Abstract
Twelve patients developed herpes simplex (HSV) hepatitis a median of 18 days after solid organ transplantation. This is earlier than cytomegalovirus hepatitis, which usually occurs 30–40 days after transplantation. Eight recipients (67%) died, and in seven, the diagnosis was made at autopsy or <48 h before death. Clinical manifestations associated with mortality were hypotension, disseminated intravascular coagulation (DIC), metabolic acidosis, gastrointestinal bleeding, and bacteremia. Laboratory abnormalities at diagnosis associated with mortality were high creatinine, low platelet counts, prolonged partial thromboplastin time, and a high percentage of band forms on the blood smear. Disseminated HSV disease was noted in four of six patients who had an autopsy and included involvement of lungs in three and the gastrointestinal tract in three. Five recipients developed DIC and all died. Pathologically, HSV hepatitis has two forms, focal and diffuse. All three patients with diffuse liver pathology died. However, three of seven with focal liver pathology survived with antiviral therapy, which suggests that early diagnosis and treatment may be lifesaving. None of these patients had received prophylactic acyclovir. It is possible that acyclovir prophylaxis may be able to prevent this disease.
Herpes simplex virus (HSV) hepatitis is considered rare [1]. It has been observed most frequently as part of disseminated HSV in immunologically compromised patients or during pregnancy. Sporadic cases have been reported from this institution in renal transplant recipients and have ended fatally [2, 3], as have most cases reported in the literature. Here we report 12 cases that occurred after solid organ transplantation over a 9-year period (1980–1988). This series is large enough to allow us to determine the approximate frequency of HSV hepatitis in our transplantation population; to present the effect of viral dissemination, the nature of the liver pathology, and the range of clinical and laboratory characteristics of the disease; and to investigate prognostic factors.
Materials and Methods
Definitions
Cases of HSV hepatitis were diagnosed by laboratory, histologic, and clinical findings of one or more of the following in liver tissue from a biopsy or autopsy: isolation of HSV, positive immunoperoxidase staining for HSV antigen, or histology showing intranuclear inclusion bodies and pathology consistent with HSV lesions. In one case there was no liver tissue to examine, and the diagnosis was made by isolation of HSV from multiple sites outside the liver, including buffy coat, and clinical evidence of fulminant hepatitis. The histologic pattern of liver involvement with HSV was used to classify hepatitis as focal or diffuse. Disseminated HSV disease was defined as involvement of two or more organs discovered at autopsy. Involvement of skin confined to the oral or genital areas alone was not considered to represent dissemination. Disseminated intravascular coagulation (DIC) was defined by clinical evidence of bleeding from multiple sites, thrombocytopenia, and prolongation of prothrombin time (PT) and partial thromboplastin time (PTT).
Laboratory methods
Tissue specimens obtained by biopsy or at autopsy were examined after routine staining and processing for detection of HSV antigen by immunoperoxidase staining and, on occasion, processed for isolation of HSV. Other specimens obtained from transplant recipients for viral isolation included throat wash, urine, buffy coat, bronchial secretions, and tissue. Specimens were inoculated onto tube monolayers of human embryonic kidney cells and observed for cytopathic effect typical of HSV. In some cases, the viral isolates were typed by direct immunofluorescence with monoclonal antibodies specific for HSV-1 and HSV-2 (Syva; Genetic Systems, Seattle).
When available, sera from recipients and donors were assayed for neutralizing antibody against HSV or for specific antibody against HSV-1 or HSV-2 using the type-specific glycoproteins gG1 and gG2 in an immunodot test adapted from methods described elsewhere [4, 5].
Statistical analysis
The χ2 test was used for analysis of proportional differences between dichotomous variables.
Results
Case identification
Cases were identified by computer search for HSV isolates in liver tissue from either biopsies or autopsies from the virology laboratory and by search of pathology records for HSV hepatitis. As shown in table 1, the liver biopsy or autopsy specimens in 10 of 11 cases stained positive for HSV by immunoperoxidase; intranuclear inclusion bodies were seen in 7 of the 10 specimens. One case was diagnosed by isolation of HSV from the liver biopsy specimen. The one case without a liver biopsy was diagnosed by liver function test abnormalities showing fulminant hepatitis, DIC, and isolation of HSV from the buffy coat, urine, and throat.
