Abstract
Host Schwann cell (SC) migration into nerve allografts is the limiting factor in the duration of immunosuppression following peripheral nerve allotransplantation, and may be affected by different immunosuppressive regimens. Our objective was to compare SC migration patterns between clinical and experimental immunosuppression regimens both over time and at the harvest endpoint. Eighty mice that express GFP under the control of the Schwann cell specific S100 promoter were engrafted with allogeneic, nonfluorescent sciatic nerve grafts. Mice received immunosuppression with either tacrolimus (FK506), or experimental T-cell triple costimulation blockade (CSB), consisting of CTLA4-immunoglobulin fusion protein, anti-CD40 monoclonal antibody, and anti-inducible costimulator monoclonal antibody. Migration of GFP-expressing host SCs into wild-type allografts was assessed in vivo every 3 weeks until 15 weeks postoperatively, and explanted allografts were evaluated for immunohistochemical staining patterns to differentiate graft from host SCs. Immunosuppression with tacrolimus exhibited a plateau of SC migration, characterized by significant early migration (< 3 weeks) followed by a constant level of host SCs in the graft (15 weeks). At the endpoint, graft fluorescence was decreased relative to surrounding host nerve, and donor SCs persisted within the graft. CSB-treated mice displayed gradually increasing migration of host SCs into the graft, without the plateau noted in tacrolimus-treated mice, and also maintained a population of donor SCs at the 15-week endpoint. SC migration patterns are affected by immunosuppressant choice, particularly in the immediate postoperative period, and the use of a single treatment of CSB may allow for gradual population of nerve allografts with host SCs.
Keywords: Nerve transplantation, Nerve allograft, Tacrolimus, FK-506, Costimulation blockade, Immunosuppression, Immunomodulation, Composite tissue allotransplantation
Introduction
Traumatic peripheral nerve injury causes significant disability and disproportionately affects the young, who may suffer the burden of functional deficit for many years (Noble, et al., 1998). Peripheral nerve allotransplantation may be the only option for meaningful restoration of sensation or function following devastating traumatic nerve injuries. As with solid organ allotransplants, an immunomodulatory or immunosuppressive strategy must be employed to prevent rejection of the nerve allograft (Mackinnon, et al., 2001). However, unlike solid organ allotransplantation, immunosuppression may eventually be discontinued in a nerve graft recipient without loss of graft function (Katsube, et al., 1998, Mackinnon, et al., 2001, Mackinnon, et al., 1992, Midha, et al., 1993, Udina, et al., 2004, Zalewski and Gulati, 1980). The cellular components of the graft are replaced by recipient cells (Midha, et al., 1994), resulting in a donor nerve scaffold repopulated by host cells; at this stage, the graft is no longer immunogenic.
Premature discontinuation of immunosuppressive therapy is associated with transient loss of graft function in the rodent model (Katsube, et al., 1998, Mackinnon, et al., 1992, Midha, et al., 1993, Udina, et al., 2004), suggesting functionally significant rejection of donor-derived cells within the nerve allograft. In particular, donor Schwann cells (SCs) are necessary to support axonal regeneration and sustained function in a donor allograft, but are a major target of the host immune system (Ansselin and Pollard, 1990, Lassner, et al., 1989). While removal of SCs can render the nerve allograft more immunologically tolerable, absence of SCs in a nerve graft significantly diminishes the ability of the graft to support nerve regeneration (Whitlock, et al., 2009, Zalewski and Gulati, 1980). Thus the survival and behavior of donor SCs following nerve allotransplantation is of substantial interest.
In the clinical cases of peripheral nerve allotransplantation described in the literature, systemic immunosuppression has been performed with the calcineurin inhibitors cyclosporine A (CsA) or tacrolimus (FK506) (Mackinnon, et al., 2001). Compared to CsA, tacrolimus has the advantages of a more permissive toxicity profile and the ability to accelerate the rate of nerve regeneration (Doolabh and Mackinnon, 1999, Gold, et al., 1995, Jost, et al., 2000, Wang, et al., 1997), and is currently the immunosuppressant of choice in peripheral nerve allotransplantation (Fox and Mackinnon, 2007). However, systemic immunosuppression has substantial risks: infection, drug toxicity, and increased long-term risk of several malignancies including lymphomas and nonmelanoma skin cancer (Adami, et al., 2003, Jensen and Mackinnon, 2000, Jensen and Mackinnon, 2000, Jensen and Mackinnon, 2000). Thus, much research has been devoted to the development of tolerance-inducing or immunomodulatory (rather than immunosuppressive) therapies for transplantation (for review, see Salama et al, 2007 (Salama, et al., 2007)).
The T-cell activation cascade has been the target of many immunomodulatory therapies. T-cell activation is essential in the immune response to alloantigenic tissue, and a number of distinct but synergistic pathways for activation have been identified (Magott-Procelewska, 2004). Blockade of CD40:CD40 ligand (CD40L) interaction with anti-CD40L monoclonal antibody (MR1) alone induces a permissive state for nerve allografts in rodent (Brenner, et al., 2004) and nonhuman primate (Brenner, et al., 2004) models, but not longterm tolerance. However, addition of CTLA4-immunoglobulin (CTLA4-Ig), an agent that blocks the CD28:B7 costimulation pathway, to anti-CD40L can induce longterm immune hyporesponsiveness, as demonstrated experimentally in skin and cardiac (Larsen, et al., 1996) and renal(Kirk, et al., 1997) allografts. A third costimulation molecule, appropriately named inducible costimulation (ICOS), is expressed by T-cells only upon activation and interacts with B7RP-1 on B-cells, macrophages and dendritic cells (Yoshinaga, et al., 1999). ICOS blockade by anti-ICOS monoclonal antibody prolonged survival of heterotopic cardiac (Ozkaynak, et al., 2001) and liver (Guo, et al., 2002) allotransplants, but again was not sufficient as a single agent. When administered in combination with anti-CD40L, however, tolerance was again seen (Nanji, et al., 2004). Triple blockade of the CD40:CD40L, CD28:B7 and ICOS:B7RP-1 interactions was recently used successfully to induce short-term tolerance in a murine model nerve allotransplantation (Ray, et al., 2009). The effective duration of this triple costimulation blockade (CSB) is unknown.
The cellular graft components – Schwann cells (SCs) in particular – are what undergo rejection with prematurely discontinued immunosuppression (Ansselin and Pollard, 1990, Lassner, et al., 1989), resulting in graft failure. A more complete understanding of the behavior of the individual populations of graft-derived and host-derived SCs in an allograft model would help refine strategies for reducing clinical immunosuppression exposure while maximizing the chance of meaningful recovery from devastating peripheral nerve injury. To that end, we here use transgenic fluorescent protein-expressing mice to investigate migratory behavior of SCs in the murine nerve allograft model. We compare tacrolimus immunosuppression, mimicking the immunosuppressive strategy used clinically, with the more experimental approach of CSB immunomodulation.
Methods and Materials
Experimental Design
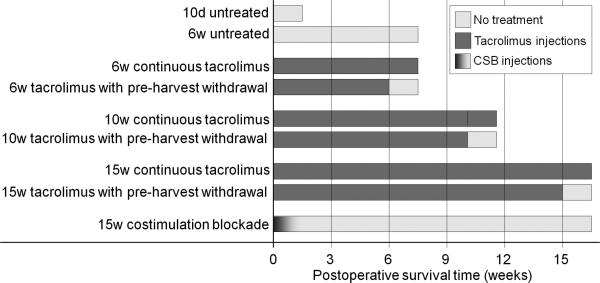
Five-millimeter wild-type BALB/c sciatic nerve allografts were placed into C57/Bl6-background mice in which green fluorescent protein (GFP) is expressed under the Schwann cell (SC) S100 promoter. Immunosuppression was administered with either tacrolimus (FK506) or T-cell triple costimulation blockade (CSB), consisting of anti-CD40 monocolonal antibodies (Brenner, et al., 2004), CTLA4 immunoglobulin fusion protein (CTLA4-Ig) (Magott-Procelewska, 2004), and anti-inducible costimulation (anti-ICOS) monoclonal antibodies (Zhang, et al., 2008). Our experimental design enabled both serial live imaging and immunohistochemical evaluation of host and donor SCs, producing a visual map of the presence of the two distinct cell populations over a 15 week postoperative course. We also introduced a 10-day immunosuppressant hiatus in some groups to allow comparison with animals undergoing continuous immunosuppression. Study endpoints were as shown in Figure 1.
