Abstract
Many studies have shown that the direction of gaze of a face covertly facilitates the response to a target presented in the matching direction. In this study we seek to determine whether there exist separate reflexive and voluntary forms of such covert social orienting and how they interact with each other. We measured the effect of the predictive value of a gaze cue on manual choice reaction times. When the predictive value of the gaze cue was zero, a facilitatory cueing effect was still observed which peaked at a Cue onset to Target onset Delay (CTD) of 150 ms and largely diminished beyond a CTD of 500 ms. When the gaze cue was 100% predictive of the future location of the target, at CTDs greater than 200, the predictive cue resulted in a significantly greater facilitation of response than occurred with a non-predictive cue. These results suggest that given enough time (about 200 ms), the social cue is interpreted and a willful or voluntary spatially-specific social cueing effect occurs. In addition, we found that a predictive cue resulted in a significant slowing of the observer’s responses up to a CTD of 200 ms. These findings show that, similar to non-social spatial orienting, there appear to be two forms of social orienting including a reflexive component and voluntary component. We suggest a model of social orienting in which the voluntary social orienting system modulates tonic inhibition of the reflexive social orienting system.
Introduction
A nonsocial cue (e.g., shape, number, arrow) presented prior to a target may either facilitate or inhibit the response to the target. The effect of the cue depends on factors such as the timing between the cue and the target (Cue onset to Target onset Delay, CTD) and the cue’s spatial location relative to the target’s location (Posner, 1980), as well as the cue’s ability to predict when and where the target will occur. Peripheral cues have been found to result in a reflexive, exogenous orienting of attention; whereas presentation of a central cue produces an endogenous, voluntary orienting (Jonides, 1981; Nakayama & Mackeben, 1989). Results from visual search paradigms have also suggested two forms of spatial attention: a reflexive or transient component and a voluntary or sustained component (Nakayama & Mackeben, 1989). In parallel, several decades of psychological research have shown that an individual’s gaze serves as a signal, used to direct the attention of others and to non-verbally express relevant social cues (Kleinke, 1986). Similar to results from the nonsocial spatial cueing paradigm, it has been shown that spatially directed social gaze cues can influence the latency of response to a following target (Friesen, Ristic & Kingstone, 2004; Frischen, Bayliss & Tipper, 2007; Tipples, 2008). An additional similarity between the nonsocial and social cueing paradigms is that whether or not the cue is spatially predictive can also influence latencies to a following target. Compared to non-social spatial orienting where the importance of many variables (e.g., central/peripheral; predictive/nonpredictive; directional/nondirectional) have been examined, many aspects of social cueing have not yet been fully explored. We begin by briefly reviewing recent studies that have been directed towards understanding the effect of spatially directed gaze cues on covert (i.e., attention) and overt (i.e., eye movement) orienting and how they relate to the non-social spatial cueing literature.
When an observer views a face with eyes averted to one side, reaction to a subsequent target is faster if the target is presented in the same direction as the eyegaze compared to an opposite direction (Driver, Davis & Ricciardelli, 1999; Friesen & Kingstone, 1998; Friesen et al., 2004; Hood, Willen & Driver, 1998; Langton, 2000; Langton & Bruce, 1999; Ristic, Friesen & Kingstone, 2002; Ristic & Kingstone, 2005; Ristic, Wright & Kingstone, 2007; Tipples, 2008). Similar facilitation is also observed with head-gaze angle (Langton, 2000). This facilitation occurs even if the observer is instructed to ignore the face and the head because it does not contain any information about the spatial location of the subsequent target. Thus, the gaze dependent facilitation is attributed to the operation of reflexive (Deaner & Platt, 2003; Driver et al., 1999; Friesen & Kingstone, 1998; Langton, 2000; Langton & Bruce, 1999) or learned (Itier, Villate & Ryan, 2007; Vecera & Rizzo, 2006) mechanisms. In contrast, others (Friesen et al 2004, Tipples 2008) have found it possible to elicit a voluntary, willful orienting of attention in experiments using gaze cues that have predictive value.
Reflexive Social versus Non-Social Orienting
In a social setting, the ability to automatically orient to the gaze cues of others could potentially serve as a means of quickly interpreting the object of another’s attention without devoting conscious effort. The mechanism underlying reflexive social orienting is believed to be distinct from that mediating reflexive non-social forms of orienting (Friesen & Kingstone, 1998). Reflexive social cueing occurs with a central face cue, whereas reflexive non-social cueing generally occurs with peripheral cues such as a brightened box (Posner, Rafal, Choate & Vaughan, 1985). Furthermore, reflexive facilitation from gaze cueing begins earlier (~100 ms; Langton & Bruce, 1999) than that seen from non-social reflexive cues, and facilitation disappears later (Friesen & Kingstone, 1998). It is also reported that the inhibitory cueing effect (termed inhibition of return, or IOR) commonly found with non-predictive non-social cues for CTDs starting at about 300 ms (Posner et al., 1985) does not occur until significantly later in response to social cues. In experiments using long CTDs between the gaze cue and the subsequent target, it has been shown that at 1440 ms there is a decay of facilitation but no IOR, even after almost 3 s (McKee, Christie & Klein, 2007). Others have found that IOR only occurred for a CTD greater than 2 s (Frischen & Tipper, 2004).
Results from a variety of electrophysiological and fMRI experiments provide evidence that neural regions involved in reflexive non-social and social cueing effects are different (Hietanen, Nummenmaa, Nyman, Parkkola & Hamalainen, 2006; Kingstone, Tipper, Ristic & Ngan, 2004). Kingstone et al. (2004) used an ambiguous non-predictive cue that could be perceived as either a car or a pair of eyes with a hat to investigate if special brain areas were involved in mediation of gaze cueing effects. In one set of cueing runs, they instructed the observers to perceive the cue as a car. In another set of cueing runs, the observers were instructed to perceive the cue as a pair of eyes with a hat. The results demonstrated equivalent behavioral facilitatory cueing effects in both sets of runs. However, fMRI activation in superior temporal sulcus (STS) occurred only for runs in which the cue was perceived as eyes with a hat. Hence, they concluded that reflexive gaze cueing was mediated by a separate neural circuit.
In another fMRI study investigating the neural regions activated in response to non-predictive arrow and gaze cues, Hietanen et al (2006) found that although there was some overlap in activation between the two stimuli, each cue type also provoked a response in unique regions. Specifically, work by this group shows that neural regions activated during cueing by a central non-predictive arrow include medial/inferior occipital gyri, medial temporal gyrus, left intraparietal area, right frontal eye field and supplementary eye field. In contrast, neural regions uniquely activated during cueing by a non-predictive gaze cue include only left inferior occipital gyrus, right medial occipital gyrus and right inferior occipital gyrus. Other neuroimaging work demonstrates that averted gaze cues lead to activation of the intraparietal sulcus (IPS), an area which is also associated with shifts of covert spatial attention from non-social cues (Hoffman & Haxby, 2000). Thus, reflexive social and non-social orienting may have some common regions of activation, but overall the neural response to non-social cueing seems to include many more regions throughout the brain and is more bilateral.
In addition, other work has indicated that faces and eye gaze are unique stimuli that the brain processes in specific regions of the brain in both non-human primates (Perrett, Hietanen, Oram & Benson, 1992) and in humans (Nummenmaa & Calder, 2009; Pelphrey, Singerman, Allison & McCarthy, 2003; Perrett et al., 1992). Perrett and colleagues (1992) have shown that cells in the superior temporal sulcus (STS) of non-human primates are activated by specific combinations of directional head and eye positions. For instance, a single STS cell may be activated when either a pair of eyes looking up, or a face looking up, is viewed. This suggests that STS cells are important not just for their commonly recognized role in processing facial identity (Perrett, Smith, Potter, Mistlin, Head, Milner & Jeeves, 1985) but may also process the direction of another person’s attention. Further, a recent cueing study of a patient with a lesion extending to the right superior temporal sulcus showed a dissociation in social and non-social cueing effects. This individual experienced a facilitation in response time after viewing a non-predictive, congruent arrow cue, but no facilitation in response to a similar gaze cue (Akiyama, Kato, Muramatsu, Saito, Umeda & Kashima, 2006). The similarities and differences in the behavioral responses and the neural substrates underlying reflexive social and non-social attentional orienting indicate that there may be a functionally parallel but anatomically distinct neural organization involved in the processing of these two forms of orienting. That is, although social cues elicit a shift in spatial attention that can be measured like other nonsocial cues, the attentional shift measured may not be the result of the same exact brain activations. Recent work (Patel, Peng & Sereno, 2010) lends support to such a conceptual framework with respect to reflexive spatial attention. Patel et al. (2010) show that the shape of cue and target can influence reflexive spatial attention and propose a model that demonstrates such findings could result from physiologically distinct activations in shape-selective populations of neurons.
Voluntary Social versus Non-social Orienting
In addition to reflexive social orienting, other work has investigated a more intentional, willful, or voluntary response to gaze cues. One function of voluntary orienting in a social setting could be to allow flexibility in the orienting of social attention (e.g., knowing when it is appropriate to look or look away). In experiments using gaze cues that were counterpredictive of target location, it has been shown that when the cue is predictive about the spatial location of the upcoming target (i.e., a voluntary cue), the response to a target presented in a cued location is quicker compared to when the gaze cue is non-predictive (Friesen et al., 2004; Tipples, 2008). In their social cueing paradigm experiments, Friesen et al. (Friesen et al., 2004) and Tipples (2008) measured manual reaction times in two types of trials which are relevant here. For comparison with non-social covert orienting, they also performed the same experiment with central arrow cues. In one type of social cueing trial (Predicted trial), the face cue was predictive and the target was most often presented at the location opposite to the direction of gaze in the face cue (counterpredicted location). In the second type of social cueing trial (Non Predicted-Non Cued or NPNC), the target was presented at a location that was not the predicted nor the cued location. The CTDs of 105 ms and 1200 ms were common in both studies (Friesen et al., 2004; Tipples, 2008) and, for social and non-social cueing experiments.
Data from the social cueing experiments of both studies showed that reaction times in Predicted trials were faster than those in NPNC trials for both the 105 ms and 1200 ms CTDs; all of these facilitatory cueing differences were significant, except the 105 ms CTD in the Friesen et al (2004) study. More importantly, for both studies, the facilitatory cueing effects due to a predictive social cue (voluntary social cueing effect) were larger at a CTD of 1200 ms compared to 105 ms. In contrast, the reflexive social cueing effect is generally larger at a CTD of 100 ms compared to 1000 ms (Friesen et al., 2004; Langton, 2000; Tipples, 2008). These differences in the cueing effect at different CTDs for voluntary and reflexive social cueing suggests that the mechanism mediating voluntary social orienting may be different from that mediating reflexive social orienting.