Table 1.
Selected demographic, pathologic, and other characteristics of patients with herpes simplex virus hepatitis
| Outcome, patient | Type of transplant | Sex/age | Days diagnosed after transplant (retransplant) | Method of diagnosis in liver (type of specimen) | Other sites of involvement | DIC | Liver pathology | Dissemination | Start of acyclovir treatment | Type of infection |
|---|---|---|---|---|---|---|---|---|---|---|
| Death | ||||||||||
| 1 | Heart | M/45 | 2 years | Immunoperoxidase (autopsy) | Lung, pancreas, adrenals, esophagus, stomach, throat | Yes | Focal | Yes | None | Reactivation |
| 2 | Liver | F/26 | 18 | Immunoperoxidase (autopsy) | Lung, larynx, trachea, bladder | Yes | Diffuse | Yes | None | Reactivation |
| 3 | Kidney | M/47 | 15 | Isolation, immunoperoxidase (autopsy) | Spleen, esophagus | Yes | Diffuse | Yes | None | Primary |
| 4 | Kidney | M/38 | 20 | Isolation, immunoperoxidase (autopsy) | Buffy coat, throat | Yes | Diffuse | No | 48 h before death | Primary |
| 5 | Kidney | M/21 | 16 | ND | Buffy coat, throat, urine | Yes | ND | NA | Day of death | Primary |
| 6 | Liver | M/36 | 21 (4) | Immunoperoxidase (biopsy) | Thigh | No | Focal | NA | 4 weeks before death (for 2 weeks) | Reactivation |
| 7 | Liver | F/42 | 46 (16) | Immunoperoxidase (autopsy) | Throat, urine | No | Focal | No | None | Reactivation |
| 8 | Liver | F/51 | 25 | Immunoperoxidase (autopsy) | Lung, larynx, trachea, esophagus, stomach, adrenals | No | Focal | Yes | None | Reactivation |
| Survival | ||||||||||
| 9 | Liver | F/24 | 30 | Immunoperoxidase (biopsy) | ND | No | Focal | NA | At diagnosis | Reactivation |
| 10 | Liver | F/26 | 5 | Isolation, immunoperoxidase (biopsy) | ND | No | Focal | NA | At diagnosis | Reactivation |
| 11 | Liver | F/54 | 14 | Immunoperoxidase (biopsy) | Throat, thigh | No | Focal | NA | At diagnosis | Primary |
| 12 | Liver | F/29 | 6 | Isolation (biopsy) | ND | No | Negative | NA | At diagnosis | Reactivation |
NOTE. DIC = disseminated intravascular coagulation; ND = not done; NA = not ascertainable.
Patient demographics and frequency of HSV hepatitis
Twelve cases of HSV hepatitis occurred in solid organ transplant recipients: eight were liver recipients, three were kidney recipients, and one was a heart recipient (table 1). These cases were diagnosed between 1980 and September 1988, during which period 3536 liver, kidney, and heart transplant operations were done. The overall frequency of HSV hepatitis was 0.3% (table 2) and did not differ among kidney, heart, and liver recipients. The male-to-female ratio was 5:7, and the mean age was 37 years (range, 21–54).
Table 2.
Incidence of herpes simplex virus (HSV) hepatitis after organ transplantation in Pittsburgh, 1980–1988.
| Type of transplant | Total no. of patients | No. of patients with HSV hepatitis | Frequency (cases per thousand) |
|---|---|---|---|
| Kidney | 1423 | 3 | 2.11 |
| Heart | 449 | 1 | 2.23 |
| Liver | 1664 | 8 | 4.81 |
| Total | 3536 | 12 | 3.39 |
Viral isolation
Of 12 cases, 8 had HSV isolated antemortem or at autopsy. Of six liver specimens sent for viral isolation, four (67%) yielded HSV. Between 1 and 13 specimens obtained from these cases were submitted for viral isolation. Seventy-nine specimens (liver biopsy culture, throat culture, urine culture, and others) were taken within 60 days of transplantation, and 17 (22%) of these were positive for HSV. The time of onset of HSV infection after transplantation could be assessed for eight recipients, and six had positive viral cultures within 20 days of transplantation. This is consistent with our previous experience that HSV shedding, HSV mucocutaneous lesions, and dissemination usually occur early after transplantation [2, 3, 6].