Figure 1.
Treatment groups. Abbreviations: d – day, w – week, untx – untreated, tac – tacrolimus, cont – continuous immunosuppression, WD – immunosuppression withdrawal (i.e. 10-day immunosuppression hiatus before sacrifice).
To support our imaging findings, we assessed quantitative and qualitative descriptors of rejection in mice at several temporal endpoints (selected on the basis of published work (Udina, et al., 2004) as well as serial live imaging findings from these experiments), in the context of both CSB and tacrolimus immunosuppression.
Animals
The transgenic mouse line used here, which is referred to as “S100”, is homozygous for an enhanced green fluorescent protein (GFP) transgene under the control of upstream regions of the human S100B gene (Zuo, et al., 2004). Mice were obtained from The Jackson Laboratory (Bar Harbor, Maine) and interbred at our institution for several years prior to this experiment. In this line of transgenic mice, named “Kosmos” by Zuo and colleagues, GFP is expressed in mature and immature SCs, as well as in the lens of the eye, adipocytes, Langerhans cells, and a small subpopulation of macrophages, among other tissues (Zuo, et al., 2004).
Eighty S100 mice received sciatic nerve allografts from 43 fluorescence-naïve inbred BALB/c mice. BALB/cs were selected as donors because they are MHC-disparate from mice of the inbred C57/Bl6 strain, and have been used previously to test immune response to allografts placed in this transgenic mouse line (Hayashi, et al., 2008) . Animals were randomly assigned to immunosuppressive regimen: 62 were treated with tacrolimus, 12 with costimulation blockade, and 6 received no immunosuppression.
All animal procedures were approved by the Animal Studies Committee of Washington University, and performed in strict accordance with their guidelines. Mice were housed in a central animal care facility with twelve hour light-dark cycles, and given food (PicoLab Rodent Diet 20 #5053, PMI Nutrition International) and water ad libitum. Animals were monitored for weight loss or other signs of distress.
Immunosuppressive regimens
Tacrolimus
: Tacrolimus (FK506; Astellas Pharmaceuticals, Deerfield, IL) was administered subcutaneously 6 days per week at ≥2 mg/kg/day according to the methods of Grand and colleagues (Grand, et al., 2002). Dosing was dependent on the weight of the heaviest mouse in the cage; as a result, the actual dose for each individual mouse ranged from 2.1 to 2.8 mg/kg/d. Mice received their first tacrolimus injection after they had recovered from anesthesia following graft implantation. We chose our 6 week endpoint to be similar to that described in Udina et al, who found functionally significant rejection after immunosuppression withdrawal at 60 days in a 6 mm murine graft model (Udina, et al., 2004). The decision to harvest mice at 15 weeks was based on serial live imaging findings indicating that no substantial change in host SC repopulation of the graft was occurring. The 10 week endpoint was intended to be an endpoint intermediate to the others in the experiment.
To study the effects of prematurely discontinued tacrolimus on allograft rejection, a subgroup of mice had their immunosuppression stopped ten days prior to harvest (“tacrolimus withdrawn”). Ten days was selected because irreversible rejection occurs in untreated rodent grafts by this time (Feng, et al., 2001). Additionally, 10 days is the minimum time course required to establish full-graft SC migration in a murine acellular graft situation (Hayashi, et al., 2007). Temporal tacrolimus administration endpoints were, therefore, as depicted in Figure 1.
T-cell triple costimulatory blockade (CSB)
The costimulation blockade regimen used here has been used previously to induce graft tolerance in the murine model (Ray, et al., 2009). It consists of a mixture of 0.5 g CTLA4-Ig fusion protein (BioXCell, West Lebanon, NH), 0.5 mg anti-inducible T-cell costimulation (anti-ICOS) monoclonal antibody (BioXCell), and 1 mg anti-CD40L monoclonal antibody (MR1, BioXCell) per mouse administered by intraperitoneal injection on postoperative days 0, 2 and 4.
Surgical procedures
Donor graft harvest
Donor mice were anesthetized by subcutaneous injection of a mixture of 75 mg/kg ketamine hydrochloride (Ketaset®, Fort Dodge Animal Health, Fort Dodge, IA) and 0.5 mg/kg medetomidine hydrochloride (Dormitor®, Orion Corporation, Espoo, Finland). The left and right hindquarters were shaved and sterilized with iodine. With the aid of a dissecting microscope, a skin incision was made parallel to the femur, and the sciatic nerve was exposed with a minimally traumatic approach, dissecting along muscle planes. At least 1 cm of sciatic nerve was harvested bilaterally and placed on sterile saline-soaked gauze until implantation. Donors were then euthanized by cervical dislocation followed by intraperitoneal injection of >200 mg/kg sodium pentobarbital (Somnasol™, Butler Animal Health Supply, Dublin, OH).
Graft implantation
Recipient mice were anesthetized as above, and the operative site shaved and sterilized on the animal's right hindlimb. The sciatic nerve was exposed as above, and the nerve severed approximately 5 mm proximal to the sciatic trifurcation. Bulging nerve fascicles were trimmed as appropriate, and the donor nerve graft was trimmed to 5 mm in length. On a piece of Parafilm M (Structure Probe Inc., West Chester, PA), a tiny volume of fibrin sealant (Tisseel, Baxter Biopharmaceuticals, Deerfield, IL) was used to perform graft coaptation according to methods that have been previously described (Whitlock, et al., 2010). To ensure that there would be no tension on the repair, no native sciatic nerve was resected prior to graft implantation, resulting in an effective 5 mm redundancy of the sciatic nerve common to all study animals.
After the fibrin sealant coaptations had cured for approximately three minutes, coaptation sites were marked with a single 11-0 nylon suture, the muscle planes were allowed to fall naturally back into place and the skin was closed with interrupted 6-0 nylon sutures. Animals were given 0.01-0.05 mg/kg subcutaneous buprenorphine HCl (Hospira Inc., Lake Forest, IL) near the operative site for postoperative pain control, and anesthesia was reversed with 0.2 mg/kg atipamezole HCl (Antisedan®, Orion Corporation). Mice were then allowed to recover on a heated bed. If they were in a group receiving immunosuppression with tacrolimus, the first injection of immunosuppressant was given once the mice had fully regained consciousness. Mice were then returned to the animal care facility.
In vivo serial imaging
Image acquisition
A subpopulation of mice in the tacrolimus group and all CSB mice underwent live imaging of their sciatic nerve based on native GFP fluorescence. In tacrolimus groups, imaging was performed after a >24 hour immunosuppressant hiatus to ensure mice would respond normally to sedation and reversal. Mice were imaged on postoperative week 3, 6, 9, 12 or 15. Mice underwent in vivo imaging and recovery only once during the experimental period (that is, no more than two survival surgeries were performed in any mouse).
