Abstract
Twenty-six adult patients with preformed IgG donor lymphocytotoxic antibodies received primary liver allografts under FK 506 immunosuppression. The effect of the crossmatch-positive state on early graft function and on the immunopathological and histo-pathological findings was compared with that of 52 crossmatch-negative control recipients. The presensitized (Crossmatch-positive) patients had prolongation of early graft dysfunction, underwent more clinically indicated biopsies and had a higher incidence of cellular rejection, both overall (p < 0.05) and within 10 days of transplantation (p < 0.01). They also had a higher incidence of graft failure in the first 180 days (p < 0.01). Hyperacute rejection with necrotizing or neutrophilic arteritis was not seen in the crossmatch-positive grafts. However, histological findings associated with presensitization included platelet margination in central veins and sinusoids in biopsy specimens 60 to 90 min after graft revascularization. Later biopsy specimens had neutrophilic portal venulitis followed by cholangiolar proliferation, acute cholangiolitis and centrilobular hepatocyte swelling that mimicked preservation injury, endothelial activation of arteries with medial changes and relapsing episodes of acute cellular rejection. These clinicopathological observations suggest that lymphocytotoxic antibodies can have a deleterious effect on liver allograft function and survival, even if they do not precipitate immediate or hyperacute rejection. (Hepatology 1992;16:671–681.)
Although the liver is known to be more resistant than other solid organs to injury from preformed graft antibodies in the recipient (1–3), this privileged state is not absolute (4, 5). Identification of the consequences of humoral antibody states on the liver has been hampered by the lack of distinctive pathological findings in many cases in which humoral rejection was suspected but was not proved. Consequently, in this study of liver recipients with preformed donor lymphocytotoxic antibodies, we have attempted to determine whether a unique, pathologically identifiable form of graft injury could be recognized and whether pathophysiological mechanisms of liver allograft injury could be deduced. A similar study on the pathological nature of ABO-mismatched livers in which the graft antibodies were isoagglutinins was published recently (6).
MATERIALS AND METHODS
Patient Selection
During the 11-mo period between November 31, 1989, and September 9, 1990, 243 adult patients ( > 16 yr) were given primary liver allografts under FK 506 and low-dose steroid therapy. The sera of 26 (11%) contained donor lymphocytotoxic antibodies. The crossmatch-negative control patients (n = 52) were those treated just before and after the crossmatch-positive cases. Most of these same cases were part of a recent clinical report (5). There were no statistically significant differences between the two cohorts with respect to age, United Network of Organ Sharing urgency of need status, original disease, donor demographic data or cold ischemic time (Table 1). More women had positive crossmatches (Table 1). The donor and recipient patients had the same ABO blood type in all cases.
Table 1.
Clinical data of crossmatch-positive patients and controls
| Characteristics | Crossmatch-Positive | Control |
|---|---|---|
| No. of patients | 26 | 52 |
| Age (yr)a (range) | 51.4 ± 9.4 (29–65) | 45.7 ± 14.0 (17–66) |
| Sex (M/F) | 11/15 | 29/23 |
| United Network of Organ Sharing classa | 3.7 ± 0.5 | 3.6 ± 0.7 |
| Blood type (A:B:AB:O) | 10:1:3:12 | 20:6:1:25 |
| Cold ischemic time (min)a (range) | 927 ± 278 (450–1631) | 838 ± 280 (265–1643) |
| Original disease | ||
| CAH/cirrhosis | 8 | 14 |
| Alcoholism | 5 | 13 |
| PBC | 4 | 4 |
| PSC | 3 | 3 |
| Biliary cirrhosis | 0 | 6 |
| AHN | 0 | 2 |
| Neoplasia | 3 | 2 |
| Others | 3 | 8 |
PSC = primary sclerosing cholangitis; AHN = acute hepatic necrosis; OLT = orthotopic liver transplantation.
Expressed as mean ± S.D.
Crossmatch Test
Recipient sera were obtained immediately before liver transplantation and tested for cytotoxic antibody activity against T lymphocytes isolated from donor lymph nodes at room temperature (37° C), followed by a 60 min incubation period with rabbit complement. Target cell lysis was determined by trypan blue exclusion. The crossmatch test was interpreted as positive when more than 50% of donor lymphocytes were killed. If the screening test was positive, the recipient serum was pretreated with dithiothreitol (DTT) for 30 min to inactivate the IgM antibodies, which have been shown to be less deleterious in kidney transplantation (7). The 26 crossmatch-positive results were after DTT treatment.
Clinical Follow-up, Immunosuppressive Regimen and Statistical Analysis
Clinical follow-up was to April 1, 1991 (Table 2), allowing potential follow-ups of 203 to 493 days for the crossmatch-positive cases and 188 to 515 days for the control patients. The clinical results have been reported in detail elsewhere (5), as have the management policies (8).
Table 2.