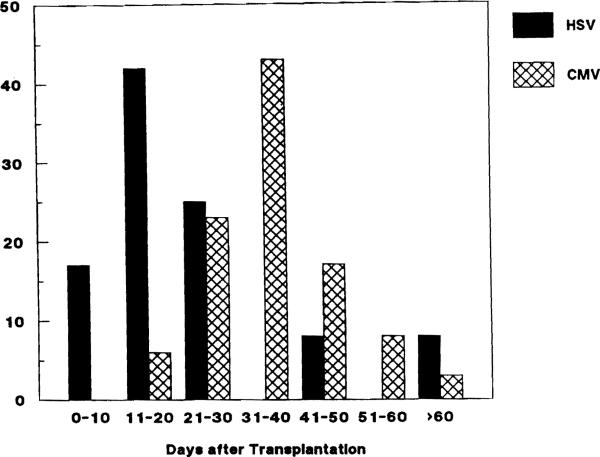
HSV hepatitis occurred as early as 5 days after transplantation (median, 18; range, 5–46). In one patient, however, HSV hepatitis occurred >2 years after transplantation. The infection appeared to have spread from an occult focus of HSV esophagitis while the patient was intubated in the intensive care unit. Thus, some small risk of HSV hepatitis persists long after transplantation. The usual timing of HSV hepatitis is earlier than cytomegalovirus (CMV) hepatitis, which peaks 30–40 days after transplantation (figure 1). The 35 cases of CMV hepatitis shown occurred at our institution between July 1987 and September 1988 (unpublished data). The cases were diagnosed by histology of liver biopsies showing inclusion bodies suggestive of CMV and isolation of CMV from at least one site. The liver biopsies were usually done to investigate elevation of liver enzymes.
Figure 1.
Diagnosis of herpes simplex virus (HSV) hepatitis (12 patients) and cytomegalovirus (CMV) hepatitis (35 patients) by days after transplantation.
Dissemination of HSV and extent of liver lesions
Visceral dissemination of HSV could be ascertained only at autopsy. Four (67%) of the six patients with autopsies had visceral disseminated HSV disease of two or more organs. Evidence of HSV was found in the lungs of three patients; two had concomitant involvement of the larynx and trachea, and two had esophageal involvement. These cases may represent contiguous spread of HSV to the lung [7]. Other organs involved included stomach, pancreas, adrenals, spleen, and bladder. Of note, three of four patients with disseminated HSV infection had overwhelming disease accompanied by DIC; only two of these had diffuse HSV hepatitis. Conversely, one patient with diffuse hepatitis had DIC but did not have dissemination at autopsy.