Under anesthesia as above, sciatic nerve exposure was obtained through a minimally traumatic muscle planes approach on the right (experimental) side only. Using an Olympus SMZ-1500 fluorescence dissecting microscope equipped with a 488 nm filter (Nikon Instruments Inc, Melville, NY), the graft and surrounding sciatic nerve were imaged with a CoolSNAP-ES CCD (Photometrics, Tucson, AZ) and MetaMorph software (Universal Imaging Corporation, Downingtown, PA). A standardized imaging routine of 2.5× magnification and 62.5 ms exposure time was used to generate a 12-bit greyscale image. Wounds were closed and the animal was allowed to recover as above.
Image processing
The standardized imaging routine described above allowed us to quantitate graft fluorescence for the transgenic animals. Using MetaMorph software, a line approximately 1200 pixels long was drawn along native nerve and graft. Whenever possible, this line was drawn to longitudinally bisect the nerve; occasionally, areas of fat or blood vessels overlying the nerve were deliberately avoided because of their ability to confound brightness values. Using the linescanning command, a brightness contour was produced using the average pixel intensity value of 20 pixels on a line perpendicular to, and centered on, the original longitudinal line, as previously described (Hayashi, et al., 2007). Absolute pixel intensities were normalized to the brightest area of normal nerve in the field and averaged along the entire length of the graft, producing a value between 0 and 1 for each mouse at each imaging timepoint.
Nerve harvest
Mice received bromodeoxyuridine (BrdU) labeling reagent (Invitrogen, Carlsbad, CA) at a volume of 0.01 mL concentrated aqueous solution per gram of body weight administered by intraperitoneal injection exactly 5 hours prior to harvest of nerve tissue. At the predetermined endpoint, mice were reanesthetized and the bilateral hindquarters were shaved and sterilized. The right (experimental side) and left (control side) sciatic nerves were exposed via the same approach as above and imaged for native GFP fluorescence according to the serial imaging routine described below. Bilateral sciatic nerves were harvested from each mouse. On the grafted side, at least 5 mm of native sciatic nerve was harvested proximal and distal to the graft. Nerves were harvested into 4% paraformaldehyde in phosphate-buffered saline (PBS) solution, where they remained for fixation overnight. They were then washed with PBS, embedded in Optimal Cutting Temperature (OCT) compound (Tissue-Tek®, Sakura Finetek USA, Torrance, CA), and stored at -80°C until use.
Immunohistochemistry
Specimens embedded in OCT were cut into longitudinal sections 20 µm thick with a cryomicrotome and mounted on Superfrost®/Plus microscope slides (Fisher Scientific, Pittsburgh, PA). Sections used for BrdU and anti-S100 staining were obtained serially.
Anti-S100
Sections were washed with phosphate-buffered saline (PBS) and soaked in acetone at -20°C for 10 m. After again washing with PBS, slides were blocked with 5% normal goat serum (Millipore, Bedford, MA) and 0.1% Triton X-100 (Sigma-Aldrich Corp., Saint Louis, MO) in PBS for one hour at 25°C or overnight at 4°C. Slides were washed with 0.1% Triton X-100 in PBS (Triton-PBS), and the primary antibody, rabbit polyclonal anti-S100 (catalogue # ab868, Abcam, Cambridge, MA), was diluted 1:100 in Triton-PBS and incubated overnight at 4°C.
After washing in Triton-PBS, the slide was incubated with secondary antibody, goat anti-rabbit IgG conjugated to Cy3 fluorophores (Jackson ImmunoResearch, West Grove, PA) diluted 1:5 in Triton-PBS, for one hour at room temperature. The slide was again washed with Triton-PBS and covered with VECTASHIELD® mounting medium with DAPI (Vector Laboratories, Burlingame, CA).
Anti-BrdU
Sections were washed with Triton-PBS and incubated in 1N HCl on ice for 10 minutes, then in 2N HCl for 10 minutes at room temperature followed by 20 minutes at 37°C. Slides were then buffered in a 0.1M sodium borate solution for 12 minutes at room temperature, washed with Triton-PBS, and blocked with 5% normal donkey serum (Millipore) and 0.02% sodium azide in Triton-PBS at room temperature for one hour. The primary antibody, sheep polyclonal anti-BrdU (catalogue #ab1893, Abcam), was diluted 1:50 in Triton-PBS and incubated overnight at 4°C.
After washing in Triton-PBS, the slide was incubated with secondary antibody, donkey anti-sheep IgG conjugated to Cy3 fluorophores (Jackson ImmunoResearch) diluted 1:500 in Triton-PBS, for one hour at room temperature. The slide was again washed with Triton-PBS and covered with VECTASHIELD® mounting medium with DAPI.
Imaging
For both immunohistochemical targets, slides were imaged on an Olympus Fluoview FV1000 confocal microscope with lasers at 488 nm for SCs (S100-GFP) and 568 nm for Cy3 pAb (anti-S100 and anti-BrdU).
Western Blot
Nerve allografts were thawed and dissected into proximal host, graft, and distal host segments for separate assessment. Segments were then homogenized on ice in lysis buffer (Halt Protease Inhibitor Cocktail [Pierce Protein Research Products, Rockford, IL] with 25mM bicine, 150mM NaCl, and 5mM EDTA at pH 7.6). Lysates were centrifuged at 13000 rpm for 15 minutes and supernatants retained for analysis.
Protein was quantitated using a 4-parameter curve fit (R2=0.999) by measuring against a known quantity of bovine serum albumin using an optical density of A562. Samples were mixed with lane marker sample buffer (Pierce Protein Research Products) and boiled at 100°C for 3 minutes. The same amount of protein (5μg) for each sample was loaded and resolved in 10% SDS-PAGE gels (Pierce Protein Research Products). Resolved proteins were transferred to PDVF membranes (Millipore) and membranes were incubated with a blocking solution containing 5% non-fat dried milk in Tris-buffered saline buffer with Tween-20 (TBS-Tween) for 30 minutes. Membranes were further incubated with rabbit primary antibodies in TBS-Tween overnight. The following primary antibodies were used: anti-caspase-3 [rabbit polyclonal; 1:500 dilution (Cell Signaling, Danvers, MA)] and anti-GAPDH [rabbit polyclonal; 1:200 dilution (Santa Cruz Biotechnology Inc., Santa Cruz, CA)]. Immunoblots were then incubated with goat anti-rabbit antibody conjugated to horseradish peroxidase (Santa Cruz Biotechnology) at a 1:5000 dilution.
Immune complexes were detected by enhanced chemiluminescence (SuperSignal West Dura Extended Duration Substrate; Pierce Protein Research Products). For quantification, immunoreactive bands were assessed using chemiluminescence imagining from a CCD camera system (Fotodyne, Hartland, WI), and analyzed for signal intensity by Fotodyne/Analyst PC Image version 5.00 (Fotodyne, Hartland, WI). Protein loading was normalized to GAPDH signals.
Statistical analysis
Data was analyzed with SigmaStat version 3.5 (Systat Software Inc., San Jose, CA). Multiple groups were compared with a one-way analysis of variance (ANOVA) if conditions of normality (assessed with the Kolmogorov-Smirnoff normality test) and equal variance (assessed with the Levene Median test) were met. If the ANOVA returned a statistically significant p value, a post-hoc Tukey HSD test was used to isolate significant differences among the data with correction for multiple comparisons. A p value of less than 0.05 was considered to indicate statistical significance. Data is presented as mean ± standard deviation where appropriate.
Results
Animals
Average weight gain in mice receiving tacrolimus over the study period (5.6 ± 1.4 g, n = 44) was substantially less than that of mice receiving triple costimulation blockade (CSB) (12.3 ± 3.1 g, n = 7). Additionally, essentially all mice receiving tacrolimus developed superficial skin ulcers at injection sites. These ulcers healed spontaneously and were not associated with abscess formation, cellulitis or other signs of infection, so were not considered sufficiently morbid to warrant euthanasia. Ulcer formation has been previously described with subcutaneous tacrolimus administration(Feng, et al., 2001).