Clinicopathological summary of crossmatch-positive primary liver allograft recipients
| Case | OLT no. | Age (yr) | Sex | UNOS | ABO | Donor (age/sex/ABO) | CIT (hr/min) | Graft |
Patient |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| status | day | status | day | ||||||||
| 1 | 2190 | 55.0 | F | 2 | O | 15/F/O | 21:30 | Fail | 8 | Dead | 24 |
| 2 | 2205 | 54.7 | F | 3 | O | 37/F/O | 14:25 | Funct | 483 | Alive | 493 |
| 3 | 2206 | 47.8 | M | 4 | A | 40/M/A | 8:30 | Funct | 493 | Alive | 483 |
| 4 | 2228 | 65.3 | F | 4 | A | 47/F/A | 27:11 | Fail | 3 | Dead | 56 |
| 5 | 2254 | 58.6 | M | 4 | O | 25/M/O | 15:39 | Funct | 457 | Alive | 457 |
| 6 | 2260 | 33.6 | M | 4 | O | 51/M/O | 15:55 | Funct | 452 | Alive | 452 |
| 7 | 2281 | 59.9 | F | 4 | A | 61/M/A | 11:48 | Funct | 438 | Alive | 436 |
| 8 | 2284 | 58.0 | M | 3 | AB | 34/M/AB | 16:05 | Funct | 435 | Alive | 435 |
| 9 | 2290 | 55.3 | M | 4 | O | 21/M/O | 21:18 | Fail | 95 | Alive | 428 |
| 10 | 2308 | 29.5 | M | 3 | AB | 54/F/AB | 11:11 | Fail | 54 | Dead | 88 |
| 11 | 2311 | 65.1 | F | 3 | B | 53/F/B | 18:27 | Fail | 245 | Dead | 245 |
| 12 | 2319 | 58.3 | F | 4 | A | 30/F/A | 7:47 | Fail | 0 | Alive | 402 |
| 13 | 2363 | 42.2 | F | 4 | O | 30/F/O | 16:22 | Funct | 370 | Alive | 370 |
| 14 | 2372 | 46.5 | M | 4 | A | 43/F/A | 18:40 | Fail | 0 | Dead | 0 |
| 15 | 2387 | 43.5 | F | 4 | O | 22/M/O | 19:01 | Funct | 353 | Alive | 353 |
| 16 | 2394 | 83.7 | F | 4 | O | 18/M/O | 7:30 | Fail | 14 | Dead | 14 |
| 17 | 2427 | 47.9 | F | 4 | O | 18/M/O | 18:36 | Fail | 17 | Dead | 17 |
| 18 | 2440 | 53.5 | F | 3 | AB | 16/F/AB | 12:33 | Funct | 305 | Alive | 305 |
| 19 | 2460 | 50.9 | M | 4 | A | 47/F/A | 18:42 | Funct | 295 | Alive | 295 |
| 20 | 2461 | 63.0 | M | 4 | O | 54/M/O | 16:51 | Fail | 19 | Dead | 19 |
| 21 | 2472 | 51.1 | M | 4 | A | 10/M/A | 14:01 | Fail | 126 | Dead | 141 |
| 22 | 2491 | 53.6 | F | 4 | O | 11/M/O | 10:26 | Funct | 273 | Alive | 273 |
| 23 | 2495 | 49.7 | F | 4 | A | 37/F/A | 18:32 | Funct | 268 | Alive | 260 |
| 24 | 2522 | 43.7 | F | 4 | A | 21/M/A | 10:44 | Funct | 252 | Alive | 252 |
| 25 | 2538 | 44.0 | F | 3 | A | 50/M/A | 15:21 | Fail | 10 | Alive | 246 |
| 26 | 2592 | 37.6 | M | 4 | O | 22/M/O | 14:33 | Funct | 203 | Alive | 203 |
Fail = failed; Funct = functioning; NA = not available; CIT = cold ischemic time; UNOS = United Network of Organ Sharing; OLT = orthotopic liver transplantation.
Daily intravenous doses of 0.1 mg/kg of FK 506 and 20 mg methylprednisolone were begun during surgery. Starting daily oral doses were 0.30 mg/kg FK 506 and 20 mg prednisone. The prednisone was tapered after 2 weeks if the postoperative course was uncomplicated. Cellular rejection episodes were treated by increasing the maintenance dose of FK 506, if this was possible without nephrotoxicity. If necessary, the FK 506 adjustments were followed with a 1 gm bolus of intravenous methylprednisolone. Refractory rejection was treated with a 5-day course of high-dose methylprednisolone, starting at 200 mg with daily decrements of 40 mg. Nonresponsive patients were given a 5-day course of 10 mg/day of OKT-3, elevation of baseline steroids above 20 mg/day or both and the addition of azathioprine.
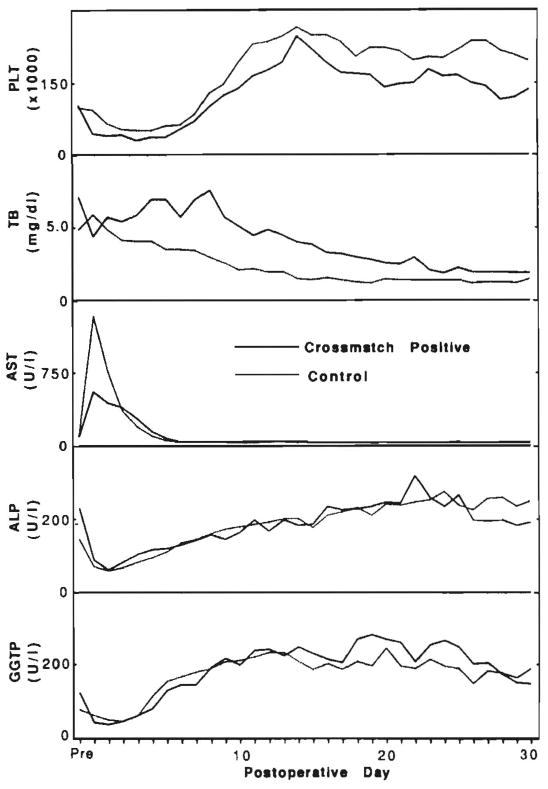
Laboratory parameters followed daily for 30 days were platelet counts, normal greater than 150 × 103/mm3; total bilirubin, normal less than 1.2 mg/dl; AST, normal less than 34 IU/L; alkaline phosphatase, normal less than 100 IU/L; and γ-glutamyl transpeptidase (GGTP), normal less than 40 IU/L. Patients with graft failure or death during the first 30 days were excluded from only the analyses of liver function and platelet counts. The results of these analyses were expressed as median values (see Fig. 1).
Fig. 1.
Postoperative platelet counts (PLT) and liver function test values for total bilirubin (TB), AST, alkaline phosphatase (ALP) and γ-glutamyl transpeptidase (GGTP). n = 18 for each point in the crossmatch-positive group and n = 46 for the crossmatch-negative patients.
The survival rates of grafts and patients were calculated by the life table method of Kaplan-Meier. Differences in survival curves were measured with the generalized Wilcoxon test. Statistical comparisons were made by Student’s t test and by χ2 analysis.
Routine and Immunopathological Studies
Liver allograft biopsy samples were obtained immediately before and 60 to 90 min after complete revascularization. Biopsies were subsequently performed when clinically indicated by an elevation of liver function test results, by changes in the color or quantity of bile production, or by the clinical suspicion that a problem in the graft was responsible for an unsatisfactory recovery. Specimens generated in the 26 crossmatch-positive cases included 7 failed allografts and 110 needle biopsy samples. In the 52 crossmatch-negative cases, there were 3 failed allografts and 191 needle biopsy specimens.
Histological sections were routinely cut at 4 μm and stained with hematoxylin and eosin. Selected sections were stained with trichrome and periodic acid–Schiff with diastase digestion. All slides were reviewed independently by two of us (A. J. D. and K. N.).