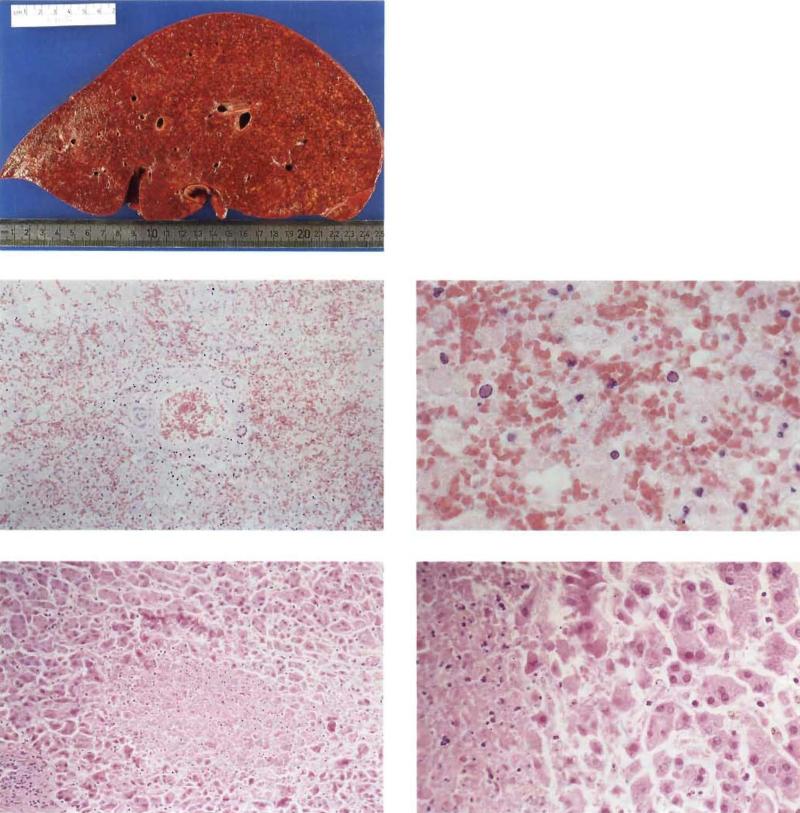
Of ten cases with histologic evidence of HSV hepatitis, three had diffuse involvement of the liver and seven had only focal involvement. All patients with diffuse hepatitis died, whereas three of seven with focal hepatitis survived. The pathology of focal and diffuse HSV hepatitis is illustrated in figure 2.
Figure 2.
Liver specimens showing diffuse and focal hepatitis. Left, macroscopic specimen (2900 g) with diffuse necrosis due to herpes simplex virus (HSV) hepatitis (patient 4, table 1). Middle and bottom: micrographs of hematoxylin- and eosin-stained sections. Middle left and right: section of above specimen showing diffuse HSV hepatitis of the portal zone (original magnification, ×42) and typical intranuclear herpetic inclusions (×170) , respectively. Bottom left and right: liver biopsy (patient 8, table 1) showing focal HSV hepatitis (×42) and typical intranuclear herpetic inclusions (×110), respectively.
Type of HSV infection
Of the 12 recipients, 8 (67%) were seropositive for HSV before transplantation and thus either developed reactivation of HSV infection or were reinfected (table 1). The other 4 (33%) were seronegative for HSV before transplantation and seemed to have primary infection.
We were able to type the viral isolates from five patients; HSV-1 was recovered from patient 3 and HSV-2 was recovered from patients 4,5, 10, and 12. However, patient 11, who was seronegative for both HSV-1 and HSV-2 before transplantation, acquired antibody to HSV-2 after transplantation. Since the donor was seropositive only for HSV-2, it is possible the infection was acquired from the donor organ, as has been previously reported [3]. Patient 2, who was seronegative for HSV-1 but seropositive for HSV-2 before transplantation, acquired antibody to HSV-1 after transplantation. It is possible that this infection was also acquired from the donor organ, since the donor was seropositive for both HSV-1 and HSV-2.
Clinical manifestations
The most common laboratory and clinical findings on the day of diagnosis were fever (temperature >38.5°C on one or more occasions before or on the day of diagnosis) in 10, abnormal chest radiograph in 11, increased percentage of band forms (>5% of total white count) in 11, elevated liver enzymes (serum aspartate aminotransferase [SGOT] and serum alanine aminotransferase [SGPT] >40 IU/l) in 10, elevated bilirubin (>1.5 mg/dl) in 11, thrombocytopenia (<100,000 platelets/ml) in 9, and abdominal pain and tenderness in 10. Patients were febrile 1-19 days (mean, 6) before the diagnosis was established. The chest radiologic changes (pleural effusion, atelectasis, consolidation) were not necessarily related to HSV pneumonia. Of three patients with histologic evidence of HSV in the lung, chest radiographs showed patchy bilateral infiltrates in one, left lower lobe density in one, and bilateral pleural effusions in one.
Three patients had skin lesions caused by HSV, one of whom died. Patient 1 had multiple ulcerated skin lesions distributed on the chest and extremities, as well as disseminated HSV at autopsy. The other three patients with evidence of disseminated HSV at autopsy did not have herpetic skin lesions. Patients 6 and 11 had vesicular lesions on their thighs from which HSV was isolated.