In the non-social cueing experiments, results from both studies were similar to those obtained in the social cueing experiments described above. In Tipples’ (2008) study, voluntary non-social cueing was significant at both 150 ms and 1200 ms. In Friesen et al. (2004), a significant voluntary social as well as non-social cueing effect was observed at CTDs of 600, 1,200, and 1,800 ms. Thus, data from both studies showed a significant voluntary non-social cueing effect at a CTD of 1200ms. This demonstrates that a similar form of voluntary orienting occurs in response to predictive social as well as non-social cues.
Interaction between Voluntary and Reflexive Orienting - Tonic Inhibition Model
In a model of non-social orienting proposed by Sereno (1992), called the Tonic Inhibition Model (TIM), there are distinct mechanisms for reflexive and voluntary non-social orienting which operate in parallel and also interact. In this model, the superior colliculus is thought to be key in mediating reflexive orienting, and prefrontal cortex is proposed to be critical in the control of voluntary orienting. Further, the basal ganglia tonically inhibit the superior colliculi, thus leading to a normal “resting” state with tonic inhibition of the reflexive orienting system. The prefrontal cortex, via projections to the basal ganglia, modulates or controls this tonic inhibition of the reflexive orienting system. An important prediction of TIM is that the activation of the voluntary system should inhibit reflexive responses. In support for this prediction, we have previously shown that a predictive voluntary spatial cue can inhibit reflexive responses to a following target using non-social cues. (Seidlits, Reza, Briand & Sereno, 2003). Additional evidence for interactions between the reflexive and voluntary spatial orienting systems may be found in a recent study (Ristic & Kingstone, 2006). Ristic and Kingstone (2006) showed that a central arrow cue is capable of eliciting reflexive spatial orienting when the cue is less predictive, and volitional spatial orienting when the predictive value of the arrow cue is increased. The reflexive spatial orienting that occurs with a central arrow cue was however distinct from that of a peripheral cue in that no IOR is observed at longer SOAs with a central arrow cue. Further, they showed that the spatial orienting by a predictive central arrow cue was substantially higher than the sum of orienting effects resulting from a predictive central number cue and the reflexive spatial orienting of a central arrow cue. Based on the assumption that voluntary orienting by a central arrow is identical to that by a central number cue, Ristic and Kingstone (2006) interpret the super-additive effect of the predictive central arrow cue on orienting as evidence for interactions between reflexive and voluntary orienting systems.
The mechanisms underlying reflexive and voluntary social orienting are even more uncertain. Further, it is not clear if the reflexive and voluntary mechanisms of social orienting interact or if they operate largely independent of each other. Friesen et al. (2004) and Tipples (2008) have presented evidence that reflexive and voluntary social orienting may interact. Because relatively more is known about mechanisms underlying non-social covert orienting, it is important to know if reflexive and voluntary social orienting mechanisms operate similarly or dissimilarly to non-social orienting mechanisms and whether reflexive and voluntary social orienting interact. Additionally, a better understanding of social orienting has the potential to benefit human populations purported to have dysfunctional gaze cueing, such as autistic individuals.
Present Study
In this study, we sought to determine if social orienting mechanisms were functionally organized similarly to the non-social orienting mechanisms. If so, then the TIM predicts that reflexive social attention, even at short CTD, could be affected if the voluntary social system is activated and control increased. To test this critical prediction of the TIM as it relates to social orienting, we investigated the relationship between voluntary and reflexive social orienting using a manual response task and a wide range of CTDs. We expected that similar to previous studies, reflexive social attention would be elicited by gaze cues that have no predictive value (Driver et al., 1999; Friesen & Kingstone, 1998; Langton & Bruce, 1999). Further, we expected that voluntary social attention would be elicited by predictive social gaze cues (Friesen et al., 2004; Tipples, 2008). However, the elicitations of reflexive and voluntary social forms of attention were expected to follow different time-courses. Based on previous gaze cueing studies, we expected that the reflexive gaze cueing effect in response to a non-predictive gaze cue would be early and of short duration, while voluntary social attention would be elicited in response to predictive cues at later SOAs. Further, if the voluntary system interacted with the reflexive system, the preparation and execution of the voluntary task might alter the reflexive response of the system. We directly test for the first time if reflexive social attention is affected by task conditions, namely, activation of voluntary mechanisms.
Methods
Design
We collected data in two conditions from the same set of observers to assess reflexive and voluntary social attention. Together, the trials for Condition 1 and Condition 2 were completed over ten (5 per Condition) sessions. One of the observers finished all the sessions of Condition 1 before running Condition 2. For the remaining observers, the two conditions were run in alternate sessions. The order of the alternating sessions was fixed for all the observers: [1212121212]. Two sessions were run in a single day. Both conditions used a standard gaze cueing paradigm in which a gaze-averted face was centrally presented followed by a peripheral presentation of a target (see Fig. 1). In both conditions, the observer was instructed to indicate as quickly as possible the spatial location of the target (i.e. left vs. right). Condition 1 consisted of two subtasks that were counterbalanced within each session. The gaze cue was 100% predictive about the spatial location of the target in both subtasks. In one subtask (Pro-Gaze or PG subtask), the target always appeared in the same direction as the gaze cue. In the second subtask (Anti-Gaze or AG subtask), the target always appeared in the opposite direction of the gaze cue. The reflexive gaze cueing effect was computed for this voluntary cueing paradigm by comparing trials in AG and PG subtasks. In Condition 2, we randomly mixed all the trials used in the PG and AG subtasks of Condition 1, thus making the gaze cue non-predictive about the spatial location of the following target. Hence, Condition 2 directly estimated the reflexive gaze cueing effect in a reflexive cueing paradigm. The voluntary gaze cueing effect was estimated by comparing identical trials from Conditions 1 and 2 (i.e., identical trials with and without cue predictability, respectively).
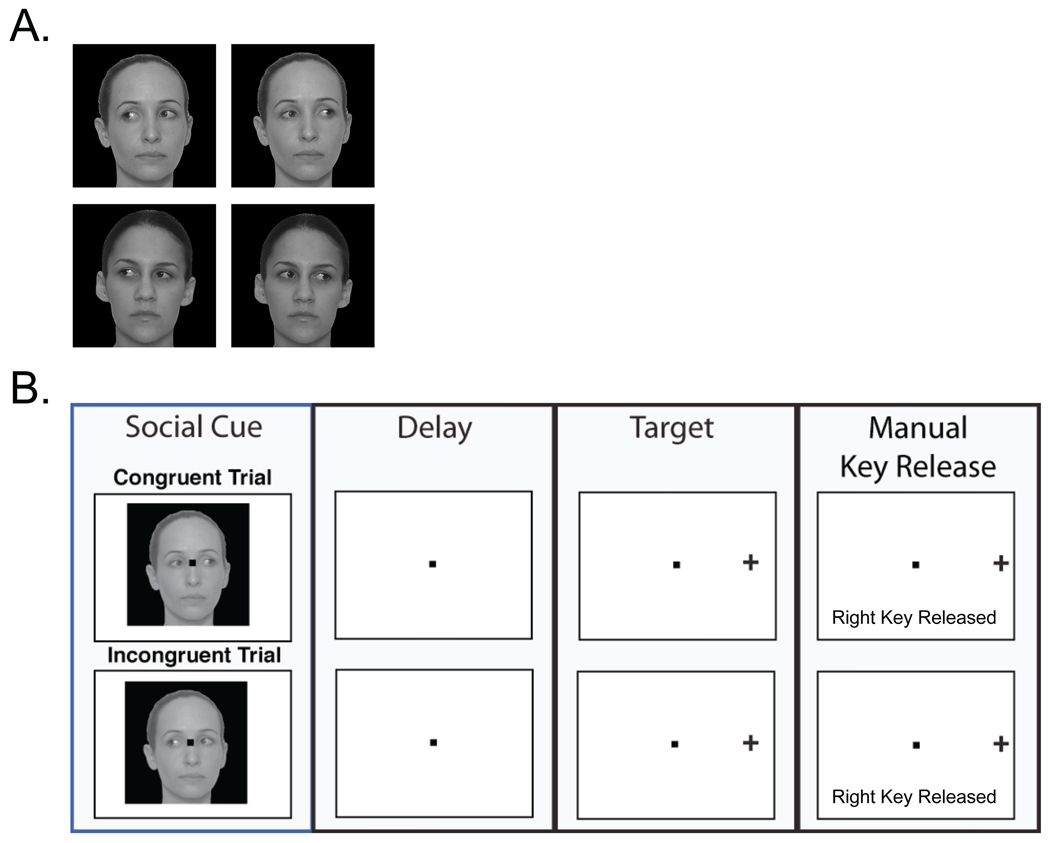
Figure 1. The set of images used as gaze cues and the illustration of the two trial types in all the experimental conditions.
A. Images were obtained using a digital camera and edited using Adobe Photoshop software. B. In a Congruent trial (top row), the target (cross) was presented in the same direction (right side of the central fixation dot) as the direction of the eye gaze in the facial cue (right side) presented earlier in the trial. In an Incongruent trial (bottom row), the target was presented in the opposite direction (right side) from the direction of the eye gaze in the cue (left side) presented earlier. The cue onset to target onset delays (CTDs) ranged from 0 to 1000 ms. An observer released the appropriate switch to indicate the location of the target (right, for both of these two example trials), as soon as the target was perceived. For illustrative and printing convenience, some colors have been inverted; during the experiment, the fixation point and the target are bright and the background is black.
Observers
Four female observers (1 author and 3 naïve) participated in both Condition 1 and 2. All subjects gave informed consent before participating in the study, which was approved by the Committee for the Protection of Human Subjects at our institution in accordance with the Declaration of Helsinki.
Apparatus
Subjects were seated 62.5 cm from the center of the computer monitor in a dark room, with their head held in place by a chin rest. Stimuli were presented on a computer screen (LCD, 15 inch, 60 Hz refresh rate, 1280×1024 pixels, 4 ms on-off response time) connected to a Macintosh G5 computer running OS-X operating system. The response to a target was obtained using a custom built box which contained two laterally displaced push button switches (response box). The analog signals produced by the switches were digitized using an analog to digital converter system (ITC-18) connected to the computer via the USB port. The analog to digital conversion sampling rate was 10 KHz thus resulting in a 100 microsecond temporal resolution of the reaction time (RT) data. The software was written in Matlab (The MathWorks, Natick, MA) and utilized the Psychtoolbox (Brainard, 1997) for visual stimulus presentation.
Stimuli
Each pixel was 1.4 arc-min. The fixation point was an 8×8 pixel square (0.19 deg) located at the center of a dark computer screen. Four gray-scale face images (faces of two individuals each with two directions of gaze aversion) shown in Fig. 1A were used for social gaze cues.
