p < 0.001) (10). Park et al. also reported that urinary iodine measurements were <100 μg/g creatinine in 78% of hypothyroid patients treated with a 2-week diet compared to 71% in those prescribed recombinant human thyrotropin after a 2-week LID (no statistical comparison performed).
The impact of an LID on RAI kinetics
There were three studies that examined RAI uptake or lesion radiation (5,7,11). Goslings examined the percentage of I-131 uptake at 24 hours in patients with metastatic follicular thyroid carcinoma before and after institution of an 8-day LID (5). The tumor uptake of I-131 increased by a factor of 1.79 (SD 0.57) after the diet (24-hour uptakes were 0.6%–8.3% before the diet and 1.5%–15.7% after the diet, with no statistical comparison) (5). Further, Goslings reported that the effective half-life of RAI increased by a factor of 1.19 (SD 0.15) after 4 days on an LID (5). Maruca et al. reported that the lesional radiation increased by 108% and 48%, in two respective patients with metastatic thyroid carcinoma after a 5-day LID (mean 247.2 μCi-days prediet and mean 500 μCi-days postdiet); serum half-life of RAI also increased in these patients (7). Pluijmen et al. reported that in using 1 mCi (37 MBq) of I-131 for pretherapy neck uptake measurements in patients (without distant metastases), before ablation, the 24-hour neck uptake was significantly higher in patients who followed a 4-day LID (mean 5.1% of activity, SD 3.8) compared to those who did not (mean 3.1%, SD 2.5) (p < 0.001) (11).
The impact of an LID on the efficacy of RAI treatment or remnant ablation
There were two studies that examined either stimulated thyroglobulin measurements or RAI uptakes in the months following postsurgical administration of I-131, and the control groups in each of these studies were comprised of historical controls (9,11). In both of these studies, the LID was stopped upon RAI administration (9,11). The duration of the LID was 10–14 days in the study by Morris et al. (9) and 4 days (with a 7-day restriction on seafood) in the study by Pluijmen et al. (11). In the study by Morris et al., patients with and without metastases (to lymph nodes or lungs) were treated postsurgically with 100–200 mCi of I-131 (9). Morris et al. reported that RAI scans performed a mean of 11.8 months after I-131 treatment (range 5–42 months) were visually negative in the thyroid bed in 68.2% (30/44) of patients treated with a 10–14-day LID compared to 62.0% (31/50) of historical controls who followed a regular diet (p = 0.53). Although not subject to statistical comparison by the primary authors, the rate of negative scans at follow-up in individuals without metastatic disease in this study was as follows: 62.1% (18/29) in the LID group and 60.0% (21/35) in the regular diet group (9). Follow-up stimulated thyroglobulin measurements were not reported by Morris et al. (9).
Pluijmen et al. examined stimulated thyroglobulin measurements and RAI activity following administration of 5 mCi (185 MBq) in patients without metastatic disease 6 months after remnant ablation (11). The mean dose activity of I-131 used for remnant ablation or I-131 treatment in this study was 76 mCi (2800 MBq) (or 162 mCi [6000 MBq] if the tumor had not been “removed radically according to surgical reports or pathological examination” as judged by the primary authors) (11). Pluijmen et al. reported that in thyroglobulin antibody-negative patients, follow-up stimulated thyroglobulin measurements (after thyroid hormone withdrawal) were <2 μg/L in 85% of those who followed the LID pretreatment compared to 69% of historical controls on a regular diet before treatment (p = 0.066) (11). In a subgroup analysis of thyroglobulin antibody-negative patients who had no gross extrathyroidal extension of tumor and negative lymph nodes at surgery, follow-up stimulated thyroglobulin measurements were <2 μg/L in 92% in the LID treatment group compared to 68% in historical controls (p = 0.012). Pluijmen et al. found that the percentage of patients who had no detectable I-131 neck activity on follow-up testing was not significantly different between the LID group (67%) compared to historical controls (73%) (p = 0.563). Further, in individuals without gross extrathyroidal tumor extension and negative lymph nodes, the absence of neck activity at follow-up was not significantly different between the low iodine treatment group (67%) and controls (62%) (p = 0.261). In thyroglobulin antibody-negative individuals, a follow-up stimulated thyroglobulin of <2 μg/L and absent I-131 neck activity was reported in 65% of patients in the LID group compared to 48% in historical controls (p < 0.001). In a subgroup of patients with no gross extrathyroidal tumor extension and negative lymph nodes, a follow-up stimulated thyroglobulin of <2 μg/L and absent I-131 neck activity was reported in 71% of patients in the LID group compared to 44% in controls (p < 0.001).