Portal tract inflammation, hepatocyte ballooning and sinusoidal neutrophilia were graded on a scale of 0 to 3. The composition of the infiltrate, when present, was labeled as polymorphonuclear, mononuclear, mixed, eosinophilic or other. Neutrophilic, mononuclear portal or central venulitis; neutrophilic, mononuclear or necrotizing arteritis; bile duct proliferation; platelet margination and thrombi; hepatocyte necrosis; and centrilobular congestion or hemorrhage were scored as present or absent. Equivocal findings were scored as negative. The distribution of necrosis was also noted. Any differences in the pathological assessment of the specimens were resolved by a joint review and consultation with other experienced pathologists.
The pathological specimens were divided into five postoperative periods (days 0 to 10, 11 to 20, 21 to 30, 31 to 60 and 61 to 120). All failed allograft tissue specimens were stained with a direct immunofluorescent (frozen tissue) method for the presence of IgG, IgM, 19A, Clq, C3, C4, α2-macroglobulin, transferrin and fibrinogen. Postreperfusion needle biopsy specimens from patients who lost their grafts because of suspected humoral rejection were stained for IgG, IgM, Clq and fibrinogen by use of an indirect immunoperoxidase technique on formalin-fixed, paraffin-embedded tissues.
Histometric Analysis of Portal Arteries
Previous studies of animal kidney allografts (9–11) and of human livers (6) have suggested that arterial spasm is an important pathophysiological mechanism of antibody-mediated graft damage. We reasoned that arteries in spasm should have an increased ratio of arterial wall thickness to vessel diameter. Therefore identical protocol sections (12) of all failed allograft specimens were blindly examined to assess the relationship between the wall thickness and the diameter of hepatic arteries. With a randomly selected area (200 mm2) of the identical hematoxylin and eosin slides, the size of the only or the largest artery in the portal tract was determined by measuring the shortest diameter of the vessel to the outer extent of the media (outer diameter) and to the internal elastic lamina (internal diameter). The thickness of the arterial wall was then calculated, and the results were expressed as a ratio.
RESULTS
Early Platelet Counts and Graft Function
Exclusive of the eight cases (31%) in which the grafts failed in fewer than 30 days, the peak AST levels immediately after transplantation in crossmatch-positive patients were actually lower than those in the control patients, of which six (17%) were excluded because of graft loss or patient death in fewer than 30 days (Fig. 1). This was partly a statistical artifact from the disproportionate culling of cases in the crossmatch-positive group. Median total bilirubin levels gradually declined in the crossmatch-negative patients throughout the 30 days, but two relative peaks were observed in the crossmatch-positive patients between days 5 and 10 and again at day 22 (Fig. 1). In the cases in which the grafts were retained for 30 days, the canalicular enzymes were similar in the two groups. As with the AST, the results were skewed by the more frequent omission from the analysis of 30-day failures in the crossmatch-positive cohort.
Peripheral platelet counts, which were similar in both groups before transplantation, were depressed in the crossmatch-positive patients relative to those of the control patients during the entire first 30 days (Fig. 1). Thrombocytopenia in presensitized liver recipients has been reported previously (13).
Incidence and Timing of Needle Biopsy Analysis
Exclusive of the intraoperative biopsies, which were uniformly distributed between the two groups, specimens were obtained significantly more frequently from the crossmatch-positive patients on postoperative days 1 to 10 (p < 0.01). Seventeen (77%) of these 26 patients provided 19 biopsy specimens during this interval vs. 19 (42%) of the 52 control patients from whom 21 biopsy specimens were obtained. The same difference was seen between days 21 and 30, corresponding to the secondary peak of allograft dysfunction in the sensitized patients. In addition to the increased frequency, the average time of the first postoperative biopsy was earlier in the crossmatch-positive patients (mean 9 ± 8 days; median 6 days) than in the control group (mean 14 ± 17 days; median 11 days; p < 0.05).
Only 25 patients in the combined groups were not subjected to biopsy during the first 14 days. Eight of these patients had early graft failure. In the other 17, the postoperative course was uneventful. Among these 17 patients with particularly benign courses, there were only two with a positive crossmatch. The first postoperative biopsies in these patients were on days 21 and 37. The other 15, who were crossmatch negative, had their first biopsy performed between days 15 and 30 (n = 11) or at day 120 (n = 4).
Biopsy Findings and Pathological Diagnoses
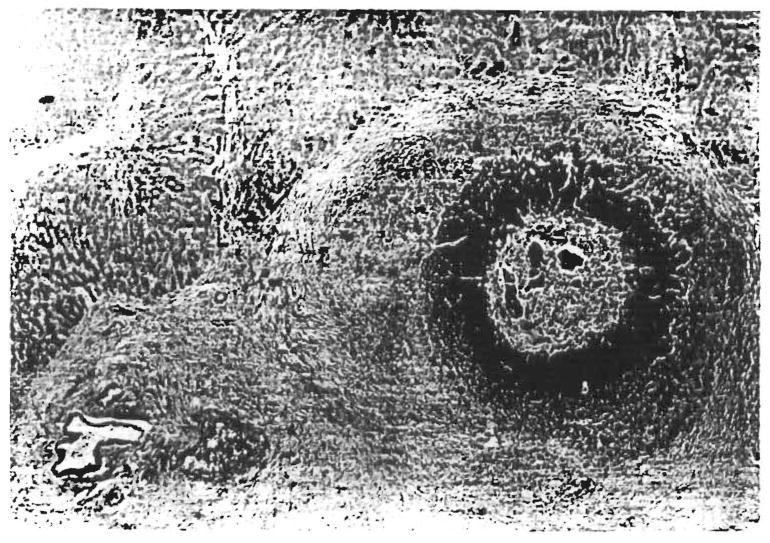
In the intraoperative biopsy specimens obtained after reperfusion, statistically significant differences were noted in the patients sensitized for vascular platelet aggregation (Fig. 2; 33% vs. 5%; p < 0.05). Later, cholangiolar proliferation (Fig. 3) was seen more frequently in the sensitized patients in the third time period (80% vs. 14%; p < 0.05), and portal venulitis, including but not limited to neutrophils (Fig. 4), was seen more frequently in the first time period (50% vs. 23%; p < 0.05). Neutrophilic or necrotizing arteritis with or without fibrinoid degeneration was not observed in any specimen.
Fig. 2.