Of the 62 mice randomized to tacrolimus treatment, 15 died prematurely, including one who was euthanized due to a non-resolving wound infection. Two mice were sacrificed for blood collection to permit interim analysis of tacrolimus drug levels, which were found to be within the therapeutic range (data not shown). One mouse experienced graft dehiscence and was excluded. The unusual burden of mortality on this population of S100 transgenic mice brings up the question of differential sensitivity to the side effects of or infectious complications related to tacrolimus; a full investigation was, however, beyond the scope of this study. One mouse receiving CSB also died of unknown causes; four were harvested at intermediate time points for hypothesis generation. All mice receiving allografts with no immunosuppression survived. Sixty-three mice (44 receiving tacrolimus, 11 treated with CSB, and 12 without immunosuppression) completed their assigned study period successfully and contributed to the data described herein.
In-vivo imaging of whole-graft fluorescence
Tacrolimus
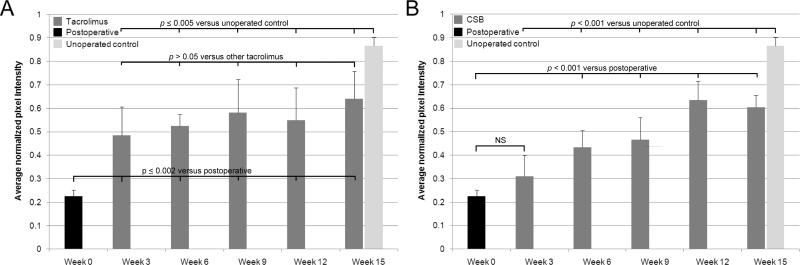
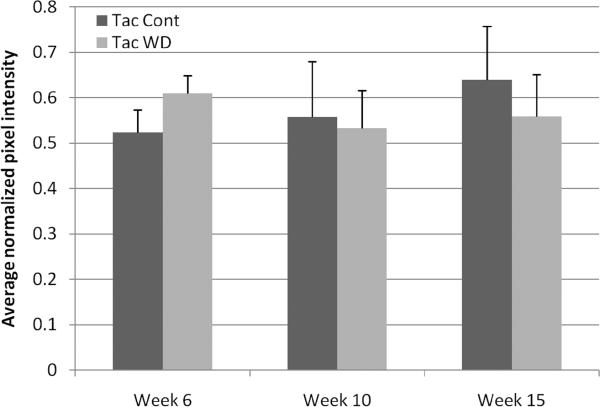
In tacrolimus-treated animals, GFP expression was visible uniformly throughout the graft in all mice as early as week 3 (Figure 2A). As assessed by one-way ANOVA followed by post-hoc Tukey HSD tests, there were no significant increases in average graft brightness after week 3 in tacrolimus-treated mice (Figure 3A), suggesting that SC migration achieves stability under this immunosuppressive regimen. Compared with normal nerve, grafts in tacrolimus-treated animals had significantly lower pixel intensity, indicating that full repopulation with host SCs had not occurred even at the 15 week endpoint (Figure 3A). Mice undergoing a 10-day immunosuppressant hiatus before sacrifice (“Tac WD”) did not have significantly different whole-graft fluorescence compared to mice with continuous immunosuppression (“Tac Cont”) (Figure 4).
Figure 2.
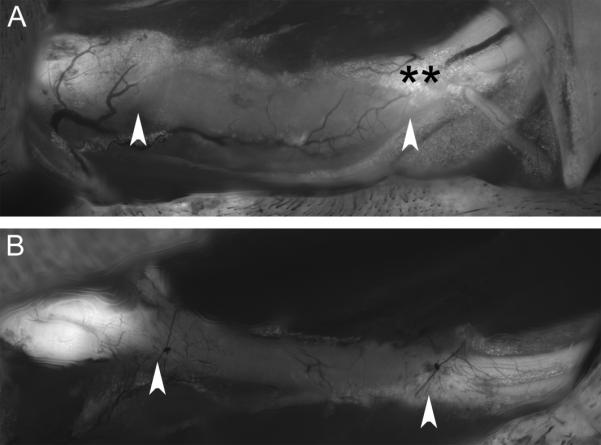
In vivo images of allografts on postoperative day 21. Original images taken with our standard imaging protocol were subjected to identical image processing routines to maintain relative pixel brightness values between the two images for comparison here. Graft boundaries are indicated with arrows. A, tacrolimus immunosuppression. Note uniform, light whole-graft fluorescence. B, costimulatory blockade immunosuppression. The graft displays fluorescence only slightly greater than the background muscle. Note the potential confounding ability of GFP-expressing adipocytes, identified with **, to increase graft fluorescence measurement if not avoided during image analysis.
Figure 3.
Comparison of whole-graft brightness values over time. Reported p values are from post-hoc Tukey HSD testing following an ANOVA which returned p < 0.001 for both the data sets in A and B. A, tacrolimus-treated animals. All intermediate imaging timepoints demonstrated greater whole-graft brightness than week 0 (image acquired immediately following graft insertion), and less brightness than unoperated control nerve. There were no significant differences between the intermediate groups. B, costimulatory blockade-treated animals. All intermediate imaging timepoints showed significantly less whole-graft brightness than unoperated control nerve. All intermediate imaging timepoints except postoperative week 3 demonstrated greater whole-graft brightness than week 0. Unlike tacrolimus-treated animals, however, there were significant differences between intermediate imaging timepoints.
Figure 4.
Comparison of endpoint whole-graft brightness in animals receiving continuous immunosuppression (“Tac Cont”) versus those with a ten-day immunosuppressant hiatus prior to sacrifice (“Tac WD”). There are no significant differences between the groups (ANOVA p > 0.05).
Triple costimulation blockade (CSB)
In mice who received postoperative CSB, accumulation of graft fluorescence progressed more gradually than in mice immunosuppressed with tacrolimus (Figure 2B, Figure 3B). While differences between adjacent imaging timepoints were rarely significant (with the exception of week 9 versus week 12, p = 0.006), nonadjacent weeks always saw significant increases in whole-graft fluorescence over time. Compared to untreated allografts or normal nerve, CSB-treated animals also had significantly lower whole-graft brightness at all time points assessed (Figure 3B), demonstrating incomplete repopulation with host GFP-expressing SCs.
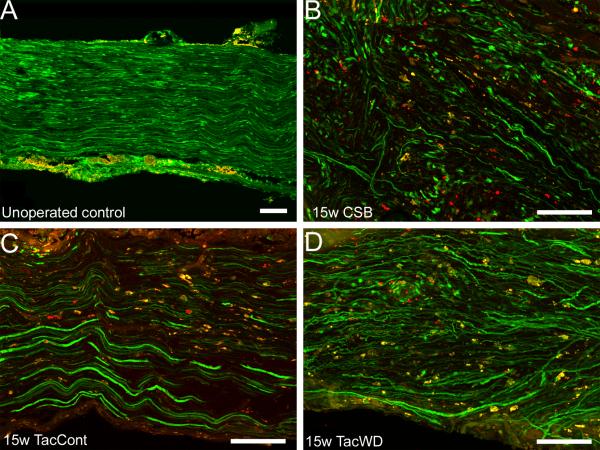
S100 antibody staining
S100 antibody staining permitted differentiation between graft and host SCs in transgenic animals. Specifically, graft SCs were single-labeled with S100 mAb (red); host SCs were labeled with S100 mAb (red) and expressed native GFP (green). Thus, the prevalence of single-labeled “red” cells would vary depending on how many graft SCs were successfully maintained by immunosuppressive therapy.