Photos of two females were taken individually with eyes straight and averted 45 deg to the left and right. Head angles were straight for each condition. All photos taken were at a resolution of 3264 × 2448 pixels. Adobe Photoshop CS2 was used for the photo editing process. Photos were scaled so that the faces were the same width and height in all the face cue images. The background was extracted. Each photo was then embedded in a black square of 9.7 deg such that the center of the fixation square was evenly spaced between the eyes and was level with the line passing through the center of the two eyes. The regions in the square that did not contain face information were dark. The resulting face cue image was presented at the center of the screen. The target was a bright cross on a dark background and was constructed in a square of 64×64 pixels (1.5 deg) and was presented 5 deg horizontally on either side of the fixation point, which was also at the center of the screen.
Stimulus Presentation and Response Sequence
Each observer initiated a trial by pressing and holding the two switches on the response box (left switch pressed by the left index finger and right switch with the right index finger). The fixation stimulus then appeared and the observer was instructed to maintain fixation on it throughout the trial. After a delay of 800 to 1200 ms, one of the four gaze cue images (randomly selected) was presented for 33 ms. After the cue onset, the target was presented after a randomly selected CTD. Seven CTDs were tested: 0, 33, 150, 200, 500, 750, and 1,000 ms. The observer was instructed to release the appropriate switch (e.g. left switch if the target appeared to the left of fixation) as soon as the target was perceived. The target remained on the screen until a response was made. A minimum delay of 0.5 s was introduced between each trial.
Trial Types
As shown in Fig. 1B for one individual face cue, there were two possible cueing trials: Congruent and Incongruent. On Congruent trials, the subsequent target (cross) was presented in the same direction as the eye gaze of the preceding facial cue. Fig. 1B, top row, illustrates an example of a Congruent trial where the cue and target are congruent (i.e., both to the right) and the correct response is right. On Incongruent trials, the subsequent target (cross) was presented in the opposite direction as the eye gaze of the preceding facial cue. Fig. 1B, bottom row, illustrates an example of an Incongruent trial where the cue and target are incongruent (i.e., cue leftward and target rightward) and the correct response is right. These types of trials were present in both Condition 1 and 2, with the only difference being whether the cue did or did not predict the target location (i.e., whether Congruent and Incongruent trial types were blocked or mixed, respectively).
Procedure
Condition 1: Voluntary (Predictive) Social Cueing Paradigm
In Condition 1, the reflexive cueing effect of social cues was investigated in the context of a voluntary social cueing paradigm. In this condition, the direction of the eyes on the face cue held predictive value as to where the target would appear. In order to accomplish this goal, Congruent and Incongruent trial types were blocked and presented separately in two subtasks: a Pro-Gaze (PG) subtask and an Anti-Gaze (AG) subtask. For the Pro-Gaze (PG) subtask, observers were informed at the beginning of testing that 100% of the time the target would appear in the same direction of the face cue (i.e., only Congruent trials were used; see Fig. 1B, top row, for an example trial). For the Anti-Gaze (AG) subtask, observers were told that 100% of the time the target would appear in the opposite direction of the face cue (i.e., only Incongruent trials were used; see Fig. 1B, bottom row, for an example trial). Thus, both subtasks manipulated voluntary attention equivalently and only differed with respect to how the gaze cue reflexively shifted gaze. In the PG subtask, the gaze cue reflexively shifted attention to the target. In the AG subtask, the gaze cue reflexively shifted attention away from the target.
Each observer completed two blocks of the PG subtask and two blocks of the AG subtask in each of five sessions of testing across separate days. Each block of trials contained 140 trials (4 cue images × 7 CTDs × 5 repetitions). In each session, the PG and AG subtask blocks were counter-balanced. The participants were divided into two groups, with one group completing the blocks in the order of [PG, AG, AG, PG] and the other performing the blocks [AG, PG, PG, AG]. For each observer, the order of blocks was the same in all the five sessions. For each subtask, the total number of trials per CTD was 200 (4 cue images × 50 repetitions). The total number of trials in Condition 1 was 2800 (4 cue images × 7 CTDs × 2 types of subtasks [PG, AG] × 50 repetitions); one subject was inadvertently tested without 0 in the set of CTDs and had only 2600 trials. Our paradigm was similar to that used by Deaner and Platt (2003) in their study of social attention in humans and monkeys. We used few observers but collected a large amount of data from each observer. This paradigm is ideally suited for direct future comparisons with monkey physiological studies (for example, see Fecteau, Bell & Munoz, 2004).
Condition 2: Reflexive (Non-Predictive) Social Cueing Paradigm
In Condition 2, the reflexive cueing effect was studied in the context of a non-predictive social cueing paradigm. In this condition, the direction of the eyes on the face cue held no predictive value as to where the target would appear and the observer was instructed to ignore the face. The same images from Condition 1 were used in Condition 2, ensuring that any differences were due solely to the predictability of the cues. To eliminate cue predictability, Congruent and Incongruent trial types were mixed together with each type of trial comprising 50% of the trials.
Each observer completed two blocks of trials in each of five sessions of testing for a total of 10 blocks. In each block, Congruent and Incongruent trial types were intermixed. Each block of trials contained 280 trials (4 cue images × 7 CTDs × 2 congruency conditions × 5 repetitions). The total number of trials in this condition was 2800 (4 cue images × 7 CTDs × 2 congruency conditions × 50 repetitions). For each congruency condition, the total number of trials per CTD was 200 (4 cue images × 50 repetitions).
Data Analyses
Data from each condition as well as for comparison across conditions were analyzed using non-parametric as well as parametric techniques. We used two different non-parametric analyses to cross-validate the conclusions of each analysis. In one analysis, we used the Wilcoxon rank-sum test to analyze within observer median cueing effects. In the second analysis, we used the randomization test to analyze the cueing effects averaged across all observers. The non-parametric techniques were used because in many cases the RT data from individual observers and individual CTDs were not distributed normally (tested using Lilliefors test). As parametric techniques are frequently used in this literature, we used the parametric technique to confirm the qualitative aspects of the results we report using non-parametric analyses. The methodological details of the three statistical techniques are summarized in the Appendix.
Comparison across Data Analyses
Despite the different analytical approaches, the findings were quite similar (see Tables 1–5). Across the thirty-five comparisons we tested across different experimental conditions, the different analytical approaches only differed in significance in one to five comparisons. In all the Tables in this paper, p-values in bold highlight any comparison that is not significant (p>.05), but that in another analysis yields a p-value for the same condition that is significant (p <.05). None of these differences affect any of our conclusions. We briefly summarize here the differences between analyses.
Table 1.
Reflexive gaze cueing effect in Condition 1
| CTD (ms) |
Gaze Cueing Effect (ms) | p - value | ||||
|---|---|---|---|---|---|---|
| Rank-sum | Rand. Test | Parametric | Rank-sum | Rand. Test | Parametric | |
| 0 | 7.15±4.08 | 13.54±5.47 | 6.73±4.18 | 0.02 | 0.01 | 0.15 |
| 33 | 12.78±3.76 | 23.12±4.82 | 14.61±4.34 | <0.001 | <0.001 | 0.01 |
| 150 | 4.73±3.64 | 9.22±4.42 | 6.42±4.03 | 0.05 | 0.04 | 0.15 |
| 200 | 2.15±3.58 | 6.05±4.77 | 4.21±3.66 | 0.21 | 0.21 | 0.28 |
| 500 | 0.30±3.46 | 1.43±4.49 | 4.06±4.12 | 0.47 | 0.75 | 0.35 |
| 750 | 2.45±3.22 | 5.64±4.69 | 0.36±3.81 | 0.23 | 0.23 | 0.93 |
| 1000 | 2.80±3.05 | 2.7±5.14 | 3.46±3.68 | 0.27 | 0.60 | 0.37 |
Table 5.
Reflexive cueing effect in Condition 2 minus that in Condition 1
| CTD (ms) |
Gaze Cueing Difference (ms) | p – value | ||||
|---|---|---|---|---|---|---|
| Rank-sum | Rand. Test | Parametric | Rank-sum | Rand. Test | Parametric | |
| 0 | −7.35±6.43 | −11.23±6.86 | −1.8±5.53 | 0.13 | 0.10 | 0.76 |
| 33 | −8.63±5.55 | −16.16±6.5 | −12.6±6.15 | 0.03 | 0.01 | 0.07 |
| 150 | 6.08±5.22 | 5.53±5.95 | 5.95±5.71 | 0.19 | 0.35 | 0.33 |
| 200 | 2.6±5.08 | −0.09±5.95 | 3.55±5.2 | 0.47 | 0.99 | 0.51 |
| 500 | 7.9±5.42 | 9.16±6.00 | 6.0±5.82 | 0.08 | 0.13 | 0.33 |
| 750 | 0.87±5.14 | 0.93±6.00 | 4.9±5.35 | 0.50 | 0.87 | 0.38 |
| 1000 | −1.4±5.04 | 0.21±6.34 | 2.69±5.16 | 0.48 | 0.97 | 0.62 |
The cueing effects from the two non-parametric analyses are qualitatively very similar. By this we mean that the cueing effects that are found to be significant using one method are also significant using the other method, with only one exception. The one exception occurs at a CTD of 200, where a significant reflexive cueing effect (p=0.04) in the reflexive paradigm using the rank-sum test, becomes marginally significant (p=0.10) using the randomization test (see Table 2). Given that the CTDs surrounding this CTD (CTDs of 150 and 500) show a significant reflexive cueing effect in both analyses, this change in level of significance at an intermediate CTD, does not affect any of our findings or conclusions. The non-parametric analyses that are based on the randomization test consider observer effect as a general error. This is not the case with the rank-sum analyses. Therefore in the remainder of this paper, we will focus our discussion mainly on the non-parametric analyses based on rank-sum tests, which analyze the data in a within-observer manner.
Table 2.