Discussion
In summary, dietary iodine restriction for 5 days to 4 weeks before RAI treatment or scanning for well-differentiated thyroid cancer has been shown to reduce urinary iodine measurements compared to non-restricted diets (6,7,9). Further, urinary iodine measurements were significantly lower after 2 weeks of iodine restriction compared to 1 week, with mean values at 2 weeks being about half of that at 1 week (10,12). LIDs have also been shown to prolong serum half-life of RAI (5,7). In this review, the most commonly used form or LID utilized a goal dietary intake of ≤50 μg/day (6–8,10), although the superiority of this dose restriction to less restrictive diets has not been adequately studied. The addition of ethacrynic acid to dietary iodine restriction does not appear to significantly impact urinary iodine measurements (5). Although studies were included from a variety of regions, including the United States (5–10), Western Europe (The Netherlands) (5,11), and Japan (12), given that urinary iodine measurements were ascertained differently and that the stringency of LIDs was variable, it is not possible, based on the data in this review, to ascertain any relationship between geographic differences in dietary iodine intake and times required to achieve adequate iodine depletion before I-131 scanning or therapy. With respect to the issue of urinary iodine excretion in patients rendered hypothyroid compared to those treated with recombinant human thyrotropin, Park et al. (10) reported that urinary iodine measurements (corrected for urine creatinine) may be reduced to a greater extent in hypothyroid individuals compared to those pretreated with recombinant human thyrotropin (10). Moreover, the clinical significance of any potential differences in urinary iodine measurements in patients undergoing thyroid hormone withdrawal compared to administration of recombinant human thyrotropin is unclear, as about 70% of patients were reported to have achieve urinary iodine measurements <100 μg/g creatinine in two respective studies after a 2-week LID (in spite of one study utilizing recombinant human thyrotropin and the other study utilizing thyroid hormone withdrawal) (10,12). Further, in a recent study by Tala Jury et al., in which an LID was not specifically prescribed, no significant difference in urinary iodine excretion was noted between individuals undergoing thyroid hormone withdrawal (mean 128, SD 187 μg/L) compared to administration of recombinant human thyrotropin (mean 139, SD 98 μg/L), before remnant ablation (13). Given these conflicting data, it is currently unclear whether urinary iodine excretion in patients rendered hypothyroid is significantly different compared to those treated with recombinant human thyrotropin, particularly if measurements are corrected for urinary creatinine, after institution of an LID. With respect to the impact of dietary iodine restriction on RAI kinetics, an LID may increase uptake of I-131 by remnant tissue or tumor and increase lesional radiation (5,7); however, the evidence for this is based on data from a very limited number of patients, largely not subject to rigorous statistical comparisons. Given that only limited data have been reported in patients with distant metastatic disease, it is not definitively proven that an LID increases the effective radiation dose to thyroid cancer metastases. There are two studies examining remnant ablation rates in the months following postsurgical I-131 remnant ablation or therapy (9,11), and each of these is limited by a comparison of patients treated with an LID to data from historical controls on an unrestricted diet (9,11). In the study by Morris et al. (9), there was no significant difference in rates of visibly negative follow-up I-131 scans in 44 patients treated with a 10–14-day LID compared to 50 historical controls (9). However, in the study by Pluijmen et al., in thyroglobulin antibody-negative individuals, the rate of absence of measurable I-131 neck activity and stimulated thyroglobulin measurements <2 μg/L at 6 months was significantly higher in 45 patients treated with a 4-day LID (with 7-day fish and seafood restriction) (65%) compared to 47 patients on an unrestricted diet (48%) (11). It is not known to what extent variability in residual remnant size may have explained the differences between the results of Morris et al. (9) and Pluijmen et al. (11). It is important to acknowledge that a methodological advantage of