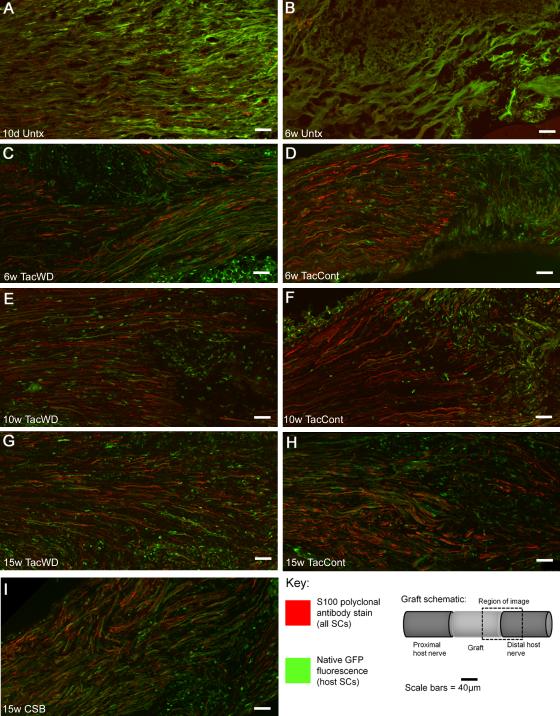
Untreated allografts, both at 10 days and 6 weeks, had no single-labeled cells, suggesting the rejection response was robust and complete (Figure 5 A-B). Grafts from tacrolimus-treated animals demonstrated no qualitative difference in concentration of donor cells as a function of postoperative duration (Figure 5C-I); this is consistent with serial live imaging results that suggested no significant increase in whole-graft GFP expression over the later postoperative course. Even at the latest endpoint, a substantial population of cells still persisted in both tacrolimus- and CSB-treated allografts (Figure 5 G-I). Mice receiving an immunosuppressant hiatus before sacrifice (Figure 5 C, E, G) did not have substantially different findings on immunohistochemistry from those receiving continuous immunosuppression, consistent with whole-graft brightness findings.
Figure 5.
Immunohistochemical staining differentiates graft from host SCs at the distal graft-host nerve border. A, B: without immunosuppression, no donor SCs are visible. C-I: Under different immunosuppressive conditions, donor cells persist both in the graft and in surrounding host nerve. Abbreviations: d – day, w – week, untx – untreated, tac – tacrolimus, cont – continuous immunosuppression, WD – immunosuppression withdrawal (i.e. 10-day immunosuppression hiatus before sacrifice).
The three-dose course of costimulatory blockade administered to study animals proved sufficiently durable to permit survival of a considerable population of donor cells until the 15 week endpoint, without the morbidity and mortality associated with daily tacrolimus injections (Figure 5I).
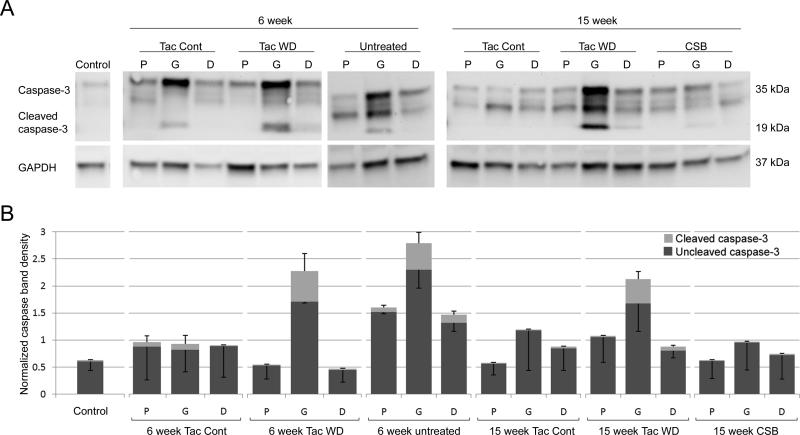
Western blot
Western blot for caspase 3 was performed on two to three samples per treatment group to provide qualitative information about apoptosis in the grafts and surrounding nerve. Because of small sample sizes, no statistical analysis was performed. Qualitatively, there were higher levels of activated caspase 3 in graft segments for groups undergoing rejection (i.e. in mice not receiving immunosuppression or after a ten-day immunosuppressant hiatus) (Figure 6). No clear elevations of activated caspase 3 were noted in native nerve proximal or distal to the graft. Mice treated with continuous tacrolimus immunosuppresson or CSB had very low levels of activated caspase 3, suggesting maintenance of donor Schwann cells.
Figure 6.
Western blot to quantitate activated caspase 3, a marker of the apoptosis cascade. A, representative Western blot gels. B, quantitative band intensity for different samples. Because sample sizes were small, no statistical analysis was performed. Abbreviations: tac – tacrolimus, cont – continuous immunosuppression, WD – immunosuppression withdrawal (i.e. 10-day immunosuppression hiatus before sacrifice), P – proximal host nerve, G – graft (donor-derived), D – distal host nerve, GAPDH – glyceraldehyde 3-phosphate dehydrogenase (control protein).
BrdU incorporation
Sciatic nerve samples were qualitatively assessed for BrdU incorporation, indicating active DNA replication within the five hours prior to sacrifice of the mouse. Cells that incorporated BrdU as DNA replication occurred were labeled red, with native S100-GFP fluorescence visible as green (Figure 7). Very rare BrdU-positive nuclei were visible in control (ungrafted) specimens (Figure 7A), indicating a low level of cell division in normal nerve. BrdU-positive profiles were more common in mice receiving continuous tacrolimus immunosuppression or CSB (Figure 7B-C), and frequent in mice who had undergone a ten-day immunosuppressant hiatus (Figure 7D). This suggests that immunosuppression withdrawal may be associated with increased cell division within the nerve graft. BrdU incorporation is not specific to Schwann cells, however, and these results are suggestive but not conclusive of increased Schwann cell division to repopulate the graft.
Figure 7.
BrdU staining patterns. Green - native S100-GFP fluorescence. Red pseudocolor - anti-BrdU antibody staining. Yellow - merge. Images taken, where appropriate, at approximately mid-graft. Scale bars in all four images represent 100 μm. A, control nerve. Nonspecific immunostaining of the epineurium with red fluorophores is seen here following image processing to maximize the appearance of red fluorophore labeling; it was also seen on internal control (i.e. no BrdU administered) specimens after image processing (data not shown). B, 15 week costimulatory blockade. C, 15 week continuous tacrolimus. D, 15 week tacrolimus with 10-day immunosuppression withdrawal. Cell division is readily demonstrated with anti-BrdU-staining (red and yellow), in 15w CSB, 15w TacCont, and 15w TacWD samples. Particularly in B and D, punctate yellow-pseudocolored overlap between red nuclear BrdU staining and green native GFP fluorescence strongly suggests that host SCs are responsible for the division seen.
Discussion
Different methods of immunosuppression have previously been shown to differentially affect Schwann cell (SC) migration (Hayashi, et al., 2008). In this work, we compared the recommended immunosuppressant for peripheral nerve allografting, tacrolimus (FK506), with the experimental therapy of T-cell triple costimulation blockade (CSB) using a novel method of macroscopic and microscopic observation to differentiate graft- from host-derived SCs. The two immunosuppressants differ substantially in the method of administration and in their biological targets, providing two contrasting pathways for achieving the common goal of nerve allograft survival.