Reflexive gaze cueing effect in Condition 2
| CTD (ms) |
Gaze Cueing Effect (ms) | p – value | ||||
|---|---|---|---|---|---|---|
| Rank-sum | Rand. Test | Parametric | Rank-sum | Rand. Test | Parametric | |
| 0 | 3.15±3.44 | 2.31±4.25 | 4.94±3.61 | 0.25 | 0.58 | 0.21 |
| 33 | 4.60±3.31 | 6.96±4.14 | 2.06±4.35 | 0.06 | 0.09 | 0.65 |
| 150 | 11.45±3.23 | 14.75±3.88 | 12.37±4.03 | <0.001 | <0.001 | 0.01 |
| 200 | 5.38±3.12 | 5.96±3.63 | 7.76±3.68 | 0.04 | 0.10 | 0.06 |
| 500 | 8.45±3.44 | 10.59±3.93 | 10.04±4.11 | 0.001 | 0.008 | 0.04 |
| 750 | 4.70±3.31 | 6.57±3.81 | 5.26±3.75 | 0.06 | 0.09 | 0.19 |
| 1000 | 1.28±3.35 | 2.91±3.77 | 0.77±3.61 | 0.43 | 0.44 | 0.84 |
The cueing effects from the non-parametric and parametric analyses differ in four (randomization) or five (rank-sum) CTDs. In general, cueing effects at fewer CTDs were significant with parametric analyses. Of the five significant cueing effects in the rank-sum non-parametric analyses that changed with parametric analyses, three became marginally significant (p=0.07), and two became non-significant (p=0.15). For the most part, the fewer statistical differences do not affect any of our findings or conclusions, because there are other CTDs surrounding the particular CTD in question that show significant effects. The one exception is the comparison across tasks (Table 5). There is only one early CTD (33 ms) that is significantly different in both non-parametric analyses, and this CTD becomes only marginally significant with the parametric analysis. The quantitative differences between non-parametric and parametric analyses occur because in most cases the RT distributions are not normal and the parametric analyses assume them to be normal. Thus, for sake of statistical propriety and brevity, we will only discuss the results of the non-parametric analyses in greater detail.
Results
Condition 1: Voluntary Paradigm (Reflexive Social Attention)
Incorrect trials (0.45%) were removed before RT analyses of data in PG and AG tasks. Combining data across all the subjects, the median reaction times for the PG and AG task are shown in Fig. 2A. Consistent with previous studies (Friesen et al., 2004; Ristic & Kingstone, 2005), we found that RTs decreased monotonically with increase in CTD in PG and AG tasks.
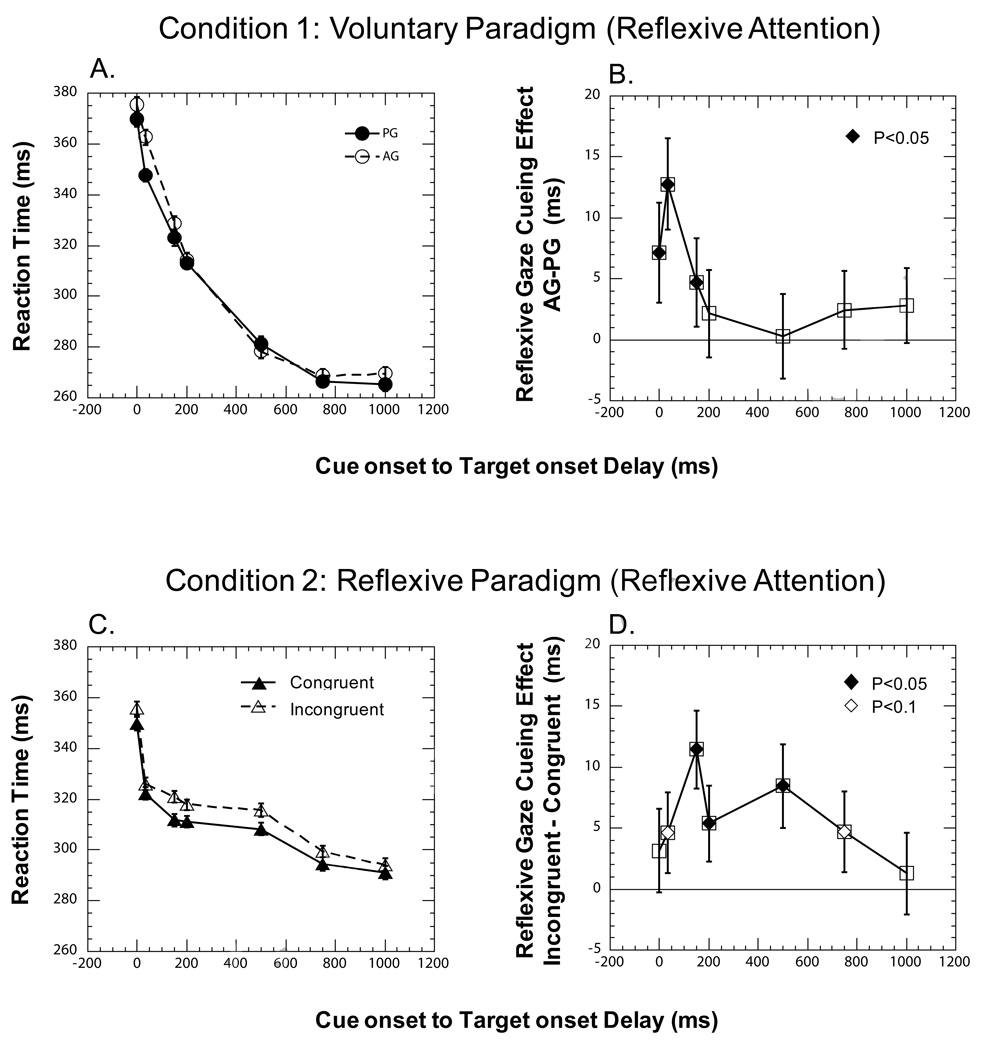
Figure 2. Median reaction times and reflexive gaze cueing effects.
A. Median reaction times from PG (solid line) and AG (dashed line) subtasks in Condition 1. The x-axis in this and all other panels represents the cue onset to target onset delays (CTDs). The y-axis in this and panel C. represents the median of reaction times (RTs) pooled across all the observers. Each error bar in this and all other panels represents standard error of median. The maximum standard error of the median was 3.1 ms and it occurred for CTD of 150 ms in the PG task. B. Reflexive gaze cueing effect in the context of a voluntary social cueing paradigm. The y-axis represents the median of the within observer difference between the RTs in AG and PG tasks pooled across all observers. Positive values on the y-axis in this and panel D. indicate a facilitatory reflexive gaze cueing effect. Filled and unfilled diamonds in this and all other panels identify cueing effects that are significantly and marginally different from zero respectively. C. Median reaction times pooled across all observers from Congruent (solid line) and Incongruent (dashed line) trials in Condition 2. The maximum standard error of the median was 2.8 ms and it occurred for CTD of 0 ms in the Congruent trials. D. Reflexive gaze cueing effect in the context of a reflexive social cueing paradigm. The y-axis represents the median of the within observer difference between the RTs in Incongruent and Congruent trials pooled across all the observers.
The reflexive gaze cueing effect, that is, median of the within subject differences in reaction times in AG and PG tasks (AG - PG), are displayed in Fig. 2B. The reflexive gaze cueing effect in the voluntary paradigm computed using non-parametric and parametric analyses are also displayed in Table 1. A positive value for a cueing effect in Table 1 (and in the following Tables) represents facilitation. RTs in the PG task were significantly shorter compared to the AG task only for CTDs of 0 (diff = 7.2 ± 4.1 ms; p=0.02), 33 (diff = 12.8 ± 3.8 ms; p<0.001) and 150 (diff = 4.7 ± 3.6 ms; p=0.05). The shorter RTs in the PG task compared to AG task are consistent with the presence of reflexive facilitation at shorter CTDs due to the gaze cue. The equality of RTs in PG and AG tasks for longer CTDs is consistent with a short duration of influence for reflexive gaze cueing under voluntary task conditions.
Condition 2: Reflexive Paradigm (Reflexive Social Attention)
Condition 2 measures the reflexive cueing effect in the context of a reflexive paradigm, where the cue has no predictive value with regards to the spatial location of the future target. Again, incorrect trials (0.7%) were removed from the data before RT analyses. The median reaction times in Congruent and Incongruent trials are shown in Fig. 2C. When there was no predictive value of the cue, participants still responded quicker to targets in Congruent trials compared to Incongruent trials.
The reflexive gaze cueing effect, i.e. median difference in reaction times in Incongruent and Congruent trials (Incongruent - Congruent) as a function of CTD, is shown in Fig. 2D. This information is also shown in Table 2, with the corresponding statistical analyses. The facilitatory effect of gaze cueing increased rapidly and was maximum for a CTD of 150 ms. The peak facilitation was 11.5 ± 3.2 ms and is significantly different from zero (p<0.001). The facilitatory effect decreased with further increase in CTD but remained significantly different from zero for CTDs of 200 (5.4 ± 3.1 ms; p=0.05) and 500 (8.5 ± 3.4 ms; p=0.005).
Reflexive gaze cueing effects obtained in this experiment are similar to those obtained in other studies (Deaner & Platt, 2003; Driver et al., 1999; Friesen & Kingstone, 1998; Friesen et al., 2004; Hood et al., 1998; Langton, 2000; Langton & Bruce, 1999; Ristic et al., 2002; Ristic & Kingstone, 2005; Ristic et al., 2007; Tipples, 2008). Similar to Friesen et al (2004), in which no IOR was observed even at an SOA of 1800 ms, we did not observe IOR for CTDs up to 1000 ms. However, we found a monotonically decreasing gaze cueing effect for CTDs greater than and equal to 500 ms, which is consistent with the possibility of observing IOR for CTDs greater than 2000 ms (Frischen & Tipper, 2004).
Condition 1 versus 2: Voluntary Social Attention (in Congruent and Incongruent Trial Types)
Having obtained the RT data in Conditions in which the predictive value of the cue is either absolute (100%, Condition 1) or of no value (50%, Condition 2), in this section we now evaluate the voluntary component of social attention. In order to measure the voluntary cueing effect, we compared identical trial types (either Congruent or Incongruent trial types) in experimental Conditions 1 (predictable) and 2 (unpredictable). Practically speaking, the voluntary cueing effect was calculated to be the median of the within subject differences in reaction times in Condition 1 and Condition 2 (Condition 2 - Condition 1), calculated separately for Congruent and Incongruent trial types (see Fig. 3B and 3D, respectively).