Eight patients (67%) died, and in seven the diagnosis was made either at autopsy or within 24–48 h of death when the patient was moribund. Table 3 shows the clinical and laboratory findings associated with death. The laboratory abnormalities that were more common in patients who died were a low platelet count, a high percentage of band forms on the blood smear, prolonged PTT (but not prolonged PT), and a high creatinine level at diagnosis. Granulocytosis (>85% neutrophils) was prominent in all patients, and the mean number of neutrophils at time of diagnosis was 7655 cells/mm3. Nine patients had platelet counts <100,000/mm3 at diagnosis (mean, 70,100/mm3). Increased levels of SGOT and SGPT were not associated with increased mortality but were markedly elevated in most patients. The median level for SGOT was 366 IU/l (range, 37–6343) and for SGPT was 175 IU/l (range 33–3865). Only two patients had normal liver enzymes (table 1, patients 7 and 12). HSV was isolated from a liver biopsy in patient 12 but there was no pathology of the liver, and patient 7 had focal hepatitis diagnosed at autopsy.
Table 3.
Laboratory and clinical data associated with mortality in 12 cases of herpes simplex virus hepatitis.
| No. of patients who died/no. with laboratory value |
|||||
|---|---|---|---|---|---|
| Data | Normal value | Median (range) for patients | ≤ median | > median | P |
| Laboratory | |||||
| No. platelets | 150,000–450,000 | 45,000 (14,000–210,000) | 6/6 | 2/6 | <.01 |
| % band forms | <5% | 25% (4%–45%) | 2/6 | 6/6 | <.02 |
| Partial thromboplastin time | 18–32 s | 38 (20.1–120) | 3/7 | 5/5 | <.05 |
| Creatinine | 0.5–1.4 mg/dl | 3.5 (0.7–6.4) | 2/6 |
6/6 |
<.02 |
| No. of patients who died/no. of patients |
|||
|---|---|---|---|
| With finding |
Without finding |
P |
|
| Clinical | |||
| Episodes of hypotension (systolic blood pressure <80 mm Hg) | 7/7 | 1/5 | <.01 |
| Metabolic acidosis (pH <7.3) | 6/6 | 2/6 | <.02 |
| Gastrointestinal bleeding (requiring blood transfusions) | 6/6 | 2/6 | <.02 |
| Coagulopathy (disseminated intravascular coagulation) | 5/5 | 3/7 | <.05 |
| Bacteremia | 6/6 | 2/6 | <.02 |
It is notable that all patients who had hypotension, metabolic acidosis, gastrointestinal bleeding, DIC, and bacteremia died (table 3). Seven of eight patients with renal failure (creatinine >1.5 mg/dl) died. The degree of renal failure at diagnosis was correlated with mortality and was multifactorial. The main contributory causes were hypotension, renal toxicity due to cyclosporine, and hepatorenal syndrome.
Immunosuppression
All patients were maintained on cyclosporine and steroids. Five of the 11 who developed HSV hepatitis within 2 months of transplantation received OKT3, and 4 died. Three of six patients without OKT3 treatment died (P > .05).
Discussion
HSV hepatitis is one of the few emergencies in infectious diseases. If not diagnosed in time, it may lead quickly to DIC and death. Sporadic case reports have described fulminant hepatitis in both immunocompromised [8–13] and normal hosts [14–19]. We present here 12 cases of herpes hepatitis from one institution that occurred in liver, heart, or kidney transplant recipients. Typically, the course of the disease was rapid and the diagnosis was difficult. Seven of eight patients who died were not diagnosed until autopsy or within 24–48 h before their deaths. The four patients who survived were diagnosed relatively early and responded to treatment with acyclovir. No patient in this series had received acyclovir prophylaxis.
There were two distinct groups of patients in this series as defined by diffuse or focal involvement of the liver. The only way to distinguish diffuse from focal HSV hepatitis is by biopsy. Diffuse hepatitis was always fatal and was associated with diffuse necrosis of the liver, coagulopathy, and DIC (patients 2–4). Most patients with focal hepatitis also died, particularly if they had DIC or dissemination, which was diagnosed at autopsy. The difference in pathogenesis between focal and diffuse hepatitis is unknown. It is unclear whether diffuse hepatitis always starts with focal infiltration that, if recognized in time, can be cured with antiviral therapy or whether there was some basic pathogenetic difference between the two groups of patients. However, the primary cause of death, at least in the first group of patients, clearly was massive involvement of the liver leading to severe coagulopathy and liver failure.