Tacrolimus complexes with FK506 binding protein 12, inhibiting the activation of the NFAT family of transcription factors and reducing the production of many downstream cytokines, including IL-2, IL-3, IL-4, IL-10, IFN-γ, and GM-CSF, thereby inhibiting T cell activation and proliferation(Feske, et al., 2001). Tacrolimus is administered in the rodent model as daily subcutaneous injections throughout the postoperative course, broadly suppressing T-cell response to both the antigens in the transplanted tissue and other (e.g. infectious) stimuli. Here, we have demonstrated that in tacrolimus-treated mice, an early period of rapid migration results in donor and host SC interaction in the graft, followed by stabilization with coexistence of graft- and host-derived SCs for at least 15 weeks after allotransplantation (Figures 3 and 4). This is consistent with published work on murine allograft SC migration utilizing mutant Shiverer mice as donors to differentiate graft from host SCs based on presence of myelin basic protein, in which persistence of allograft SCs was noted until at least 14 weeks under cyclosporine immunosuppression (Midha, et al., 1994). Here, we use morphologically and functionally normal (Kang, et al., 2007, Zuo, et al., 2004) GFP-expressing SCs and obtain similar endpoint results.
These results suggest that, following the rapid migration seen in the immediate postoperative period, further replacement of donor by host SCs occurs slowly – if at all – with continuous tacrolimus immunosuppression. Prior work has demonstrated that cessation of immunosuppression results in eventual rejection of many (though perhaps not all (Katsube, et al., 1998)) remaining donor SCs. In our study, discontinuation of tacrolimus for 10 days before sacrifice appeared to increase production of activated caspase 3 and BrdU incorporation (DNA replication) within the graft, suggestive of donor cell rejection and early stages of repopulation with host cells. Although no increase in whole-graft pixel intensity was seen 10 days after tacrolimus withdrawal, we hypothesize that migration of host SCs into the allograft lags behind the loss of donor cells. Previously we have shown that paucity or absence of SCs is a powerful stimulus for migration (Hayashi, et al., 2007, Midha, et al., 1994). The results of the present study suggest that withdrawal of tacrolimus immunosuppression is essential to stimulate the process of repopulation of the allograft with host SCs and the ultimate achievement of nonimmunogenicity.
It is important to consider the effects of immunosuppression dosing fluctuations after allotransplantation. As mentioned in the methods section, the animals in the tacrolimus group were dosed based on the heaviest animal in the cage. The dosing protocol resulted in a variation in dose from 2.1 to 2.8 mg/kg/day , ensuring the tacrolimus dose in all animals was maintained at or above the standard immunosuppressive threshold of 2 mg/kg/day. We could not find a study that directly assessed the impact of small fluctuations (33%) above the accepted immunosuppressive dose, as were a consequence of our dosing strategy. Tacrolimus doses of up to 5 mg/kg/day have no additional immunosuppressive effect (Udina, et al., 2004). However, tacrolimus has several known tissue effects besides immunosuppression. In the rat model, tacrolimus at 2 mg/kg/d retards dermal wound healing compared to placebo; however, the effects do not appear to follow a dose-response curve (Schaffer, et al., 1998). The neuroregenerative effects of tacrolimus are seen at 0.5 mg/kg/day; quadrupling that dose does not cause further enhancement (Yang, et al., 2003). Although a wide body of allotransplantation literature has concluded that subimmunosuppressive tacrolimus doses are markedly detrimental to allogeneic SCs, no study has specifically investigated the effect of tacrolimus dose on SC behavior. In light of this uncertainty, the dosing fluctuations seen in this experiment, although small, should be taken into consideration when interpreting the results of this study.
In contrast to tacrolimus, the goal of triple costimulation blockade (CSB) is to induce long-term tolerance to allogeneic tissue by inhibiting sensitization of host T-cells to donor alloantigens in the immediate postoperative period. CSB is administered as a short course of intraperitoneal injections given on postoperative days 0, 2 and 4. In CSB-treated mice, graft fluorescence gradually increased throughout the 15-week study period. Immunosuppressive effect persisted until the 15-week endpoint, as measured by Western blot for activated caspase 3, and as evidenced indirectly by the substantial population of donor SCs remaining in the graft and surrounding host nerve. Increasing whole-graft fluorescence and an elevated rate of cell division within the graft strongly suggest that the effect in fact wears off gradually with the progression of time, consistent with gradual elimination from the body, gradual diminution of the blockade efficacy, or some combination of the two. Mice receiving costimulatory blockade experienced none of the morbidity (including skin ulcers and relatively poor weight gain) and far less mortality than those mice in the tacrolimus groups. We also hypothesize that gradual withdrawal of immunosuppression, as may occur if CSB effect wears off gradually, may provide a gentle stimulus for migration of host SCs into the graft as the donor cell population slowly undergoes rejection. More work is needed to characterize the effective length of single-course CSB immunosuppression; we show here that its duration of effect is at least 15 weeks.
Both tacrolimus and CSB permitted the survival of a substantial population of graft-derived SCs until the 15 week endpoint. SCs are particularly involved in the immunoreactivity of a nerve allograft (Ansselin and Pollard, 1990, Lassner, et al., 1989), and persistence of donor SCs in the graft poses a problem when discontinuing immunosuppression. Histological assessment of allografts with prematurely discontinued immunosuppression shows reduction in the number of large, well-myelinated axons, with a concomitant increase in the number of large thinly- or non-myelinated axons compared to normal controls (Midha, et al., 1993). This is consistent with rejection of donor SCs, and consequent failure of myelination, in the graft region.
This study demonstrates that SC migration patterns during the postoperative course are in part dependent on the method of immunosuppression selected, and may suggest strategies for immunosuppression withdrawal if continuous immunosuppression (i.e. tacrolimus) is used. The time course of rejection after immunosuppression withdrawal is crucial; if it occurs suddenly, as in our animals who received allografts but no immunosuppression, destruction of donor cells is rapid and complete. In one published case report of human nerve allotransplantation, subtherapeutic immunosuppression very early in the postoperative course led to rapid, irreversible rejection (Mackinnon, et al., 2001). This contrasts with rodent experiments describing viability of temporary immunosuppression (Katsube, et al., 1998, Mackinnon, et al., 1992, Midha, et al., 1993, Udina, et al., 2004), as well as the other human patients described in the work by Mackinnon et al (Mackinnon, et al., 2001). The emerging therapy of composite tissue allotransplantation (CTA, e.g. hand and face transplantation) also depends upon immunosuppression or immunomodulation for its success. In contrast to nerve allotransplantation, host SC migration in a CTA occurs only at the single interface between host and CTA tissue and may thus be more limited by distance. As new experimental immune tolerance regimens are employed in CTA, they may eliminate episodes of early acute rejection, retarding migration of host SCs into the graft. In the absence of successful host SC repopulation, a sudden prolonged episode of rejection following complete nerve regeneration could irreversibly damage the function of the CTA (Moore, et al., 2009), rendering an otherwise technically successful CTA useless.
A limitation of this study relates to the nature of the transgenic GFP-expressing mouse model used. Because of the wide GFP expression profile of S100 mice – adipocytes, a small subpopulation of macrophages, etc. – it is impossible to determine conclusively from the live imaging photographs which cell type is responsible for the observed fluorescence, particularly at the three week endpoint where confirmatory immunohistochemistry was not performed. We have previously shown that, in a cold-preserved nerve allograft model, only the minority of macrophages are labeled with GFP; additionally, GFP-expressing Schwann cells are approximately three times as bright as the infrequent GFP-expressing macrophages(Hayashi, et al., 2007). We would also anticipate that macrophages that had recently phagocytosed components of a GFP-labeled SC would also appear to express GFP; it is a limitation of this model that that phenomenon cannot be experimentally eliminated in the live-imaging technique. However, the presence of spindle-shaped GFP-expressing SCs throughout the graft at 6 weeks by native GFP fluorescence and anti-S100 immunohistochemistry strongly suggests that the GFP-expressing cells visible in the graft at three weeks are likely to be migratory host SCs.