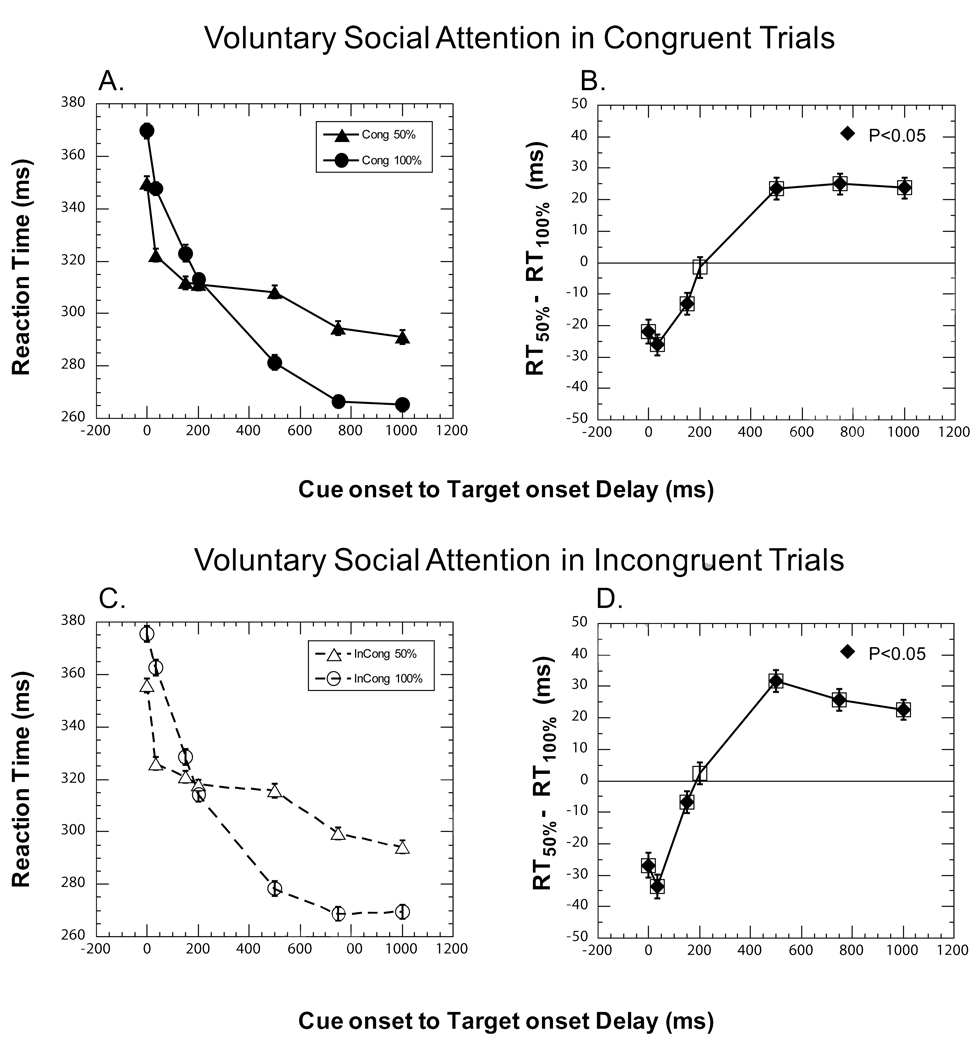
Figure 3. Median reaction times and voluntary gaze cueing effects.
A. & C. Median reaction times (RTs) from Predictive (circle) and Non-predictive (triangle) trials for Congruent (panel A., solid symbols) and Incongruent (panel B., open symbols) trials. Predictive trials (100%, circle symbol) were trials presented in Condition 1 and Non-predictive trials (50%, triangle symbol) were identical trials presented in Condition 2. The y-axis represents the median of RTs pooled across all the observers. B. & D. Voluntary gaze cueing effect in Congruent and Incongruent trials of Conditions 1 and 2. Panel B. shows cueing effects in Congruent trials and panel D. shows cueing effects in Incongruent trials. The y-axis represents the median of the within observer difference between the RTs in Non-predictive and Predictive trials pooled across all the observers. Positive values on the y-axis indicate a facilitatory voluntary gaze cueing effect.
First, however, the data were analyzed to see if the order of Condition 1 and Condition 2 sessions affected the voluntary gaze cueing effects. For the three observers that performed the two conditions in an interleaved manner, we compared the results of the analyses with and without the data from the first session of Condition 1 so that sessions were biased with all sessions of Condition 1 preceding all sessions of Condition 2 (i.e., order 1212121212) or all sessions of Condition 2 preceding Condition 1 (i.e., order 212121212). None of the findings we report below were altered by biasing the order of Condition 1 and 2. In addition, we also compared the analyses with and without the data from the one observer who did not run the two conditions in an interleaved manner. Again, none of the findings we report below were altered by removal of this subject. Thus the results reported below are from all the observers and all the sessions.
In Fig. 3, the median reaction times from Condition 1 (50%) and 2 (100%) are shown separately for Congruent (Fig. 3A) and Incongruent trials (Fig. 3C). The voluntary gaze cueing effects as a function of CTD in Congruent and Incongruent trials are shown in Figs. 3B and 3D respectively (e.g., Gaze cueing effect in (1) Congruent trials: congruent trials of Condition 2 (50% predictive) versus identical trials in PG task of Condition 1 (100% predictive) and (2) Incongruent trials: incongruent trials of Condition 2 (50% predictive) versus identical trials in AG task of Condition 1 (100% predictive)).
Given that these graphs represent the difference of RT in trials with a non-predictive cue minus the RT of trials with a predictive cue, the benefit of a predictive gaze cue is represented by a positive value. The voluntary gaze cueing effects are also tabulated in Tables 3 (Congruent trials) and 4 (Incongruent trials). In both Congruent and Incongruent trials, the predictive value of the gaze cue significantly affected the reaction times for virtually all CTDs tested. Beyond a CTD of 200 ms, responses were quicker with a predictive compared to non-predictive social cue and presumably reflects the successful operation of the voluntary social orienting mechanism which must first interpret the trial specific cue and then exert its influence. However, up to CTD of 200 ms, responses were quicker with a non-predictive compared to a predictive social cue. This observation suggests a suppression of reflexive mechanisms of social orienting for short CTDs. The general suppression of reflexive mechanisms may be due to increased tonic inhibition by the voluntary mechanisms of social orienting that are engaged in the preparation and execution of the voluntary cueing task.
Table 3.
Effect of cue’s predictive value in Congruent trials
| CTD (ms) |
Gaze Cueing Effect (ms) | p – value | ||||
|---|---|---|---|---|---|---|
| Rank-sum | Rand. Test | Parametric | Rank-sum | Rand. Test | Parametric | |
| 0 | −21.8±3.8 | −18.45±4.83 | −19.75±3.91 | <0.001 | <0.001 | 0.002 |
| 33 | −26.15±3.43 | −25.93±4.07 | −26.19±4.32 | <0.001 | <0.001 | <0.001 |
| 150 | −13.1±3.49 | −15.41±3.90 | −14.25±4.03 | <0.001 | <0.001 | 0.01 |
| 200 | −1.5±3.32 | −4.41±4.20 | −4.33±3.7 | 0.45 | 0.30 | 0.27 |
| 500 | 23.53±3.37 | 18.45±4.18 | 23.83±4.13 | <0.001 | <0.001 | <0.001 |
| 750 | 25.05±3.29 | 17.97±4.03 | 23.4±3.78 | <0.001 | <0.001 | <0.001 |
| 1000 | 23.77±3.32 | 14.68±4.57 | 26.34±3.65 | <0.001 | 0.001 | <0.001 |
Table 4.
Effect of cue’s predictive value in Incongruent trials
| CTD (ms) |
Gaze Cueing Effect (ms) | p – value | ||||
|---|---|---|---|---|---|---|
| Rank-sum | Rand. Test | Parametric | Rank-sum | Rand. Test | Parametric | |
| 0 | −26.8±4.06 | −29.69±4.9 | −21.53±3.91 | <0.001 | <0.001 | 0.009 |
| 33 | −33.55±3.75 | −42.09±5.00 | −38.74±4.39 | <0.001 | <0.001 | <0.001 |
| 150 | −6.8±3.47 | −9.88±4.43 | −8.3±4.06 | 0.01 | 0.03 | 0.07 |
| 200 | 2.38±3.37 | −4.50±4.17 | −0.79±3.66 | 0.46 | 0.28 | 0.84 |
| 500 | 31.75±3.58 | 27.61±4.37 | 29.81±4.11 | <0.001 | <0.001 | <0.001 |
| 750 | 25.8±3.46 | 18.90±4.55 | 28.29±3.79 | <0.001 | <0.001 | <0.001 |
| 1000 | 22.55±3.26 | 14.88±4.40 | 23.66±3.66 | <0.001 | <0.001 | 0.001 |
Reflexive Cueing Effects in Condition 1 versus 2
The social reflexive system is operating under different task conditions in Conditions 1 (Voluntary Paradigm) and 2 (Reflexive Paradigm). We tested whether the magnitude of cueing effects exhibited by the social reflexive system are different under these different task conditions. For each CTD, we compared the magnitude of the reflexive cueing difference in Condition 1 (RT differences between Pro-Gaze and Anti-Gaze trials) with the magnitude of the reflexive cueing difference for the same types of trials in Condition 2 (RT differences between Congruent and Incongruent trials).
The difference between the reflexive gaze cueing effects in Conditions 1 and 2 are computed in Table 5. The magnitude of reflexive gaze cueing effects were significantly different in Conditions 1 and 2 for a CTD of 33 ms (p=0.03) and marginally significant for a CTD of 500 ms (p=0.08). At a CTD of 33 ms, the reflexive gaze cueing effect in the voluntary paradigm (Condition 1) was larger than that in the reflexive paradigm (Condition 2) by 8.6 ms (SE = 5.6 ms). In contrast, at a 500 CTD, there was a trend towards a smaller cueing effect (−7.9 ms, SE = 5.4) in the voluntary paradigm (Condition 1) than reflexive paradigm (Condition 2). Thus, the reflexive gaze cueing effects are dependent on the task in a dynamic manner.
General Discussion
In the present study, using a design that is more compatible for cross-species comparisons, we report results that are consistent with previous work and also extend our understanding of reflexive and voluntary social orienting. By utilizing an experimental design with two conditions where the only variable was gaze cue predictability, we were able to demonstrate important properties of both voluntary and reflexive social cueing. Clarifying these different social orienting mechanisms and understanding how they interact is critical for developing a better understanding of social disorders such as autism.
Reflexive Social Orienting
We measured the reflexive effect of a social gaze cue on choice reaction times in two paradigms. In Condition 1, choice reaction times were measured in a voluntary cueing paradigm in which the social gaze cue was 100% predictive of the location of the target. We found that up to a CTD of 150 ms, responses are quicker if the target appeared at the gaze-directed location (independent of the predicted location) as compared to the opposite location (Fig. 2B). This difference in reaction times despite identical predictive value of the social gaze cue is indicative of a fast and short-lived reflexive covert social orienting that operates even during a voluntary task. In Condition 2, choice reaction times were measured in a reflexive cueing paradigm in which the social gaze cue was non-predictive about the location of the target. We found that responses were quicker if the target was presented in the cue-predicted location compared to opposite locations for CTDs ranging from 100 to 500 ms (Fig. 2D). In the range of CTDs tested, we did not find any evidence for inhibition of return (Fig. 2D). The facilitatory gaze cueing effect for a CTD of 100 ms in the reflexive cueing paradigm is once again indicative of a fast reflexive social orienting. The temporal properties of the reflexive social orienting found in our reflexive cueing paradigm are consistent with previous findings of reflexive social orienting (Driver et al., 1999; Friesen & Kingstone, 1998; Friesen et al., 2004; Hood et al., 1998; Langton, 2000; Langton & Bruce, 1999; Ristic et al., 2002; Ristic & Kingstone, 2005; Ristic et al., 2007; Tipples, 2008).