the study by Pluijmen et al. (11) was the utilization of independent confirmation and blinding of nuclear medicine physicians reviewing RAI scans. However, Pluijmen et al. excluded from their analysis an unknown number of patients who had been prescribed the low iodine whose urinary iodine excretion was above 49.4 μg/day (11), suggesting that noncompliant patients may have been excluded. It is also important to recognize that in any studies utilizing historical control data, that differences in outcomes may have potentially been attributed to sources other than the intervention of study (such as advances in surgical techniques, or other factors). It is not proven that ablation outcomes are superior using a 2-week LID compared to a shorter diet, as the positive study by Pluijmen diet utilized only a 4-day diet (with 7 days of fish and seafood restriction) (11). It is possible that the incorporation of a more sensitive outcome definition for successful remnant ablation, such as stimulated thyroglobulin measurements, allowed more statistical power for the study by Pluijmen et al. (11) to detect a difference in ablation rates between groups, relative to the study by Morris et al. (9). Of note, in a recent study by Tala Jury et al., there was no significant relationship between urinary iodine excretion and successful remnant ablation, although the study was conducted in patients not specifically prescribed an LID (13). Also, in terms of RAI treatment (or remnant ablation), it remains unclear whether continuing the LID after RAI administration may result in superior ablation outcomes (as opposed to continuing the diet until post-treatment scanning), as both the studies by Morris et al. (9) and Pluijmen et al. (11) stopped the diet upon RAI administration. Unfortunately, there are still no studies examining long-term recurrence or mortality rates in patients treated with an LID compared to an unrestricted diet.
The potential adverse effects of temporary dietary iodine restriction was examined in only two studies in this review (7,8). In the study of three patients by Maruca et al., nausea and diarrhea were routinely experienced by patients, but this was attributed to the concomitant prescription of high-dose sodium chloride (12 g/day) (7). In this study, hypothyroid patients were treated in the hospital with a 5-day LID (<25 μg/day), 12 g of sodium chloride per day, diuretics, and 3 L of water per day (7). In the study by Maxon et al. (8), the authors reported that in 19 hypothyroid patients treated with an LID (45–50 μg/day), none of the individuals experienced nausea, but they did complain that the diet was “boring.” The potential issue of radiation toxicity attributable to prolonged retention of I-131 in LID-treated patients was not examined by any of the studies in the review and deserves further study. However, theoretically, as LIDs prolong retention of RAI, the whole-body radiation dose per mCi of I-131 administered may create the potential for radiation toxicity when high-dose activities of therapeutic I-131 are used, particularly in patients who may have delayed excretion due to advanced age or renal dysfunction. Although not eligible for this review, there are several notable case reports of patients developing symptomatic hyponatremia while on an LID (14,15). Krishnamurthy and McDougall described symptomatic hyponatremia (serum sodium of 115–116 nM) occurring in a 70-year-old man who underwent thyroid hormone withdrawal and was prescribed a 2-week LID, as well as a 81-year-old woman who received recombinant human thyrotropin and hydrochlorothiazide with an LID (the latter diet duration was unspecified) (14). Also, Shakir et al. reported symptomatic hyponatremia (serum sodium of 110–121 nM) in five patients, aged 66–87 years, who underwent thyroid hormone withdrawal and whose LIDs ranged from 1 to 6 weeks in duration (15). In the report by Shakir et al., none of the patients were taking any antihypertensive agents, but four of the five patients had either lung or cerebral metastases (15). In the studies included in our review, serum sodium was not routinely measured in patients. Although it is important to be aware of the potential for hyponatremia developing in patients prescribed LIDs, particularly in elderly patients who may be restricting all salt (including noniodized salt), it is certainly possible that other factors such as concomitant severe hypothyroidism or diuretic use played a contributory role.