Postreperfusion needle biopsy specimen from orthotopic liver transplantation no. 2284 demonstrating platelet margination along the central vein endothelium and in the lumen. This finding was most common in the crossmatch-positive patients immediately after reperfusion and was identical to that observed in sensitized rodents (Nakamura K, et al, Manuscript submitted, 1992). (H & E; original magnification × 768.)
Fig. 3.

Needle biopsy specimen of liver allograft from orthotopic liver transplantation no. 2309, 17 days after transplantation. This biopsy specimen was obtained 9 days after treatment of acute cellular rejection. Note the marked cholangiolar proliferation. Centrilobular hepatocyte swelling was also present. These two findings are often attributed to preservation or ischemic injury but were much more common in the crossmatch-positive patients despite similar preservation times. (H & E; original magnification × 192.)
Fig. 4.

Needle biopsy specimen of liver allograft from orthotopic liver transplantation no. 2284, 9 days after transplantation. Although portal localization of mononuclear cells has been emphasized as a component of cellular rejection, neutrophils, as a component of the population, were more common in the crossmatch-positive patients. (H & E; original magnification × 480.)
By use of previously published histological criteria (14–16), a higher percentage of crossmatch-positive patients had at least one biopsy specimen that demonstrated acute cellular rejection during the first 30 days compared with control patients. This difference was statistically significant (p < 0.05) during only the first 10 days. In addition, the first biopsy-proved diagnosis of acute cellular rejection occurred on day 9 ± 6 (median 6 days) in the crossmatch-positive patients compared with day 14 ± 6 in the control group (p < 0.05).
A scatter plot of the cold ischemic time vs. the severity of preservation injury indicated that during the first and second periods (p < 0.05), the crossmatch-positive cases more often demonstrated a more severe form of preservation injury as previously defined (12). This finding was true even though no difference was noted in the cold ischemic time for the two cohorts (Fig. 5).
Fig. 5.
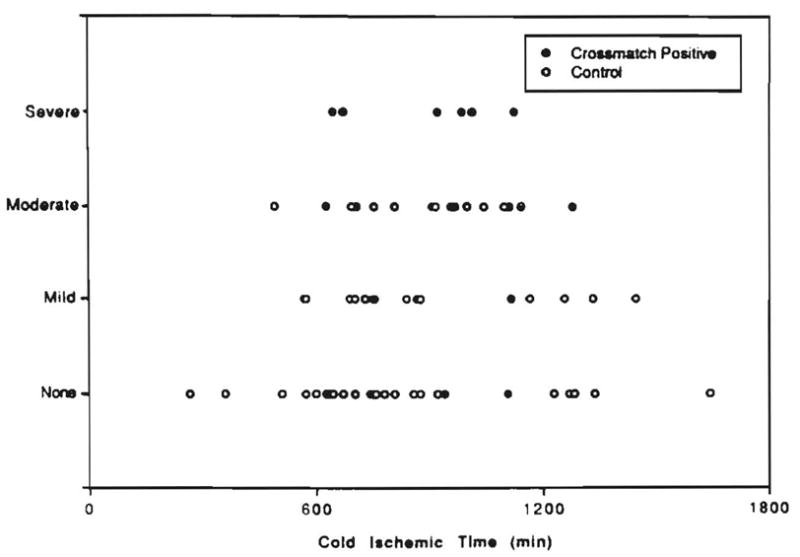
Relationship between the cold ischemic time and the severity of preservation injury present in allograft biopsy specimens.
Clinicopathological Correlations
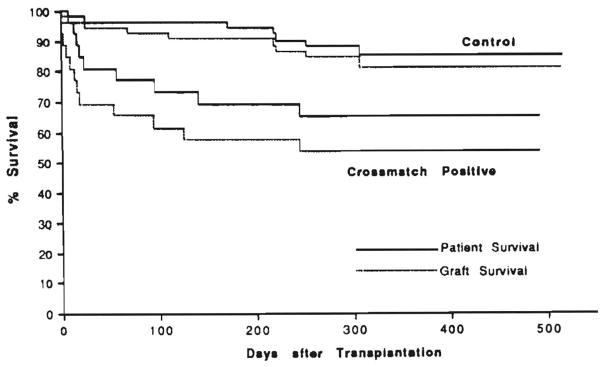
A statistically significant difference was seen for both primary graft (p < 0.01) and patient (p > 0.05) survival (Fig. 6). At the time this article was written (August 1991), 9 (35%) of the 26 crossmatch-positive patients and 7 (15%) of the 52 control patients had died.
Fig. 6.
Primary graft and patient survival curves for the crossmatch-positive patients vs. controls.
The pathological findings in grafts retrieved at the time of retransplantation were analyzed separately from those obtained at autopsy.
Grafts Retrieved at Retransplantation
In the crossmatch-negative group, 3 (6% of the original) grafts failed, leading to retransplantation within 180 days; the time of failure and retransplantation was 94 ± 87 days. By comparison 7 (27%) grafts in the crossmatch-positive group failed after a mean of 42 (±51) days.
The pathological findings in the failed allografts from both groups are summarized in Table 3. No correlation was detected between the underlying liver disease and graft failure. In the crossmatch-positive patients, all but two of the grafts showed portal inflammation with neutrophilia and cholangiolar proliferation. Focal large hilar bile duct, necrosis with biliary sludge (Fig. 7), or both and organized intrahepatic portal vein and arterial thrombi were also present in 5 of the 7 failed grafts from crossmatch-positive patients.
Table 3.
Pathological findings in failed liver allograft obtained at the time of retransplantation
| Patient no. (OLT no.) | Crossmatch | CIT (min) | Graft survival (days) | Pathological findings |
|---|---|---|---|---|
| 12 (2319) | Positive | 467 | 0 | Widespread coagulative necrosis with intrahepatic venular thrombi |
| 4 (2228) | Positive | 1631 | 3 | Arterial thrombosis; imperfect arterial anastomosis |
| 1 (2198) | Positive | 1298 | 6 | Severe predominantly neutrophilic and lymphoblastic portal infiltrate with focal intrahepatic vascular thrombi and infarcts |
| 25 (2538) | Positive | 921 | 10 | Hepatic artery thrombosis at anastomosis (technical); severe acute cellular rejection with prominent neutrophilia |
| 10 (2309) | Positive | 671 | 54 | Focal bile duct necrosis with prominent neutrophilic portal inflammation and biliary sludge |
| 9 (2290) | Positive | 1278 | 95 | Focal intrahepatic large bile duct necrosis with biliary sludge; recent and remote intrahepatic thrombi in portal veins; superimposed cytomegalo virus infection (rare) |
| 21 (2472) | Positive | 841 | 126 | Focal intrahepatic bile duct necrosis with organized intraarterial thrombi and small bile duct loss |
| 115 (2283) | Negative | 1843 | 2 | Portal vein thrombosis and focal hilar hepatic artery necrosis (technical) |
| 120 (2313) | Negative | 647 | 68 | Acute cellular rejection with centrilobular necrosis and fibrosis |
| 109 (2240) | Negative | 698 | 111 | Severe atherosclerosis of arterial graft with superimposed thrombosis |
CIT = cold ischemic time; OLT = orthotopic liver transplantation.