The Effect of Task on Reflexive Social Orienting
The effect of task (voluntary vs. reflexive) on reflexive social orienting, as indicated by the differences in the reflexive cueing effects in Conditions 1 and 2 (Table 5), was significant for a CTD of 33 ms and marginally significant for a CTD of 500 ms. These differences in reflexive social orienting depending on context demonstrate an interaction between voluntary and reflexive social orienting.
Consistent with the idea of suppression of the reflexive system during the voluntary paradigm (implemented in the TIM, Sereno, 1992, and discussed earlier), note that at early CTDs RTs are slowed in the voluntary paradigm compared to the reflexive paradigm (in both congruent and incongruent trial types; see Fig. 2A versus 2C). Nevertheless, at a CTD of 33 ms, the reflexive gaze cueing effect in the voluntary paradigm (Condition 1; Fig. 2B) was larger than that in the reflexive paradigm (Condition 2; Fig. 2D). The higher reflexive cueing effect in the voluntary paradigm when the reflexive system should be suppressed by the voluntary system can be explained if, as the suppression of the reflexive system is increased, its gain is reduced non-uniformly for cued and uncued trials. In the appendix, we show that neurons exhibit such non-linearity in their responses thus making such an explanation biologically plausible.
In contrast, at a 500 ms CTD, there was a trend towards a smaller reflexive cueing effect in the voluntary paradigm (Condition 1) than reflexive paradigm (Condition 2). That is, the reflexive gaze cueing effect in the voluntary paradigm (Condition 1; Fig. 2B) is smaller than that in the reflexive paradigm (Condition 2; Fig. 2D). The differences between the early and late CTDs are not unexpected. By 500 ms, the changes in the reflexive orienting system during the voluntary paradigm that are due to a general increase in inhibition of the reflexive system are now also influenced by knowledge about the cue. That is, by 500 ms in the voluntary paradigm where the cue is 100% predictive of target location, RTs should be facilitated by this information. Consistent with this idea, note that at longer CTDs RTs are faster in the voluntary paradigm compared to the reflexive paradigm (in both congruent and incongruent trial types; see Fig. 2A versus 2C). Thus, although superficially, the smaller reflexive cueing effect in the voluntary paradigm when the reflexive system should be suppressed by the voluntary system seems logical, this change in reflexive social cueing may be the result of the increase in inhibition of the reflexive system, the additional processing required for interpreting the voluntary cue, and/or some interaction. Thus, without either carefully manipulating these factors separately or making explicit these effects and interactions in a model and testing outcome, the mechanisms underlying these behavioral findings are difficult to pin down. Nevertheless, taken together, these findings demonstrate that reflexive social cueing effects are dependent on cueing paradigm and that the cueing paradigm can significantly alter these reflexive cueing effects, even at short CTDs before the cue is processed.
Voluntary Social Orienting
We estimated the voluntary social cueing effect by comparing the response times when the cue was 100% predictive of the location of the target (Condition 1) to that when the cue was non-predictive about the target’s location (Condition 2). Due to the fact that the only difference in these experiments was whether the gaze cue held predictive information for the viewer, comparison of reaction times from these two experiments allowed us to estimate what effect voluntary attention had on the time it took the participants to respond. In our experiments, a significant facilitatory voluntary social cueing effect occurred from 500 ms up to 1000 ms (Fig. 3B and 3D). These data are consistent with voluntary social and non-social facilitatory cueing effects observed in Friesen et al. (2004). We interpret this cueing effect to represent the action of a voluntary social orienting system. The voluntary social orienting system is slow to exert its facilitatory influence primarily because a certain amount of time is needed to analyze the voluntary cue. Interestingly, for CTDs shorter than 200 ms, observers were slower to respond when the cue predicted the location of the target compared to when it did not. This phenomenon is observed for Congruent as well as Incongruent trials. This means that in the range of CTDs where fast automatic social orienting occurs, a voluntary form of covert social orienting also occurred. We attribute this early voluntary social orienting for short CTDs to a general inhibitory action of the voluntary social orienting system upon the reflexive social orienting system that is gradually overcome as the cue is processed and interpreted. A similar increased inhibitory interaction between voluntary and reflexive systems has been proposed for non-social orienting (Khatoon, Briand & Sereno, 2002) and modulation of a subcortical tonic inhibitory interaction forms the basis of the Tonic Inhibition Model (Sereno, 1992). Thus we propose that a similar functional architecture underlies reflexive and voluntary covert orienting for both social and non-social cues (cf. Haber, 2008; Haber, Kunishio, Mizobuchi & Lynd-Balta, 1995).
Similar to our results, previous studies (Friesen et al., 2004; Tipples, 2008) using counter predictive cues have shown facilitatory voluntary social cueing effects. Both of these studies show that generally the benefit of making the gaze cue predictive increases as CTD increases. However, neither of the previous studies (Friesen et al., 2004; Tipples, 2008) reported a cost associated with making the cue predictive, as found in our study. We believe that this difference between our study and previous studies may be due to differences in the experimental paradigms. In both previous studies (Friesen et al., 2004; Tipples, 2008), data for predictive and non-predictive trials were collected in a single experiment in which these trials were mixed together. Counter-predictive trials constituted 75% of the total trials while there were only 15% non-predictive uncued trials. In the present study, data were collected in separate blocks of trials, with a cue that was either perfectly predictive or non-predictive and with equal number of predictive and non-predictive trials. Thus, we were able to more precisely evaluate the effects of the predictive social cue on reflexive social cueing effects.
Social versus Non-Social Reflexive Orienting
Our findings of reflexive social orienting are consistent with most previous studies of normal adults. In addition, our results (Figs. 2B and 2D) show that even a short presentation (33 ms) of a face cue with eyes averted can elicit at short CTDs a robust reflexive social orienting to a target that appears in the same direction as the gaze (gaze-directed location) similar to non-social orienting. We find that the early facilitation of social orienting (under conditions of no cue predictability; Fig. 2D) is similar to that in non-social orienting. However, differences in social and non-social orienting in a non-predictive context emerge at longer CTDs. In a non-predictive context, reflexive social orienting peaks around 150 ms and lasts until about 500 ms. In contrast, reflexive non-social orienting is short lasting and is normally absent beyond about 250 ms (Posner, 1980). Another difference is that IOR is not observed in a non-predictive reflexive social cueing paradigm. This is in contrast with the occurrence of IOR with non-social cues in the same range of CTDs (Posner, 1980). These results are consistent with another study of reflexive social orienting (McKee et al., 2007) even though in that study a considerably longer cue duration was used.
It should also be noted that reflexive social cues are normally presented at a spatial location distinct from that of the target (more like endogenous spatial cues) while in most studies of reflexive nonsocial orienting using exogenous cues, the reflexive spatial cue is presented at a location in close proximity to that of the target. However, more recent studies have shown that reflexive spatial orienting can also occur when the spatial cue is presented at a central location and the target is presented at a different, eccentric location (Ristic & Kingstone, 2006). This reflexive spatial facilitation occurring after a central arrow cue may have similarities to the reflexive social cueing that accrues after central presentation of a gaze cue. Interestingly, both the central arrow cue and gaze cue do not result in IOR, a characteristic feature that occurs with a reflexive cue that spatially overlaps the target location. These behaviorally observed differences in reflexive social and non-social cueing paradigms are important and may reflect the underlying neural mechanisms of reflexive orienting (Sereno, Lehky, Patel & Peng, in press).
Vecera and Rizzo (2006) found that E.V.R., a patient with frontal lobe damage, had normal orienting for non-predictive non-social (spatial) cues but significantly abnormal orienting for non-predictive social and predictive symbolic cues. In this study, orienting was measured in E.V.R in response to social (schematic faces), central symbolic (word) and non-social (peripheral flashes) cues. Given that non-predictive gaze cues did not produce facilitation in E.V.R, they suggested that social orienting is mediated by the frontal lobe, which is normally associated with voluntary processes. However, our results of this study as well as previous work reviewed earlier suggest that both reflexive and voluntary forms of social orienting exist and their mechanisms interact with each other. One possibility for this discrepancy is that as Frischen et al. (2007) have pointed out, there are substantial individual differences in manifestation of covert cueing effects when the cues are non-predictive. Thus, it is difficult to make a judgment based on the observations of E.V.R. (Vecera & Rizzo, 2004; Vecera & Rizzo, 2006), without knowing what the patient’s orienting abilities were prior to frontal damage. Another possible explanation is that the CTD for which a facilitatory reflexive gaze cueing effect in response to a non-predictive gaze cue occurred was outside the two CTDs (200 and 700 ms) tested by Vecera and Rizzo (2006). We found that the peak reflexive social cueing effect occurs at a CTD of 150 ms. In support of this, Hietanen et al (2006) used fMRI to examine brain activation in response to gaze cues in a reflexive, non-predictive cueing paradigm similar to Vecera et al (2006) and found that with a 200 ms CTD, frontal area activation did not rise above its baseline values in normals. Further, in normals, the magnitude of the peak facilitatory reflexive social gaze cueing effect is very small compared to peak facilitatory cueing effect obtained with non-social cues. Thus this study by Vecera and Rizzo may have lacked the conditions or sensitivity to detect non-predictive social cueing effects. Finally, other studies have implicated occipital gyrus (Hietanen et al., 2006), IPS (Hoffman & Haxby, 2000) and STS (Akiyama et al., 2006; Kingstone et al., 2004) in reflexive social orienting. Thus, there is little evidence to date to support the notion that the frontal lobe is the sole and direct mediator of reflexive social orienting.