Lethal HSV hepatitis, coagulopathy, and DIC have been reported in both neonates and adults [20, 21]. In our series, five patients (42%) developed DIC, and all died. Other clinical situations associated with mortality were episodes of hypotension, metabolic acidosis, bacteremia, gastrointestinal bleeding, and renal failure. Several reported cases of HSV hepatitis with hypotension and hemodynamic instability were found at autopsy to have adrenal necrosis [22, 23]. In our series, two patients had diffuse hepatitis and coagulative necrosis with inclusion bodies in the adrenals (patients 1 and 8). Although herpetic skin lesions help in diagnosis, they were the exception rather than the rule, and the clinician should not rely on their presence [14, 24]. Only one patient had herpetic skin lesions over the trunk and limbs (patient 1); he also had disseminated disease and died.
When a clinician notes a transplant recipient who has fever, progressive transaminase elevation, abdominal symptoms, and coagulopathy in the first month after transplantation, herpes hepatitis should be considered [14]. The empiric use of intravenous acyclovir in such patients, pending the results of a liver biopsy and viral cultures, seems warranted and may be lifesaving [25].
Bacteremia, which is common after liver transplantation [26], occurred in 50% of the patients and was correlated with mortality. Other laboratory abnormalities associated with a fatal outcome were platelet counts below the median of 45,000/mm3 and values above the medians for creatinine (3.5 mg/dl), PTT (38 s), and percentage of band forms (25%) at diagnosis. Elevated liver enzymes at diagnosis did not correlate with mortality.
A comparison of CMV hepatitis with HSV hepatitis is important because both occur almost exclusively in the early posttransplant period. The clinical pictures of these diseases are distinct; CMV hepatitis is usually “smoldering” and rarely fulminant [27]. Also, with CMV hepatitis, one usually finds a low white blood cell count with atypical lymphocytosis and no leukocytosis or shift to the left. While CMV hepatitis is usually seen toward the end of the first and beginning of the second month after transplantation, HSV hepatitis usually occurs earlier. The earliest case in this series occurred only 5 days after transplantation.
The mortality from primary HSV infection in seronegative patients was 75% (3/4) and in seropositive patients was 63% (5/8). The difference was not statistically significant. The development of fulminant disease was more frequent in seronegative patients (3/4) than in seropositive patients (2/8). However, this study shows that HSV hepatitis can occur as a reactivation infection. This is important because most HSV infections after transplantation represent reactivation of latent virus [6]. We found that 78% of patients in a large series of liver transplant recipients were seropositive for HSV before transplantation [28].
The role of transmission has been addressed previously at this institution. One case was a 47-year-old man who developed fulminant HSV-1 hepatitis with disseminated intravascular coagulation 12 days after cadaveric renal transplantation (patient 3, table 1). We were unable to implicate the transplanted kidney as the source of HSV infection because both the donor and the recipient before transplantation were seronegative for HSV [2]. More recently, we have documented transmission of HSV from donor organ to recipient [3, 29]. In one instance [29], two renal transplant recipients had a common donor and both developed primary disseminated skin infection with HSV-2. In the second instance [3], two recipients of cadaveric renal transplant developed a fatal fulminant HSV-2 hepatitis. In both instances, the viral isolates from recipients were identical by restriction endonuclease polymorphism pattern. Thus the infections were assumed to arise from a common source, the donor kidney. The donors were seropositive for both HSV-1 and HSV-2.
Prophylaxis against HSV infection after kidney and marrow transplantations has been shown to be effective [30, 31]. We instituted acyclovir prophylaxis in our kidney and liver transplant recipients in February 1989. No additional cases of HSV hepatitis have been seen since the last case of this series in July 1988. Thus, between October 1988 and April 1990 1144 additional transplant operations were done in Pittsburgh, and there were no cases of HSV hepatitis. These data offer support for the use of acyclovir prophylaxis in the early posttransplant period.