Fig. 7.
Section of hilum of failed liver allograft from orthotopic liver transplantation no. 2474. Note the bile duct necrosis with bile leakage and intraluminal biliary sludge. An artery with the characteristic changes described above is present in the lower left corner. (H & E; orginal magnification × 77.)
In the grafts from these sensitized patients, the most conspicuous vascular findings were in the arteries and peribiliary vascular plexus. Arterial findings included a thickened media with medial myocyte vacuolization and marked endothelial cell hypertrophy, at times with platelet margination coating the luminal surface (Fig. 8). Platelet margination was present in all but two of the grafts from sensitized patients but not in any of the controls. Occasionally, recanalized arterial thrombi also were seen but necrotizing or neutrophilic arteritis was not seen. Vascular channels in the peribiliary plexus were distended by macrophages, RBCs and fewer neutrophils. Nearby, large bile duct walls were focally necrotic and contained biliary sludge.
Fig. 8.
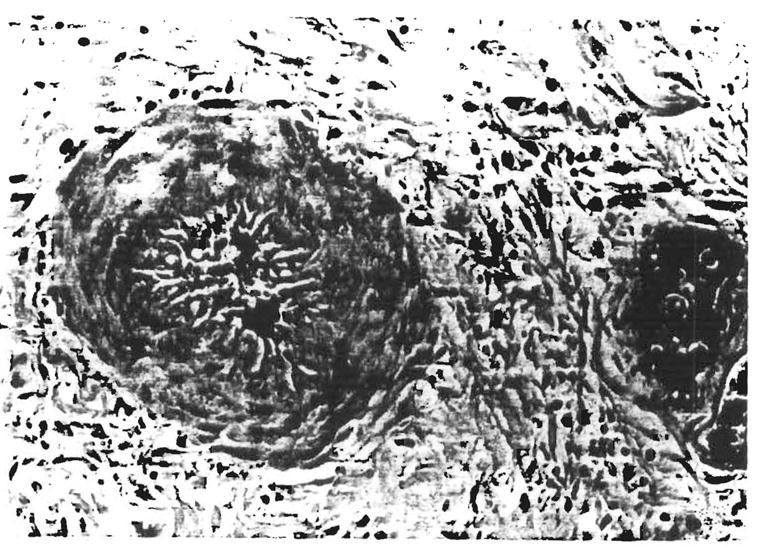
Section of failed liver allograft from orthotopic liver transplantation no. 2309, 54 days after transplantation. Note the marked medial thickening, luminal narrowing and medial myocyte vacuolization (indirect evidence of spasm). This finding was present in many, but not all, arteries in sections from the deep hilum of the liver. (H & E; original magnification × 480.)
Grafts Retrieved at Autopsy
Four patients underwent postmortem examination; three were in the crossmatch-positive group (two after retransplantation), and one was in the control group. The primary cause of death for each is listed in Table 4. No major differences in the causes of death were noted between the two groups.
Table 4.
Cause of death
| Patient no. (OLT no.) | Crossmatch | Patient survival (days) | Graft in place at time of death | Cause of death |
|---|---|---|---|---|
| 14 (2372) | Positive | 0 | First | Surgical complicationa |
| 16 (2394) | Positive | 14 | First | Gastrointestinal bleedinga |
| 17 (2427) | Positive | 17 | First | Candida sepsis and cerebral bleedinga |
| 20 (2461) | Positive | 19 | First | Candida sepsis |
| 1 (2190) | Positive | 24 | Second | Pseudomonas sepsis and respiratory failurea |
| 4 (2220) | Positive | 56 | Second | Hepatorenal failure with posttransplantation lymphoproliferative disorder |
| 10 (2309) | Positive | 96 | Second | Pseudomonas sepsis and kidney failurea |
| 21 (2472) | Positive | 141 | Second | Sepsis (Candida and Staphylococcus) |
| 11 (2311) | Positive | 245 | First | Sepsis with mycotic aneurysma |
| 149 (2553) | Negative | 8 | First | Patient never awoke from comaa |
| 126 (2384) | Negative | 25 | First | Sepsisa |
| 109 (2240) | Negative | 171 | Second | Aspergillosis |
| 127 (2381) | Negative | 219 | First | Pneumoniaa |
| 134 (2456) | Negative | 222 | First | Recurrent hepatitis Ba |
| 144 (2500) | Negative | 253 | First | Intracerebral hemorrhage and metastatic diseasea |
| 110 (2292) | Negative | 307 | First | Cryptococcal meningitisa |
OLT = orthotopic liver transplantation.
Autopsy not done.
Arterial Wall Thickness
Arterial wall thickness and diameter ratios were measured in the grafts retrieved at retransplantation (Fig. 9). The ratios were significantly higher in the crossmatch-positive patients than in the control patients (p < 0.05).
Fig. 9.
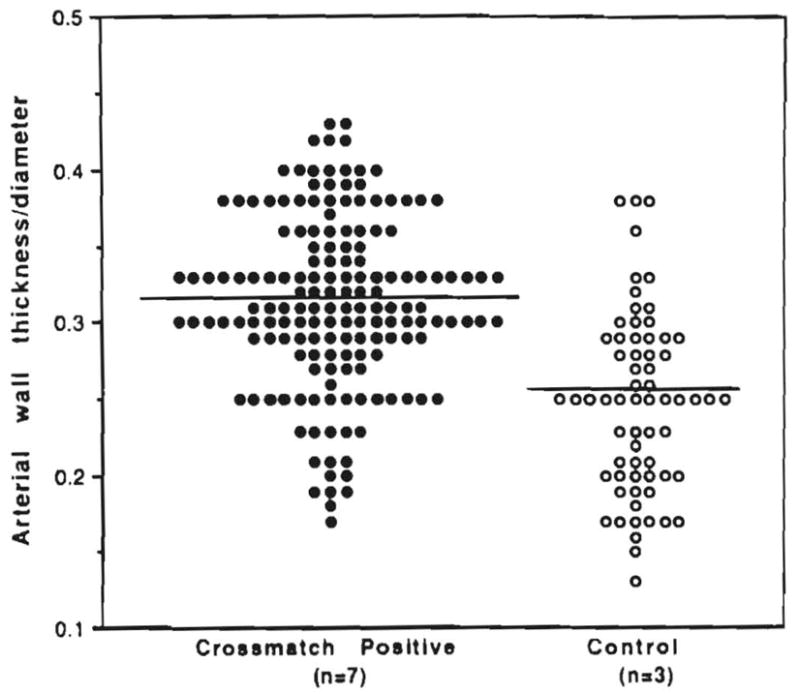
Ratio of arterial wall thickness to vessel diameter in failed grafts from crossmatch-positive patients vs. controls.