Conclusions
In our work, we have demonstrated that the use of completely predictive or non-predictive gaze cues evokes two different types of social orienting—reflexive and voluntary. In addition, we also provide evidence that the two underlying systems interact, and suggest that the voluntary system may tonically inhibit the reflexive system. These findings enhance the current knowledge of social gaze cueing; they also suggest that more detailed models of specific mechanisms and interactions are needed both for testing specific hypotheses as well as exploring the consequences of different architectures. A better understanding of the underlying mechanisms of the social orienting system could provide critical insight into disorders where social orienting is believed to be abnormal, such as in Autism Spectrum Disorders (ASD). One facet of Autism Spectrum Disorders is a deficit in engaging in non-verbal social communication (joint attention, recognizing emotion). Autistic individuals use a random, disorganized pattern of viewing faces whether given a social or non-social task (Pelphrey, Sasson, Reznick, Paul, Goldman & Piven, 2002). It has long been recognized that a core feature of autism includes diminished overt orienting to faces and eyes (e.g., Klin, Jones, Schultz, Volkmar & Cohen, 2002). If autistic individuals orient to faces and eyes in an abnormal manner, then a first question of any study should be to control and equate sensory input (foveation and fixation duration). That is, to what extent are autistic individuals compared to normal subjects receiving the same gaze-cue related information in a given study? Extra care in study design, such as directing and monitoring fixation or, after fixation, short stimulus durations to guarantee equivalent fixation durations would be critical. Some have argued that individuals with autism avoid eye contact because they experience uncomfortably intense emotional reactions when looking at faces (Dalton, Nacewicz, Johnstone, Schaefer, Gernsbacher, Goldsmith, Alexander & Davidson, 2005). It is possible that autistic individuals are more sensitive to reflexive social cues but have deficits in voluntary social orienting (i.e., are unable to interpret a social cue in a given context). This could partly explain the observed inability of autistic individuals to judge the emotions of others. Many researchers have investigated social orienting abnormalities in children, and these studies have produced conflicting results (Nation & Penny, 2008). By being able to separate out reflexive social gaze following and voluntary social gaze following as we do in the present study, one can tease apart and separately measure reflexive and voluntary social orienting. Although Ristic et al. (2005) show a lack of reflexive social orienting and normal voluntary social orienting in high functioning autistic children, the use of a schematic face cue may have reduced the social relevance of the face stimuli for autistic children and allowed or even engendered non-social feature analysis of the visual cues. We believe that careful control and testing of voluntary and reflexive components of social orienting in autistic individual versus normal controls could lead to a better understanding of the specific abnormalities of social orienting in autistic individuals, and possibly illuminate the neural basis of those behavioral abnormalities.
Acknowledgment
This work was supported by NAAR, NSF BCS 0924636, and P30EY010608 grants. We thank Dr. Alice Chuang for performing the mixed model analyses of our data. We thank the reviewers for their valuable comments and suggestions for improving the manuscript. JLH acknowledges support from NIH Training Grant T32NS07467 during a portion of this work.
Appendix
As mentioned earlier, the data from Conditions 1 and 2 were analyzed using two non-parametric and one parametric technique.
a. Non-parametric analysis of cueing effects using rank-sum method
Let us take an example of computing the reflexive cueing effect, at a single CTD in the voluntary paradigm, i.e. in Condition 1. All other cueing effects were computed similarly by choosing the data sets from appropriate types of trials. We describe the whole procedure as a series of steps.
For each observer i, pool RTs in PG task from different sessions and store them in a vector Xi and pool RTs in AG task and store them in a vector Yi. Because the number of errors was small, the length of Xi and Yi was approximately 200 elements.
For each observer i, randomly (uniform probability) draw 200 samples of RTs from Xi and Yi each and store them in vectors Pi and Qi respectively.
For each observer i, compute Ri = Qi − Pi.
Create a vector S by combining Ris for i = 1…N, where N is the number of observers.
Compute the median of S (mj), the standard error of median of S (ej) using the kernel density method and the p-value using the Wilcoxon signed rank test to determine if the median of S is significantly different from zero (wj).
Repeat steps 2 to 5 1000 times, i.e. j = 1…1000. On each iteration, store mj, ej and wj in vectors M, E and W respectively.
Compute the median cueing effect (shown in fig. 2B) as the median of M, the standard error of the median cueing effect as the median of E and the p-value of the median cueing effect as the median of W.
It should be noted here that the median of RT differences of any two distributions is not identical to the difference in medians of the two RT distributions. Therefore, for example, the RT data plotted in Fig 2a cannot be directly subtracted to yield cueing effects plotted in Fig. 2b. We have mathematically verified that the above non-parametric analysis technique is identical to ANOVA if the RT distributions are normal.
b. Non-parametric analysis of cueing effects using randomization method
Let us take the same example of computing the cueing effect as in section a above, i.e. reflexive cueing effect in the voluntary paradigm.
For each observer i, pool RTs in PG task from different sessions and store them in a vector Xi and pool RTs in AG task and store them in a vector Yi.
Create a vector X by combining Xis and a vector Y by combining Yis for i = 1…N, where N is the number of observers. Let the lengths of X and Y be lx and ly respectively.
Compute the means of X (μx) and Y (μy) and compute the cueing effect as μy–μx (shown in Table 1).
Create a vector S by combining X and Y. The length of S will be lx + ly.
Randomly (uniform probability) draw lx samples from S and store them in P, and ly samples from S and store them in Q.
Compute means of P (μp) and Q (μq) and compute zj = μq − μp.
Repeat steps 5 and 6 10000 times, i.e. j=1…10000. On each iteration, store zj in a vector Z.
Standard error of the cueing effect (shown in Table 1) is equal to the standard deviation of Z. The probability that the cueing effect is zero (shown in Table 1) is equal to the proportion of elements of Z in which the absolute value of the element is greater than the absolute value of the cueing effect, i.e. |μy − μx|.
A correction for multiple comparisons was not performed because data for different CTDs are not entirely independent. Also note that all RTs (after removal of trials with errors) were used in both non-parametric analyses (a and b). In other words, no filtering of data was performed prior to analyses. Therefore some quantitative differences are expected in the two non-parametric analyses simply because one analysis (a) uses median and the other (b) uses mean as the measure of central tendency.
c. Parametric analysis of cueing effects
The first step in analyzing the data with a parametric method was to trim the RT outliers. Note that no trimming was performed in the non-parametric analyses. For each observer, trial type and CTD, the RT distribution combined across all the sessions was iteratively trimmed to include only those RTs that were within 2.5 standard deviations of the mean. The iterative procedure is necessary because the mean and SD of the RT distribution are both unduly affected by outliers. This trimming removed 7.7% of all error free trials.
A mixed model repeated measures analysis was performed on the trimmed data using SAS for Windows (V9., Cary, NC) by a biostatistician. A mixed effect model for repeated measures analysis was used instead of the traditional repeated measures ANOVA because the mixed model analysis has a higher accuracy in modeling the correlation structure in the data and thus yields more accurate test results. The effect of trial type with four levels (Congruent_exp1, Incongruent_exp1, Congruent_exp2, Incongruent_exp2) on the RT was analyzed for each CTD. Since the experimental unit was the observer, a first order autoregression structure was assumed for observations within each observer. Planned contrasts between the above trial types (for Condition 1: Incongruent_exp1 – Congruent_exp1; For Condition 2: Incongruent_exp2 – Congruent_exp2) yielded the cueing effects and the corresponding significance levels reported in Tables 1 and 2.
d. Parametric analysis of voluntary gaze cueing effects
Planned contrasts using the same mixed model analyses described in the previous section were used to estimate the voluntary cueing effects for each CTD. Planned contrasts between (a) the RTs in Congruent trials in Condition 1 and 2 and (b) the RTs in Incongruent trials in Condition 1 and 2, yielded the voluntary cueing effects shown in Tables 3 and 4.
e. Parametric analysis of difference between reflexive gaze cueing effects in Conditions 1 and 2
Planned contrasts using the same mixed model analyses described in the previous section were used to estimate the difference between the reflexive cueing effects in Condition 1 and 2. Planned contrasts between the reflexive gaze cueing effects in Condition 1 (RTs in Incongruent trials minus those in Congruent trials in Condition 1) and Condition 2 (RTs in Incongruent trials minus those in Congruent trials in Condition 2) yielded the cueing effect differences shown in Table 5. Positive values for a difference in Table 5 indicate that the reflexive gaze cueing effect is larger in the reflexive paradigm (Condition 2) compared to that in the voluntary paradigm (Condition 1).
f. Simulated reflexive cueing in voluntary and reflexive paradigms
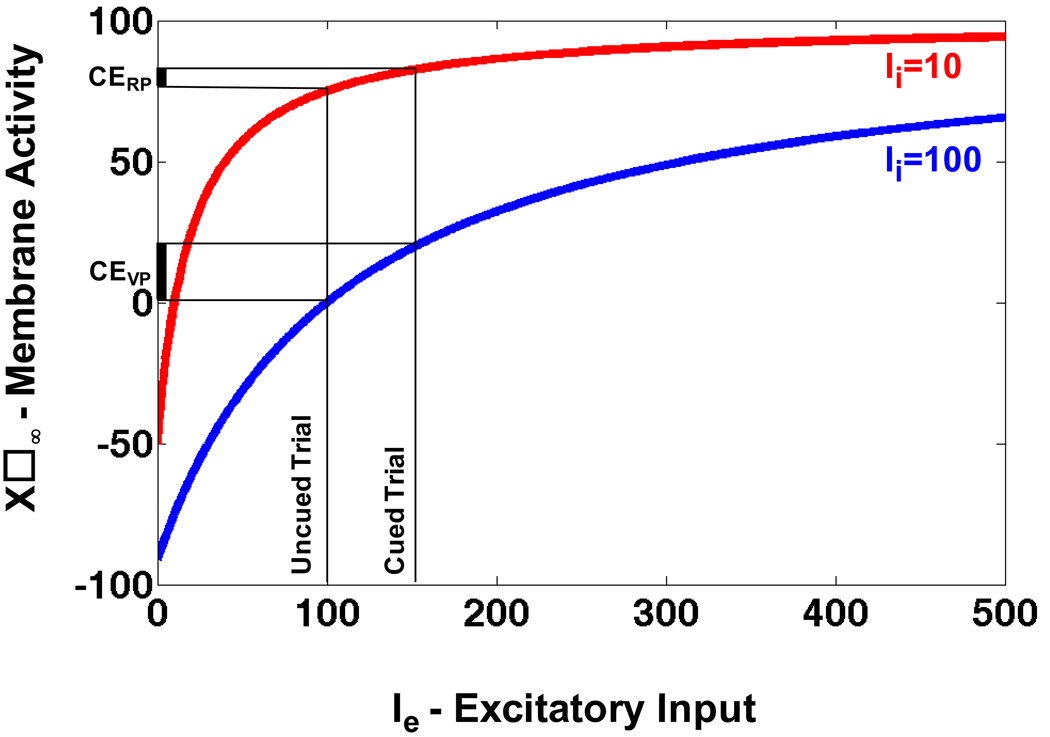
To illustrate how gain of a neuron changes non-uniformly for a range of inputs and how non-uniform gain changes can explain the reflexive cueing data in reflexive and voluntary paradigms, let us examine the output of a model neuron under two simulated conditions: a) When the neuron receives a strong inhibitory signal from another neuron. b) When the neuron receives a weak inhibitory signal from another neuron. Simulated condition ‘a’ mimics the operation of the reflexive system in our voluntary paradigm, and ‘b’ mimics the operation of the reflexive system in our reflexive paradigm. The model neuron’s dynamic is characterized by the shunting equations (Grossberg, 1972) and is given below:
| (1) |
where, x, A, B, D, Ie and Ii are the membrane activity, passive decay constant, upper bound for membrane activity, lower bound for membrane activity, total excitatory input and total inhibitory input respectively. The steady-state membrane activity of the neuron is given below:
| (2) |
The steady-state membrane activity x∞ is plotted as a function of the excitatory input (Ie) for two values of inhibitory inputs (Ii = 10.and Ii = 100) in Fig. A1. For figure A1, A = 10, B = 100, and D = −100. Assume that the neuron receives a stronger input in cued trials compared to uncued trials and that the response time in both trial types is inversely proportional to the membrane activity. As shown in Fig. A1, the difference between the steady-state membrane activity in uncued and cued trials, i.e. the facilitatory cueing effect, is larger when the inhibition is stronger (CEVP) compared to when it is weaker (CERP). In other words, the facilitatory cueing effect is larger in the simulated voluntary compared to reflexive paradigm. Also notice that the opposite could happen if the inputs corresponding to the cued and uncued trials were both sufficiently lower than those shown in Fig. A1.