Animal models of peripheral nerve allografting have been extensively described in the literature; however, nerve allografting in humans is reserved for otherwise irreparable nerve injury. Surgeons have historically been reluctant to expose patients to the risks of systemic immunosuppression for nonessential allotransplantation (Morris, et al., 2004), an issue which has received substantial attention in the lay press since the first human partial face transplant was performed in 2005 (Devauchelle, et al., 2006). Unlike transplantation of other tissues – solid organs, or composite tissues like the hand or face – the immunosuppressive period for peripheral nerve allotransplantation is finite. The limited clinical experience with peripheral nerve allografting in the peer-reviewed literature has been predominantly positive. Most patients successfully completed the required period of immunosuppression without undue morbidity and recovered sensation and function that otherwise would have been unattainable. Particularly in young patients, whose lifetime burden from the functional and sensory deficits following peripheral nerve injury is of particular concern, a finite period of immunosuppression may be worthwhile if it allowed them the hope of recovery from an otherwise devastating injury.
We demonstrate here that SC migration plateaus with continuous tacrolimus immunosuppression, suggesting that withdrawal of tacrolimus immunosuppression is likely required for full repopulation of an allograft with host cells. We also demonstrate the modality of immunosuppression affects SC migration, and hypothesize that gradual replacement of donor with host SCs as seen with triple costimulatory blockade might provide the gentle stimulus necessary for graft repopulation without a catastrophic rejection event. Further characterization of the events leading to host cell repopulation of donor nerve, and the withdrawal of immunosuppression that this permits, will provide important information for more effective clinical application of nerve allotransplantation.
Acknowledgments
This work was funded by a grant from the National Institute of Health (5RO1NS033406) and ELW was supported by the Howard Hughes Medical Institute as a Medical Research Fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adami J, Gabel H, Lindelof B, Ekstrom K, Rydh B, Glimelius B, Ekbom A, Adami HO, Granath F. Cancer risk following organ transplantation: a nationwide cohort study in Sweden. Br J Cancer. 2003;89:1221–1227. doi: 10.1038/sj.bjc.6601219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansselin AD, Pollard JD. Immunopathological factors in peripheral nerve allograft rejection: quantification of lymphocyte invasion and major histocompatibility complex expression. J Neurol Sci. 1990;96:75–88. doi: 10.1016/0022-510x(90)90058-u. [DOI] [PubMed] [Google Scholar]
- 3.Brenner MJ, Jensen JN, Lowe JB, 3rd, Myckatyn TM, Fox IK, Hunter DA, Mohanakumar T, Mackinnon SE. Anti-CD40 ligand antibody permits regeneration through peripheral nerve allografts in a nonhuman primate model. Plast Reconstr Surg. 2004;114:1802–1814. doi: 10.1097/01.prs.0000143575.88064.d0. discussion 1815-1807. [DOI] [PubMed] [Google Scholar]
- 4.Brenner MJ, Tung TH, Mackinnon SE, Myckatyn TM, Hunter DA, Mohanakumar T. Anti-CD40 ligand monoclonal antibody induces a permissive state, but not tolerance, for murine peripheral nerve allografts. Exp Neurol. 2004;186:59–69. doi: 10.1016/j.expneurol.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Devauchelle B, Badet L, Lengele B, Morelon E, Testelin S, Michallet M, D'Hauthuille C, Dubernard JM. First human face allograft: early report. Lancet. 2006;368:203–209. doi: 10.1016/S0140-6736(06)68935-6. [DOI] [PubMed] [Google Scholar]
- 6.Doolabh VB, Mackinnon SE. FK506 accelerates functional recovery following nerve grafting in a rat model. Plast Reconstr Surg. 1999;103:1928–1936. doi: 10.1097/00006534-199906000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Feng FY, Ogden MA, Myckatyn TM, Grand AG, Jensen JN, Hunter DA, Mackinnon SE. FK506 rescues peripheral nerve allografts in acute rejection. J Neurotrauma. 2001;18:217–229. doi: 10.1089/08977150150502631. [DOI] [PubMed] [Google Scholar]
- 8.Feske S, Giltnane J, Dolmetsch R, Staudt LM, Rao A. Gene regulation mediated by calcium signals in T lymphocytes. Nat Immunol. 2001;2:316–324. doi: 10.1038/86318. [DOI] [PubMed] [Google Scholar]
- 9.Fox IK, Mackinnon SE. Experience with nerve allograft transplantation. Semin Plast Surg. 2007;21:242–249. doi: 10.1055/s-2007-991194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gold BG, Katoh K, Storm-Dickerson T. The immunosuppressant FK506 increases the rate of axonal regeneration in rat sciatic nerve. J Neurosci. 1995;15:7509–7516. doi: 10.1523/JNEUROSCI.15-11-07509.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grand AG, Myckatyn TM, Mackinnon SE, Hunter DA. Axonal regeneration after cold preservation of nerve allografts and immunosuppression with tacrolimus in mice. J Neurosurg. 2002;96:924–932. doi: 10.3171/jns.2002.96.5.0924. [DOI] [PubMed] [Google Scholar]
- 12.Guo L, Li XK, Funeshima N, Fujino M, Nagata Y, Kimura H, Amemiya H, Enosawa S, Tsuji T, Harihara Y, Makuuchi M, Suzuki S. Prolonged survival in rat liver transplantation with mouse monoclonal antibody against an inducible costimulator (ICOS). Transplantation. 2002;73:1027–1032. doi: 10.1097/00007890-200204150-00003. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi A, Koob JW, Liu DZ, Tong AY, Hunter DA, Parsadanian A, Mackinnon SE, Myckatyn TM. A double-transgenic mouse used to track migrating Schwann cells and regenerating axons following engraftment of injured nerves. Exp Neurol. 2007;207:128–138. doi: 10.1016/j.expneurol.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashi A, Moradzadeh A, Tong A, Wei C, Tuffaha SH, Hunter DA, Tung TH, Parsadanian A, Mackinnon SE, Myckatyn TM. Treatment modality affects allograft-derived Schwann cell phenotype and myelinating capacity. Exp Neurol. 2008;212:324–336. doi: 10.1016/j.expneurol.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi A, Moradzadeh A, Tong AY, Wei C, Tuffaha SH, Hunter D, Tung TH, Parsadanian A, Mackinnon SE, Myckatyn TM. Treatment modality affects allograft-derived Schwann cell phenotype and myelinating capacity. Exp Neurol. 2008 doi: 10.1016/j.expneurol.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen JN, Mackinnon SE. Composite tissue allotransplantation: a comprehensive review of the literature--part 1. J Reconstr Microsurg. 2000;16:57–68. [PubMed] [Google Scholar]
- 17.Jensen JN, Mackinnon SE. Composite tissue allotransplantation: a comprehensive review of the literature--Part II. J Reconstr Microsurg. 2000;16:141–157. [PubMed] [Google Scholar]
- 18.Jensen JN, Mackinnon SE. Composite tissue allotransplantation: a comprehensive review of the literature--part III. J Reconstr Microsurg. 2000;16:235–251. [PubMed] [Google Scholar]
- 19.Jost SC, Doolabh VB, Mackinnon SE, Lee M, Hunter D. Acceleration of peripheral nerve regeneration following FK506 administration. Restor Neurol Neurosci. 2000;17:39–44. [PubMed] [Google Scholar]