Immunofluorescence and Immunoperoxidase Findings
Only one crossmatch-positive graft that failed on the same day of transplantation contained detectable, specific immune deposits (see Table 3). Relatively faint granular IgG, Clq and C3 deposits were present, predominantly in the sinusoids, with focal weak deposits in hepatic arteries. Portal and central veins generally tested negative for these deposits. No significant immune deposits were present in any of the failed grafts in the other crossmatch-positive cases except for weak, questionable positivity on the basement membrane and on the epithelial cells of bile ducts.
Biopsy specimens obtained in the early postoperative period (1 to 10 days) either tested negative or showed faint granular sinusoidal deposits similar to those described above. However, the immunoperoxidase technique used on the needle biopsy specimens may have been less sensitive than the direct immunofluorescence technique used on the failed allografts above. No immune deposits were detected in any of the crossmatch-negative grafts with direct immunofluorescence on frozen tissue.
DISCUSSION
In kidney allografts the adverse effect of preformed donor antibodies was first shown with the demonstration that ABO-mismatched kidneys often were rejected immediately by a process presumed to involve graft antibody RBC isoagglutinins (17). Lymphocytotoxic antibodies (18–21) and possibly reticuloendothelial-specific (22) antibodies have also been shown to have similar effects. The target of destruction is the kidney microvasculature, which is ruined and plugged with thrombi so quickly that the descriptive term hyperacute rejection has been applied. Disparate xenografts are thought to be rejected by a similar mechanism, and the preformed antibodies, formed blood elements and clotting factors are sequestered in the transplant (23–25).
Although the same rapid sequestration occurs in the transplanted liver in such experiments (23, 24), hyper-acute rejection of liver allografts is difficult to produce experimentally (26, 27) and rarely occurs clinically (1–5). Nevertheless, the adverse effect of preformed antibody states on the transplanted liver has been increasingly acknowledged, first with the recognition that the prognosis after liver transplantation is degraded if ABO-incompatible donors are used (2, 6, 28, 29) and then with isolated descriptions of primary liver graft nonfunction in patients with lymphocytotoxic antibodies (2, 30–32). Subsequently, an increased graft loss at a later time after surgery in cytotoxic crossmatch-positive cases was reported (4, 5). In addition, controlled experimental animal studies have shown that accelerated liver rejection can be reliably induced by prior recipient sensitization (33, 34). Finally, Weber et al. (13) demonstrated a remarkable increase in the need for blood and blood product transfusions in patients with crossmatch-positive liver donors.
Despite this evidence, the role of lymphocytotoxic antibodies in the cause of humoral rejection after liver transplantation has been confused in the past by several factors. One factor has been the failure to differentiate between the lymphocytotoxic antibodies of the IgG class, which predispose to humoral rejection, and the IgM antibodies, which may not. The possibly irrelevant IgM antibodies can be eliminated as an artifact by treating the test sera with DTT (7, 35), as was done in the present study. We have also recently shown the greater destructive potential of IgG alloantibodies in a small-animal model of hepatic humoral rejection (Furuya et al., Unpublished observations, January 1992).
Our study provides pathological confirmation that IgG lymphocytotoxic antibodies can adversely affect the human liver allograft, with an array of morphological consequences. Although cellular rejection is frequently superimposed, the histopathological pattern of injury on needle biopsy cannot be described as unique because many of the characteristic alterations are similar to those observed in sepsis and preservation injury (12). The changes include the appearance of cholangioles with acute cholangiolitis, centrilobular hepatocyte swelling and hepatocanalicular cholestasis, often combined with cellular rejection. Additional findings described below are present in the hilum of failed allografts that were not accessible by biopsy evaluation.
The pathophysiology of the observed changes could not be fully explained by use of the uncontrolled, clinically directed biopsy samplings. However, the acute injury appears to be related to mechanical occlusion of the microvasculature (36) and functional narrowing of the arterial tree because of immunologically mediated vasoconstriction (6, 32), as has been reported in hyper-acutely rejecting kidneys (9–11, 37). This injury can manifest as focal intrahepatic infarcts and large bile duct necrosis followed later by periductal fibrosis and stricturing. The appearance of cholangioles with acute cholangiolitis is considered by us to be a stereotypic regenerative response to injury, particularly when the periportal hepatocytes are involved.
Nevertheless, the difficulty of studying pathophysiology from noncontrolled clinical tissue samples remains. We have therefore developed a clinically relevant small-animal model to address many of the issues raised by this study (Nakamura et al., Unpublished observations, February 1992).
The large liver mass and the presence of a second (portal) source of blood flow may partially explain its ability to withstand the initial insult of a positive crossmatch better than do the kidney and other organs that have an exclusively arterial supply and undergo infarction as an end result. However, it appears that the liver may behave as two organs. The first is the hepatic parenchyma with a sinusoidal vasculature, which may be more resistant because of its unique antigenic composition, the presence of Kupffer cells and possibly the release of soluble mediators from Kupffer cells, which could influence the inflammatory response.
The biliary tree, on the other hand, may behave more similarly to kidney and heart grafts because of its arterial only type circulation and the presence of a more conventional arteriolar and capillary network. A continued antibody-mediated insult to the arterial tree and peribiliary vascular plexus could account for the ductopenia without cholangiolar proliferation seen later in grafts that have survived despite a positive crossmatch (38, 39).