Acknowledgment
We thank Lucille Guevarra for HSV antibody assays and Betty Edwards for secretarial assistance.
Grant support: National Institutes of Health (AI-19377).
References
- 1.Chase RA, Pottage JC, Jr, Haber MH, Kistler G, Jensen D, Levin S. Herpes simplex viral hepatitis in adults: two case reports and review of the literature. Rev Infect Dis. 1987;9:329–33. doi: 10.1093/clinids/9.2.329. [DOI] [PubMed] [Google Scholar]
- 2.Taylor RJ, Saul SH, Dowling JN, Hakala TR, Peel RL, Ho M. Primary disseminated herpes simplex infection with fulminant hepatitis following renal transplantation. Arch Intern Med. 1981;141:1519–21. [PubMed] [Google Scholar]
- 3.Koneru B, Tzakis AG, Depuydt LE, et al. Transmission of fatal herpes simplex infection through renal transplantation. Transplantation. 1988;45:653–6. doi: 10.1097/00007890-198803000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee FK, Coleman RM, Pereira L, Bailey PD, Tatsuno M, Nahmias AJ. Detection of herpes simplex virus type 2-specific antibody with glycoprotein G. J Clin Microbiol. 1985;22:641–4. doi: 10.1128/jcm.22.4.641-644.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee FK, Pereira L, Griffin C, Reid E, Nahmias AJ. A novel glycoprotein for detection of herpes simplex virus type 1-specific antibodies. J Virol Methods. 1986;14:111–8. doi: 10.1016/0166-0934(86)90041-8. [DOI] [PubMed] [Google Scholar]
- 6.Pass RF, Whitley RJ, Whelchel JD, Diethelm AG, Reynolds DW, Alford CA. Identification of patients with increased risk of infection with herpes simplex virus after renal transplantation. J Infect Dis. 1979;140:487–92. doi: 10.1093/infdis/140.4.487. [DOI] [PubMed] [Google Scholar]
- 7.Ramsey PG, Fife KH, Hackman RC, Meyers JD, Corey L. Herpes simplex virus pneumonia. Clinical, virologic, and pathologic features in 20 patients. Ann Intern Med. 1982;97:813–20. doi: 10.7326/0003-4819-97-6-813. [DOI] [PubMed] [Google Scholar]
- 8.Montgomerie JZ, Becroft DMO, Croxson MC, Doak PB, North JDK. Herpes simplex virus infection after renal transplantation. Lancet. 1969;2:867–71. doi: 10.1016/s0140-6736(69)92327-7. [DOI] [PubMed] [Google Scholar]
- 9.Lee JC, Fortuny IE. Adult herpes simplex hepatitis. Hum Pathol. 1972;3:277–81. doi: 10.1016/s0046-8177(72)80081-9. [DOI] [PubMed] [Google Scholar]
- 10.Elliott WC, Houghton DC, Bryant RE, Wicklund R, Barry JM, Bennett WM. Herpes simplex type 1 hepatitis in renal transplantation. Arch Intern Med. 1980;140:1656–60. [PubMed] [Google Scholar]
- 11.Anuras S, Summers R. Fulminant herpes simplex hepatitis in an adult: report of a case in renal transplant recipient. Gastroenterology. 1976;70:425–8. [PubMed] [Google Scholar]
- 12.Holdsworth SR, Atkins RC, Scott DF, Hayes K. Systemic herpes simplex infection with fulminant hepatitis post-transplantation. Aust N Z J Med. 1976;6:588–90. doi: 10.1111/j.1445-5994.1976.tb04002.x. [DOI] [PubMed] [Google Scholar]
- 13.Walker DP, Longson M, Lawler W, Mallick NP, Davies JS. Disseminated herpes simplex virus infection with hepatitis in an adult renal transplant recipient. J Clin Pathol. 1981;34:1044–6. doi: 10.1136/jcp.34.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connor RW, Lorts G, Gilbert DN. Lethal herpes simplex virus type 1 hepatitis in a normal adult. Gastroenterology. 1979;76:590–4. [PubMed] [Google Scholar]