- 20.Kang H, Tian L, Son YJ, Zuo Y, Procaccino D, Love F, Hayworth C, Trachtenberg J, Mikesh M, Sutton L, Ponomareva O, Mignone J, Enikolopov G, Rimer M, Thompson W. Regulation of the intermediate filament protein nestin at rodent neuromuscular junctions by innervation and activity. J Neurosci. 2007;27:5948–5957. doi: 10.1523/JNEUROSCI.0621-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katsube K, Doi K, Fukumoto T, Fujikura Y, Shigetomi M, Kawai S. Successful nerve regeneration and persistence of donor cells after a limited course of immunosuppression in rat peripheral nerve allografts. Transplantation. 1998;66:772–777. doi: 10.1097/00007890-199809270-00012. [DOI] [PubMed] [Google Scholar]
- 22.Kirk AD, Harlan DM, Armstrong NN, Davis TA, Dong Y, Gray GS, Hong X, Thomas D, Fechner JH, Jr., Knechtle SJ. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci U S A. 1997;94:8789–8794. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, Cho HR, Aruffo A, Hollenbaugh D, Linsley PS, Winn KJ, Pearson TC. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 24.Lassner F, Schaller E, Steinhoff G, Wonigeit K, Walter GF, Berger A. Cellular mechanisms of rejection and regeneration in peripheral nerve allografts. Transplantation. 1989;48:386–392. doi: 10.1097/00007890-198909000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Mackinnon SE, Doolabh VB, Novak CB, Trulock EP. Clinical outcome following nerve allograft transplantation. Plast Reconstr Surg. 2001;107:1419–1429. doi: 10.1097/00006534-200105000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Mackinnon SE, Midha R, Bain J, Hunter D, Wade J. An assessment of regeneration across peripheral nerve allografts in rats receiving short courses of cyclosporin A immunosuppression. Neuroscience. 1992;46:585–593. doi: 10.1016/0306-4522(92)90146-s. [DOI] [PubMed] [Google Scholar]
- 27.Magott-Procelewska M. Costimulatory pathways as a basic mechanisms of activating a tolerance signal in T cells. Ann Transplant. 2004;9:13–18. [PubMed] [Google Scholar]
- 28.Midha R, Mackinnon SE, Becker LE. The fate of Schwann cells in peripheral nerve allografts. J Neuropathol Exp Neurol. 1994;53:316–322. doi: 10.1097/00005072-199405000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Midha R, Mackinnon SE, Evans PJ, Best TJ, Hare GM, Hunter DA, Falk-Wade JA. Comparison of regeneration across nerve allografts with temporary or continuous cyclosporin A immunosuppression. J Neurosurg. 1993;78:90–100. doi: 10.3171/jns.1993.78.1.0090. [DOI] [PubMed] [Google Scholar]
- 30.Moore AM, Ray WZ, Chenard KE, Tung T, Mackinnon SE. Nerve Allotransplantation as it Pertains to Composite Tissue Transplantation. Hand (N Y) 2009;4:239–244. doi: 10.1007/s11552-009-9183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris PJ, Bradley JA, Doyal L, Earley M, Hagan P, Milling M, Rumsey N. Facial transplantation: a working party report from the Royal College of Surgeons of England. Transplantation. 2004;77:330–338. doi: 10.1097/01.TP.0000113810.54865.BE. [DOI] [PubMed] [Google Scholar]
- 32.Nanji SA, Hancock WW, Anderson CC, Adams AB, Luo B, Schur CD, Pawlick RL, Wang L, Coyle AJ, Larsen CP, Shapiro AM. Multiple combination therapies involving blockade of ICOS/B7RP-1 costimulation facilitate long-term islet allograft survival. Am J Transplant. 2004;4:526–536. doi: 10.1111/j.1600-6143.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- 33.Noble J, Munro CA, Prasad VS, Midha R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma. 1998;45:116–122. doi: 10.1097/00005373-199807000-00025. [DOI] [PubMed] [Google Scholar]
- 34.Ozkaynak E, Gao W, Shemmeri N, Wang C, Gutierrez-Ramos JC, Amaral J, Qin S, Rottman JB, Coyle AJ, Hancock WW. Importance of ICOS-B7RP-1 costimulation in acute and chronic allograft rejection. Nat Immunol. 2001;2:591–596. doi: 10.1038/89731. [DOI] [PubMed] [Google Scholar]
- 35.Ray WZ, Kasukurthi R, Papp EM, Moore AM, Yee A, Hunter DA, Solowski NL, Mohanakumar T, Mackinnon SE, Tung TH. The role of T helper cell differentiation in promoting nerve allograft survival with costimulation blockade. J Neurosurg. 2009 doi: 10.3171/2009.7.JNS09187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salama AD, Womer KL, Sayegh MH. Clinical transplantation tolerance: many rivers to cross. J Immunol. 2007;178:5419–5423. doi: 10.4049/jimmunol.178.9.5419. [DOI] [PubMed] [Google Scholar]
- 37.Schaffer MR, Fuchs N, Proksch B, Bongartz M, Beiter T, Becker HD. Tacrolimus impairs wound healing: a possible role of decreased nitric oxide synthesis. Transplantation. 1998;65:813–818. doi: 10.1097/00007890-199803270-00008. [DOI] [PubMed] [Google Scholar]
- 38.Udina E, Gold BG, Navarro X. Comparison of continuous and discontinuous FK506 administration on autograft or allograft repair of sciatic nerve resection. Muscle Nerve. 2004;29:812–822. doi: 10.1002/mus.20029. [DOI] [PubMed] [Google Scholar]
- 39.Wang MS, Zeleny-Pooley M, Gold BG. Comparative dose-dependence study of FK506 and cyclosporin A on the rate of axonal regeneration in the rat sciatic nerve. J Pharmacol Exp Ther. 1997;282:1084–1093. [PubMed] [Google Scholar]
- 40.Whitlock EL, Kasukurthi R, Yan Y, Tung TH, Hunter DA, Mackinnon SE. Fibrin glue mitigates the learning curve of microneurosurgical repair. Microsurgery. 2010;30:218–222. doi: 10.1002/micr.20754. [DOI] [PubMed] [Google Scholar]
- 41.Whitlock EL, Tuffaha SH, Luciano JP, Yan Y, Hunter DA, Magill CK, Moore AM, Tong AY, Mackinnon SE, Borschel GH. Processed allografts and type I collagen conduits for repair of peripheral nerve gaps. Muscle Nerve. 2009;39:787–799. doi: 10.1002/mus.21220. [DOI] [PubMed] [Google Scholar]
- 42.Yang RK, Lowe JB, 3rd, Sobol JB, Sen SK, Hunter DA, Mackinnon SE. Dose-dependent effects of FK506 on neuroregeneration in a rat model. Plast Reconstr Surg. 2003;112:1832–1840. doi: 10.1097/01.PRS.0000091167.27303.18. [DOI] [PubMed] [Google Scholar]
- 43.Yoshinaga SK, Whoriskey JS, Khare SD, Sarmiento U, Guo J, Horan T, Shih G, Zhang M, Coccia MA, Kohno T, Tafuri-Bladt A, Brankow D, Campbell P, Chang D, Chiu L, Dai T, Duncan G, Elliott GS, Hui A, McCabe SM, Scully S, Shahinian A, Shaklee CL, Van G, Mak TW, Senaldi G. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- 44.Zalewski AA, Gulati AK. Survival of nerve and Schwann cells in allografts after cyclosporin A treatment. Exp Neurol. 1980;70:219–225. doi: 10.1016/0014-4886(80)90022-9. [DOI] [PubMed] [Google Scholar]
- 45.Zhang QW, Rabant M, Schenk A, Valujskikh A. ICOS-Dependent and -independent functions of memory CD4 T cells in allograft rejection. Am J Transplant. 2008;8:497–506. doi: 10.1111/j.1600-6143.2007.02096.x. [DOI] [PubMed] [Google Scholar]
- 46.Zuo Y, Lubischer JL, Kang H, Tian L, Mikesh M, Marks A, Scofield VL, Maika S, Newman C, Krieg P, Thompson WJ. Fluorescent proteins expressed in mouse transgenic lines mark subsets of glia, neurons, macrophages, and dendritic cells for vital examination. J Neurosci. 2004;24:10999–11009. doi: 10.1523/JNEUROSCI.3934-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]