The strength of this review of dietary iodine restriction in preparation for I-131 treatment or scanning is its systematic nature, the critical appraisal of the existing evidence, and the inclusion of studies in which some form of controlled comparison was reported. Some limitations of this review include the English-language restriction, the inclusion of relatively less methodologically rigorous studies (such as studies incorporating historical controls, studies incompletely reporting relevant data, and the lack of availability of intention-to-treat analyses in some studies), relatively small sample sizes in many of the included studies, and the lack of pooled analyses. Given the variety of outcome measurements utilized in included studies, methodologic heterogeneity precluded meaningful pooling of outcomes.
Currently, American, British, and European clinical practice guidelines variably, respectively, recommend the institution of an LID for durations ranging from 1 to 4 weeks before RAI treatment or scanning (1–4). On the basis of a review of the available current evidence, an LID of ≤50 μg iodine/day for 1–2 weeks appears to be the most studied, although rigorous head-to-head comparisons have not been performed with less restrictive diets of shorter duration. It is not known if an LID may result in improved long-term outcomes in thyroid cancer, either in those undergoing remnant ablation or in those undergoing treatment of residual, recurrent, or distant metastatic disease. Ideally, randomized controlled trials should be conducted to identify the optimal daily dose restriction (if any) for dietary iodine, diet duration (if any), and impact on long-term outcomes, particularly relating to remnant ablation or treatment of residual or metastatic disease. Close examination of side effects, including hyponatremia, should be performed in the context of such studies. Further, it is currently unclear whether individuals receiving recombinant human thyrotropin may benefit from a longer LID duration (or different stringency of restriction) compared to hypothyroid patients, and this issue deserves further study. Severe side effects of LIDs, such as symptomatic hyponatremia, were not reported in the studies in our review, although they were not systematically examined by the majority of primary authors. Clinicians recommending an LID should be aware of this risk and consider stopping concurrent diuretic treatment (or measuring serum sodium if continued), as well as encouraging noniodized salt intake. In the meantime, although subject to some uncertainty, it appears reasonable for clinicians to recommend dietary iodine restriction for 1–2 weeks before RAI treatment or scanning, based on the best available evidence examining urinary iodine excretion, radioisotope kinetics, and ablation outcomes (including stimulated thyroglobulin measurements and post-RAI uptake results). Although not specifically examined in the studies in this review, the avoidance and the explicit assessment of exposure to vitamins, supplements, or iodinated contrast are additional important clinical considerations before RAI treatment or scanning for thyroid cancer.
Acknowledgments
This work and Anna M. Sawka have been supported by the Canadian Institutes of Health Research New Investigator Program (CNI-80701). Anna M. Sawka currently holds a Cancer Care Ontario Chair in Health Services Research.
Disclosure Statement
The authors have no commercial associations to declare that might create a conflict of interest in connection with this article.