In a previous publication (40), we outlined restrictive criteria for the histopathological diagnosis of hyperacute rejection. These criteria include rapid graft failure, consistent and predictable histopathological and immunofluorescent changes, demonstrable presensitized state with lymphocytotoxic antibodies and elution of donor-specific antibodies from the failed graft. It is clear that all of these conditions rarely can be documented, as was emphasized earlier (30), especially the demonstration of convincing immunofluorescence findings. The lack of significant deposits may be related to the less-sensitive immunoperoxidase technique used for postreperfusion biopsies. However, the failed allografts were examined by the more-sensitive direct immunofluorescent technique with frozen tissue and were also generally negative.
Even with the treatment conditions used during this study, the majority of livers were able to pass through the perioperative period despite the handicap imposed by a positive cytotoxic crossmatch. In view of the association of preformed antibodies with an enhanced immunological injury, biopsy findings like those described above should elicit augmented immunosuppression because early and aggressive therapy can significantly alter the posttransplant course in crossmatch-positive patients. In addition, prophylactic treatment with prostaglandin to protect the microvasculature is under clinical trial, with encouraging preliminary results (41). Thus instead of avoiding transplantation in highly sensitized patients who may not be able to wait for a crossmatch-negative donor, who is unlikely to be found, modification of the standard treatment protocol appears to be a supportable option.
Acknowledgments
This work was supported by grants from the Department of Veterans Affairs, the National Institutes of Health (project grant no. DK 29961), the Pathology Education and Research Foundation and the Diviaion of Transplantation Surgery.
References
- 1.Starzl TE, Ishikawa M, Putnam CW, Porter KA, Picache R, Husberg BS, Halgrimson CG, et al. Progress in and deterrents to orthotopic liver transplantation, with special reference to survival, resistance to hyperacute rejection, and biliary duct reconstruction. Transplant Proc. 1974;6(4 suppl 10):129–139. [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Koep LJ, Halgrimson CG, Hood J, Schroter GPJ, Porter KA, Weil R., III Fifteen years of clinical liver transplantation. Gastroenterology. 1979;77:375–388. [PMC free article] [PubMed] [Google Scholar]
- 3.Iwatsuki S, Rabin BS, Shaw BW, Jr, Starzl TE. Liver transplantation against T cell-positive warm crossmatches. Transplant Proc. 1984;16:1427–1429. [PMC free article] [PubMed] [Google Scholar]
- 4.Takaya S, Duquesnoy R, Iwaki Y, Demetris J, Yagihashi A, Bronsther O, Iwatsuki S, et al. Positive crossmatch in primary human liver allografts under cyclosporine or FK 506 therapy. Transplant Proc. 1991;23:396–399. [PMC free article] [PubMed] [Google Scholar]
- 5.Takaya S, Bronsther O, Iwaki Y, Nakamura K, Abu-Elmagd K, Yagihashi A, Demetria AJ. The adverse impact on liver transplantation of using positive cytotoxic crossmatch donors. Transplantation. 1992;53:400–406. doi: 10.1097/00007890-199202010-00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demetris AJ, Jaffe R, Tzakis A, Ramsey G, Todo S, Belle S, Esquivel C, et al. Antibody mediated rejection of human orthotopic liver allografts: a study of liver transplantation across ABO blood group barriers. Am J Pathol. 1988;132:489–502. [PMC free article] [PubMed] [Google Scholar]
- 7.Iwaki Y, Lau M, Terasaki PI. Successful transplants across T-warm IgM positive crossmatches. Clin Transpl. 1988;2:81–94. [Google Scholar]
- 8.Todo S, Fung JJ, Starzl TE, Tzakis A, Demetris AJ, Kormos R, Jain A, et al. Liver, kidney, and thoracic organ transplantation under FK 506. Ann Surg. 1990;212:295–305. doi: 10.1097/00000658-199009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dempster WJ. The nature of experimental second-set kidney transplant rejection. Br J Exp Pathol. 1971;52:172–185. [PMC free article] [PubMed] [Google Scholar]
- 10.Busch GJ, Martins CP, Hollenberg NK, Wilson RE, Colman RW. A primate model of hyperacute renal allograft rejection. Am J Pathol. 1975;79:31–56. [PMC free article] [PubMed] [Google Scholar]
- 11.Terada Y, Ueno A. Hyperacute renal allograft rejection in the rabbit. Transplantation. 1983;35:205–208. doi: 10.1097/00007890-198303000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Demetris AJ, Jaffe R, Starzl TE. A review of adults and pediatric post-transplant liver pathology. Pathol Annu. 1987;22:347–386. [PubMed] [Google Scholar]
- 13.Weber T, Marino IR, Kang YG, Esquivel C, Starzl TE, Duquesnoy R. Intraoperative blood transfusions in highly alloimmunized patients undergoing orthotopic liver transplantation. Transplantation. 1989;45:797–801. doi: 10.1097/00007890-198905000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demetris AJ, Lasky S, Van Thiel DH, Starzl TE, Dekker A. Pathology of hepatic transplantation: a review of 62 adult allograft recipients immunosuppressed with a cyclosporine/steroid regimen. Am J Pathol. 1985;118:151–161. [PMC free article] [PubMed] [Google Scholar]
- 15.Eggink HF, Hofstee N, Gips CH, Krom RAF, Houthoff HJ. Histopathology of serial graft biopsies from liver transplant recipients. Am J Pathol. 1984;114:18–31. [PMC free article] [PubMed] [Google Scholar]
- 16.Snover DC, Sibley RK, Freese DK, Sharp HL, Bloomer JR, Najarian JS, Ascher NL. Orthotopic liver transplantation: a pathological study of 63 serial liver biopsies from 17 patients with special reference to the diagnostic features and natural history of rejection. Hepatology. 1984;4:1212–1222. doi: 10.1002/hep.1840040620. [DOI] [PubMed] [Google Scholar]
- 17.Starzl TE, Marchioro TL, Holmes JH, Waddell WR. The incidence, cause, and significance of immediate and delayed oliguria or anuria after human renal transplantation. Surg Gynecol Obstet. 1964;119:819–827. [PMC free article] [PubMed] [Google Scholar]