Figure A1. Non-linear gain changes in a model neuron.
The steady-state membrane activity of a model neuron with shunting dynamics is shown as a function of excitatory input (Ie) for two levels of inhibitory input (Ii=10 and Ii=100). Gain of the neuron is defined as the slope of the curves and it varies as a function of Ie and Ii.
We do not want to imply that a single neuron is involved in determining reaction times in our experiments, rather we want to illustrate that a non-linearity of the type observed in neurons can explain the difference in reflexive gaze cueing effects in the two paradigms. Note also that the reflexive and voluntary systems are dynamical systems and in addition to the difference in the processing of the cue in the two paradigms, the comparison of reflexive cueing effects in the two paradigms is expected to be CTD dependent.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyama T, Kato M, Muramatsu T, Saito F, Umeda S, Kashima H. Gaze but not arrows: a dissociative impairment after right superior temporal gyrus damage. Neuropsychologia. 2006;44(10):1804–1810. doi: 10.1016/j.neuropsychologia.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10(4):433–436. [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, Alexander AL, Davidson RJ. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8(4):519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaner RO, Platt ML. Reflexive social attention in monkeys and humans. Curr Biol. 2003;13(18):1609–1613. doi: 10.1016/j.cub.2003.08.025. [DOI] [PubMed] [Google Scholar]
- Driver J, Davis G, Ricciardelli P. Gaze perception triggers visuospatial orienting in a reflexive manner. Visual Cognition. 1999;6(5):509–540. [Google Scholar]
- Fecteau JH, Bell AH, Munoz DP. Neural correlates of the automatic and goal-driven biases in orienting spatial attention. J Neurophysiol. 2004;92(3):1728–1737. doi: 10.1152/jn.00184.2004. [DOI] [PubMed] [Google Scholar]
- Friesen CK, Kingstone A. The eyes have it! Reflexive orienting is triggered by nonpredictive gaze. Psychonomic Bulletin and Review. 1998;5:490–495. [Google Scholar]
- Friesen CK, Ristic J, Kingstone A. Attentional effects of counterpredictive gaze and arrow cues. J Exp Psychol Hum Percept Perform. 2004;30(2):319–329. doi: 10.1037/0096-1523.30.2.319. [DOI] [PubMed] [Google Scholar]
- Frischen A, Bayliss AP, Tipper SP. Gaze cueing of attention: visual attention, social cognition, and individual differences. Psychol Bull. 2007;133(4):694–724. doi: 10.1037/0033-2909.133.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischen A, Tipper SP. Orienting attention via observed gaze shift evokes longer term inhibitory effects: implications for social interactions, attention, and memory. J Exp Psychol Gen. 2004;133(4):516–533. doi: 10.1037/0096-3445.133.4.516. [DOI] [PubMed] [Google Scholar]
- Grossberg S. A neural theory of punishment and avoidance, II: Quantitative theory. Mathematical Biosciences. 1972;15:253–285. [Google Scholar]
- Haber S. Parallel and integrative processing through the Basal Ganglia reward circuit: lessons from addiction. Biol Psychiatry. 2008;64(3):173–174. doi: 10.1016/j.biopsych.2008.05.033. [DOI] [PubMed] [Google Scholar]
- Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E. The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci. 1995;15(7 Pt 1):4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hietanen JK, Nummenmaa L, Nyman MJ, Parkkola R, Hamalainen H. Automatic attention orienting by social and symbolic cues activates different neural networks: an fMRI study. Neuroimage. 2006;33(1):406–413. doi: 10.1016/j.neuroimage.2006.06.048. [DOI] [PubMed] [Google Scholar]
- Hoffman EA, Haxby JV. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nat Neurosci. 2000;3(1):80–84. doi: 10.1038/71152. [DOI] [PubMed] [Google Scholar]
- Hood BM, Willen JD, Driver J. Adult's eyes trigger shifts of visual attention in human infants. Psychological Science. 1998;9(2):131–134. [Google Scholar]
- Itier RJ, Villate C, Ryan JD. Eyes always attract attention but gaze orienting is task-dependent: evidence from eye movement monitoring. Neuropsychologia. 2007;45(5):1019–1028. doi: 10.1016/j.neuropsychologia.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Jonides J. Attention and performance. IX. Hillsdale, NJ: Erlbaum; 1981. Voluntary vs. automatic control over the mind's eye's movement; pp. 187–203. [Google Scholar]
- Khatoon S, Briand KA, Sereno AB. The role of response in spatial attention: direct versus indirect stimulus-response mappings. Vision Res. 2002;42(24):2693–2708. doi: 10.1016/s0042-6989(02)00327-9. [DOI] [PubMed] [Google Scholar]
- Kingstone A, Tipper C, Ristic J, Ngan E. The eyes have it!: an fMRI investigation. Brain Cogn. 2004;55(2):269–271. doi: 10.1016/j.bandc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- Kleinke CL. Gaze and eye contact: a research review. Psychol Bull. 1986;100(1):78–100. [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry. 2002;59(9):809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Langton SR. The mutual influence of gaze and head orientation in the analysis of social attention direction. Q J Exp Psychol A. 2000;53(3):825–845. doi: 10.1080/713755908. [DOI] [PubMed] [Google Scholar]
- Langton SR, Bruce V. Reflexive visual orienting in response to the social attention of others. Visual Cognition. 1999;6:541–568. [Google Scholar]
- McKee D, Christie J, Klein R. On the uniqueness of attentional capture by uninformative gaze cues: facilitation interacts with the Simon effect and is rarely followed by IOR. Can J Exp Psychol. 2007;61(4):293–303. doi: 10.1037/cjep2007029. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Mackeben M. Sustained and transient components of focal visual attention. Vision Res. 1989;29(11):1631–1647. doi: 10.1016/0042-6989(89)90144-2. [DOI] [PubMed] [Google Scholar]
- Nation K, Penny S. Sensitivity to eye gaze in autism: is it normal? Is it automatic? Is it social? Dev Psychopathol. 2008;20(1):79–97. doi: 10.1017/S0954579408000047. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L, Calder AJ. Neural mechanisms of social attention. Trends Cogn Sci. 2009;13(3):135–143. doi: 10.1016/j.tics.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Patel SS, Peng X, Sereno AB. Shape effects on reflexive spatial selective attention and a plausible neurophysiological model. Vision Res. 2010;50(13):1235–1248. doi: 10.1016/j.visres.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J. Visual scanning of faces in autism. J Autism Dev Disord. 2002;32(4):249–261. doi: 10.1023/a:1016374617369. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Singerman JD, Allison T, McCarthy G. Brain activation evoked by perception of gaze shifts: the influence of context. Neuropsychologia. 2003;41(2):156–170. doi: 10.1016/s0028-3932(02)00146-x. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Hietanen JK, Oram MW, Benson PJ. Organization and functions of cells responsive to faces in the temporal cortex. Philos Trans R Soc Lond B Biol Sci. 1992;335(1273):23–30. doi: 10.1098/rstb.1992.0003. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Smith PA, Potter DD, Mistlin AJ, Head AS, Milner AD, Jeeves MA. Visual cells in the temporal cortex sensitive to face view and gaze direction. Proc R Soc Lond B Biol Sci. 1985;223(1232):293–317. doi: 10.1098/rspb.1985.0003. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32(1):3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rafal RD, Choate LS, Vaughan J. Inhibition of return: neural basis and function. Cognitive Neuropsychology. 1985;2:211–228. [Google Scholar]
- Ristic J, Friesen CK, Kingstone A. Are eyes special? It depends on how you look at it. Psychon Bull Rev. 2002;9(3):507–513. doi: 10.3758/bf03196306. [DOI] [PubMed] [Google Scholar]
- Ristic J, Kingstone A. Taking control of reflexive social attention. Cognition. 2005;94(3):B55–B65. doi: 10.1016/j.cognition.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Ristic J, Kingstone A. Attention to arrows: pointing to a new direction. Q J Exp Psychol (Colchester) 2006;59(11):1921–1930. doi: 10.1080/17470210500416367. [DOI] [PubMed] [Google Scholar]
- Ristic J, Mottron L, Friesen CK, Iarocci G, Burack JA, Kingstone A. Eyes are special but not for everyone: the case of autism. Brain Res Cogn Brain Res. 2005;24(3):715–718. doi: 10.1016/j.cogbrainres.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Ristic J, Wright A, Kingstone A. Attentional control and reflexive orienting to gaze and arrow cues. Psychon Bull Rev. 2007;14(5):964–969. doi: 10.3758/bf03194129. [DOI] [PubMed] [Google Scholar]
- Seidlits SK, Reza T, Briand KA, Sereno AB. Voluntary spatial attention has different effects on voluntary and reflexive saccades. ScientificWorldJournal. 2003;3:881–902. doi: 10.1100/tsw.2003.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno AB. Programming saccades: the role of attention. In: Rayner K, editor. Eye Movements and Visual Cognition. New York: Springer; 1992. pp. 89–107. [Google Scholar]
- Sereno AB, Lehky SR, Patel SS, Peng X. A neurophysiological correlate and model of reflexive spatial attention. In: Srinivasan N, Kar BR, Pandey J, editors. Advances in Cognitive Science. p. 2. in press. [Google Scholar]
- Tipples J. Orienting to counterpredictive gaze and arrow cues. Percept Psychophys. 2008;70(1):77–87. doi: 10.3758/pp.70.1.77. [DOI] [PubMed] [Google Scholar]
- Vecera SP, Rizzo M. What are you looking at? Impaired 'social attention' following frontal-lobe damage. Neuropsychologia. 2004;42(12):1657–1665. doi: 10.1016/j.neuropsychologia.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Vecera SP, Rizzo M. Eye gaze does not produce reflexive shifts of attention: evidence from frontal-lobe damage. Neuropsychologia. 2006;44(1):150–159. doi: 10.1016/j.neuropsychologia.2005.04.010. [DOI] [PubMed] [Google Scholar]