References
- 1.Cooper DS. Doherty GM. Haugen BR. Kloos RT. Lee SL. Mandel SJ. Mazzaferri EL. McIver B. Pacini F. Schlumberger M. Sherman SI. Steward DL. Tuttle RM. the American Thyroid Association Guidelines Task Force 2009 Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 2.British Thyroid Association, Royal College of Physicians. Guidelines for the Management of Thyroid Cancer. second. 2007. www.british-thyroid-association.org. [Jul 7;]. 2009. www.british-thyroid-association.org
- 3.Pacini F. Schlumberger M. Dralle H. Elisei R. Smit JWA. Wiersinga W. the European Thyroid Cancer Task Force. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006;154:787–803. doi: 10.1530/eje.1.02158. [DOI] [PubMed] [Google Scholar]
- 4.American Association of Clinical Endocrinologists, American College of Endocrinology, American Association of Endocrine Surgeons. AACE/AAES medical/surgical guidelines for clinical practice: management of thyroid carcinoma. Endocr Pract. 2001;7:202–220. [PubMed] [Google Scholar]
- 5.Goslings BM. Proceedings: effect of a low iodine diet on 131-I therapy in follicular thyroid carcinomata; J Endocrinol; 1975. 30P. [PubMed] [Google Scholar]
- 6.Lakshmanan M. Schaffer A. Robbins J. Reynolds J. Norton J. A simplified low iodine diet in I-131 scanning, therapy of thyroid cancer. Clin Nucl Med. 1988;13:866–868. doi: 10.1097/00003072-198812000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Maruca J. Santner S. Miller K. Santen RJ. Prolonged iodine clearance with a depletion regimen for thyroid carcinoma: concise communication. J Nucl Med. 1984;25:1089–1093. [PubMed] [Google Scholar]
- 8.Maxon HR. Thomas SR. Boehringer A. Drilling J. Sperling MI. Sparks JC. Chen IW. Low iodine diet in I-131 ablation of thyroid remnants. Clin Nucl Med. 1983;8:123–126. doi: 10.1097/00003072-198303000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Morris LF. Wilder MS. Waxman AD. Braunstein GD. Reevaluation of the impact of a stringent low-iodine diet on ablation rates in radioiodine treatment of thyroid carcinoma. Thyroid. 2001;11:749–755. doi: 10.1089/10507250152484583. [DOI] [PubMed] [Google Scholar]
- 10.Park JT. Hennessey JV. Two-week low iodine diet is necessary for adequate outpatient preparation for radioiodine rhTSH scanning in patients taking levothyroxine. Thyroid. 2004;14:57–63. doi: 10.1089/105072504322783858. [DOI] [PubMed] [Google Scholar]
- 11.Pluijmen MJ. Eustatia-Rutten C. Goslings BM. Stokkel MP. Arias AM. Diamant M. Romijn JA. Smit JWA. Effects of low-iodide diet on postsurgical radioiodide ablation therapy in patients with differentiated thyroid carcinoma. Clin Endocrinol (Oxf) 2003;58:428–435. doi: 10.1046/j.1365-2265.2003.01735.x. [DOI] [PubMed] [Google Scholar]
- 12.Tomoda C. Uruno T. Takamura Y. Ito Y. Miya A. Kobayashi K. Matsuzuka F. Amino N. Kuma K. Miyauchi A. Reevaluation of stringent low iodine diet in outpatient preparation for radioiodine examination and therapy. Endocr J. 2005;52:237–240. doi: 10.1507/endocrj.52.237. [DOI] [PubMed] [Google Scholar]
- 13.Tala Jury HP. Castagna MG. Fioravanti C. Cipri C. Brianzoni E. Pacini F. Lack of association between urinary iodine excretion and successful thyroid ablation in thyroid cancer patients. J Clin Endocrinol Metab. 2010;95:230–237. doi: 10.1210/jc.2009-1624. [DOI] [PubMed] [Google Scholar]
- 14.Krishnamurthy VR. McDougall IR. Severe hyponatremia: a danger of low-iodine diet. Thyroid. 2007;17:889–892. doi: 10.1089/thy.2007.0094. [DOI] [PubMed] [Google Scholar]
- 15.Shakir MK. Krook LS. Schraml FV. Hays JH. Clyde PW. Symptomatic hyponatremia in association with a low-iodine diet and levothyroxine withdrawal prior to I131 in patients with metastatic thyroid carcinoma. Thyroid. 2008;18:787–792. doi: 10.1089/thy.2008.0050. [DOI] [PubMed] [Google Scholar]