- 15.Rubin MH, Ward DM, Painter CJ. Fulminant hepatic failure caused by genital herpes in a healthy person. JAMA. 1985;253:1299–301. [PubMed] [Google Scholar]
- 16.Young EJ, Killam AP, Greene JR., Jr Disseminated herpes virus infection associated with primary genital herpes in pregnancy. JAMA. 1976;235:2731–3. doi: 10.1001/jama.235.25.2731. [DOI] [PubMed] [Google Scholar]
- 17.Eron L, Kosinski K, Hirsch MS. Hepatitis in an adult caused by herpes simplex virus type 1. Gastroenterology. 1976;71:500–4. [PubMed] [Google Scholar]
- 18.Goyette RE, Donowho EM, Jr, Hieger LR, Plunkett GD. Fulminant herpesvirus hominis hepatitis during pregnancy. Obstet Gynecol. 1974;43:191–6. [PubMed] [Google Scholar]
- 19.Werthein RA, Brooks BJ, Jr, Rodriguez FH, Jr, Lesesne HR, Jennette JC. Fatal herpetic hepatitis in pregnancy. Obstet Gynecol. 1983;62:38S–42S. [PubMed] [Google Scholar]
- 20.Miller DR, Hanshaw JB, O'Leary DS, Hnilicka JV. Fatal disseminated herpes simplex virus infection and hemorrhage in the neonate. J Pediatr. 1970;76:409–15. doi: 10.1016/s0022-3476(70)80481-4. [DOI] [PubMed] [Google Scholar]
- 21.Keane JT, Malkinson FD, Bryant J, Levin S. Herpesvirus hominis hepatitis and disseminated intravascular coagulation. Arch Intern Med. 1976;136:1312–7. [PubMed] [Google Scholar]
- 22.Joseph TJ, Vogt PJ. Disseminated herpes with hepatoadrenal necrosis in an adult. Am J Med. 1974;56:735–9. doi: 10.1016/0002-9343(74)90643-3. [DOI] [PubMed] [Google Scholar]
- 23.Francis TI, Osuntokun BO, Kemp GE. Fulminant hepatitis due to herpes hominis in an adult human. Am J Gastroenterol. 1972;57:329–32. [PubMed] [Google Scholar]
- 24.Berglin E, Blohme I, Frisk B, Hedman L, Jeansson S, Brynger H. A case of lethal herpes simplex hepatitis in a diabetic renal transplant recipient. Transplant Proc. 1982;14:765–9. [PubMed] [Google Scholar]
- 25.Lagrew DC, Jr, Furlow TG, Hager WD, Yarrish RL. Disseminated herpes simplex virus infection in pregnancy. Successful treatment with acyclovir. JAMA. 1984;252:2058–9. [PubMed] [Google Scholar]
- 26.Kusne S, Dummer JS, Singh N, et al. Infections after liver transplantation. An analysis of 101 consecutive cases. Medicine. 1988;67:132–43. doi: 10.1097/00005792-198803000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bronsther O, Makowka L, Jaffe R, et al. Occurrence of cytomegalovirus hepatitis in liver transplant patients. J Med Virol. 1988;24:423–34. doi: 10.1002/jmv.1890240409. [DOI] [PubMed] [Google Scholar]
- 28.Singh N, Dummer JS, Kusne S, et al. Infections with cytomegalovirus and other herpesviruses in 121 liver transplant recipients: transmission by donated organ and the effect of OKT3 antibodies. J Infect Dis. 1988;158:124–31. doi: 10.1093/infdis/158.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dummer JS, Armstrong J, Somers J, et al. Transmission of infection with herpes simplex virus by renal transplantation. J Infect Dis. 1987;155:202–6. doi: 10.1093/infdis/155.2.202. [DOI] [PubMed] [Google Scholar]
- 30.Pettersson E, Hovi T, Ahonen J, et al. Prophylactic oral acyclovir after renal transplantation. Transplantation. 1985;39:279–81. doi: 10.1097/00007890-198503000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Gold D, Corey L. Acyclovir prophylaxis for herpes simplex virus infection. Antimicrob Agents Chemother. 1987;31:361–7. doi: 10.1128/aac.31.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]