- 18.Terasaki PI, Marchioro TL, Starzl TE. Histocompatibility testing. Washington, DC: National Academy of Sciences–National Research Council; 1965. Sero-typing of human lymphocyte antigens: preliminary trials on long-term kidney homograft survivors; pp. 83–96. [Google Scholar]
- 19.Kissmeyer-Nielsen F, Olsen S, Peterson VP, Fjeldborg O. Hyper-acute rejection of kidney allografts associated with preexisting humoral antibodies against donor cells. Lancet. 1966;2:662–665. doi: 10.1016/s0140-6736(66)92829-7. [DOI] [PubMed] [Google Scholar]
- 20.Williams GM, Hume DM, Hudson RP, Morris PJ, Kano K, Milgrom F. Hyperacute renal-homograft rejection in man. N Engl J Med. 1968;279:611–618. doi: 10.1056/NEJM196809192791201. [DOI] [PubMed] [Google Scholar]
- 21.Starzl TE, Lerner RA, Dixon FJ, Groth CG, Brettschneider L, Terasaki PI. Shwartzman reaction after human renal transplantation. N Engl J Med. 1968;278:642–648. doi: 10.1056/NEJM196803212781202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cerilli J, Brasile L, Galouzis T, Lempert N, Clarke J. The vascular endothelial cell antigen system. Transplantation. 1985;39:286–289. doi: 10.1097/00007890-198503000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Giles GR, Boehmig JH, Lilly J, Amemiya H, Takagi H, Coburg AJ, Hathaway WE, et al. Mechanism and modification of rejection of heterografts between divergent species. Transplant Proc. 1970;2:522–537. [PMC free article] [PubMed] [Google Scholar]
- 24.Simpson KM, Bunch DL, Amemiya H, Boehmig HJ, Wilson CB, Dixon FJ, Coburg AJ, et al. Humoral antibodies and coagulation mechanisms in the accelerated or hyperacute rejection of renal homografts in sensitized canine recipients. Surgery. 1970;68:77–87. [PMC free article] [PubMed] [Google Scholar]
- 25.Starzl TE, Tzakis A, Makowka L, Banner B, Demetris AJ, Ramsey G, Duquesnoy R, et al. The definition of ABO factors in transplantation: relation to other humoral antibody states. Transplant Proc. 1987;19:4492–4497. [PMC free article] [PubMed] [Google Scholar]
- 26.Houssin D, Bellon B, Brunaud MD, Gugenheim J, Settaf A, Mriggi F, Emond J. Interactions between liver allografts and lymphocytotoxic alloantibodies in inbred rats. Hepatology. 1986;6:994–998. doi: 10.1002/hep.1840060531. [DOI] [PubMed] [Google Scholar]
- 27.Gugenheim J, Le Thai B, Rouger P, Gigou M, Gane P, Vial MC, Charpentier B, et al. Relationship between the liver and lymphocytotoxic alloantibodies in inbred rats: specific absorption by nonparenchymal liver cells. Transplantation. 1988;45:474–478. doi: 10.1097/00007890-198802000-00046. [DOI] [PubMed] [Google Scholar]
- 28.Gordon RD, Iwatsuki S, Esquivel CO, Tzakis A, Todo S, Starzl TE. Liver transplantation across ABO blood groups. Surgery. 1986;100:342–348. [PubMed] [Google Scholar]
- 29.Gugenheim J, Samuel D, Reynes M, Bismuth H. Liver transplantation across ABO blood group barriers. Lancet. 1990;336:519–523. doi: 10.1016/0140-6736(90)92082-s. [DOI] [PubMed] [Google Scholar]
- 30.Starzl TE, Demetria AJ, Todo S, Kang Y, Tzakis A, Duquesnoy R, Makowka L, et al. Evidence for hyperacute rejection of human liver grafts: the case of the canary kidneys. Clin Transpl. 1989;3:37–45. [PMC free article] [PubMed] [Google Scholar]
- 31.Hanto DW, Snover DC, Sibley RK, Noreen HJ, Gajl-Pezalska KF, Najarin JS, Ascher NL. Hyperacute rejection of a human orthotopic liver allograft in a presensitized recipient. Clin Transpl. 1978;1:304–310. [Google Scholar]
- 32.Bird G, Friend P, Donaldson P, O’Grady J, Portmann B, Caine R, Williams R. Hyperacute rejection in liver transplantation: a case report. Transplant Proc. 1989;21:3742–3744. [PubMed] [Google Scholar]
- 33.Knechtle S, Kolbeck PC, Tsuchimoto S, Coundouriotis A, Sanfilippo F, Bollingher RR. Hepatic transplantation into sensitized recipients. Transplantation. 1987;43:8–12. doi: 10.1097/00007890-198701000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Gubernatis G, Lauchart W, Jonker M, Steinhoff G, Bornscheuer A, Neuhaus P, van Es AA, et al. Signs of hyperacute rejection of liver grafts in rhesus monkeys after donor-specific presensitization. Transplant Proc. 1987;19:1082–1083. [PubMed] [Google Scholar]
- 35.Okuno T, Kondelis N. Evaluation of dithiothreitol (DTT) for inactivation of IgM antibodies. J Clin Pathol. 1978;31:1152–1155. doi: 10.1136/jcp.31.12.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kakizoe S, Yanaga K, Starzl TE, Demetris AJ. Evaluation of protocol pre-transplant and post-reperfusion biopsies from human orthotopic liver allografts: considerations of “preservation” and early immunologic injury. Hepatology. 1990;11:932–941. doi: 10.1002/hep.1840110605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ito S, Camussi C, Tetta C, Milrom F, Andres G. Hyperacute renal allograft rejection in the rabbit. Lab Invest. 1984;51:148–161. [PubMed] [Google Scholar]
- 38.Batts KP, Moore SB, Perkins JD, Weisner RH, Grambsch PM, Krom RA. Influence of positive lymphocyte crossmatch and HLA matching on vanishing bile duct syndrome in human liver allografts. Transplantation. 1988;45:376–379. doi: 10.1097/00007890-198802000-00026. [DOI] [PubMed] [Google Scholar]
- 39.Donaldson PT, Alexander GJM, O’Grady J, Newberger J, Portmann B, Thick M, Davis H, et al. Evidence of an immune response to HLA class I antigens in the vanishing bile duct syndrome after liver transplantation. Lancet. 1987;1:945–948. doi: 10.1016/s0140-6736(87)90293-5. [DOI] [PubMed] [Google Scholar]
- 40.Starzl TE, Demetris AJ. Liver Transplantation. Chicago: Year Book; 1991. pp. 57–70. [Google Scholar]
- 41.Takaya S, Iwaki Y, Starzl TE. Value of high dose steroids and prostaglandin E1 for liver transplantation in positive cytotoxic crossmatch cases. Transplantation. 1992 doi: 10.1097/00007890-199211000-00031. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]