Fungal infections remain an important cause of morbidity and mortality following solid organ and bone marrow transplantation (1–3). Use of amphotericin B, the antifungal agent of choice at present, is frequently associated with a high incidence of side effects. In organ transplant patients particularly, the nephrotoxicity of amphotericin may be amplified when combined with immunosuppressive agents such cyclosporine or FK506.
Fluconazole, a new antifungal agent with low toxicity and excellent oral bioavailability has been shown to be effective in both the treatment and prophylaxis of fungal infections in immunocompromised patients (4, 5). However, fluconazole is known to inhibit the cytochrome P-450 enzyme system in humans and animals. Coadministration of fluconazole with cyclosporine, a drug that is primarily metabolized in the liver, results in increased cyclosporine blood concentrations in transplant patients (6). Since FK506 is also known to be primarily eliminated by hepatic metabolism, coadministration of fluconazole to patients on FK506 therapy is expected to increase FK506 concentrations in patients (7). In rats, fluconazole is known to increase FK506 blood concentrations (8). In this communication we report our observations on the interaction between FK506 and fluconazole in transplant patients.
Twenty organ transplant patients under FK506 immunosuppression were evaluated while receiving a course of fluconazole therapy. The patient population included 11 patients who received livers (OLTX); 6 patients who received kidneys (KTX), 2 patients who received hearts (HTX) and one bone marrow patient. The immunosuppressive regimen included FK506 and low-dose steroids (20 mg/day). FK506 was given initially as a continuous i.v. infusion at 0.1 mg/kg/day, with conversion to the oral dose of 0.15 mg/kg every 12 hr with the return of normal bowel function. Subsequent dose adjustments were guided by the quality of the graft, the presence of any evidence of rejection, signs of toxicity, and trough FK506 plasma concentrations (normal range 0.5 to 2 ng/ml).
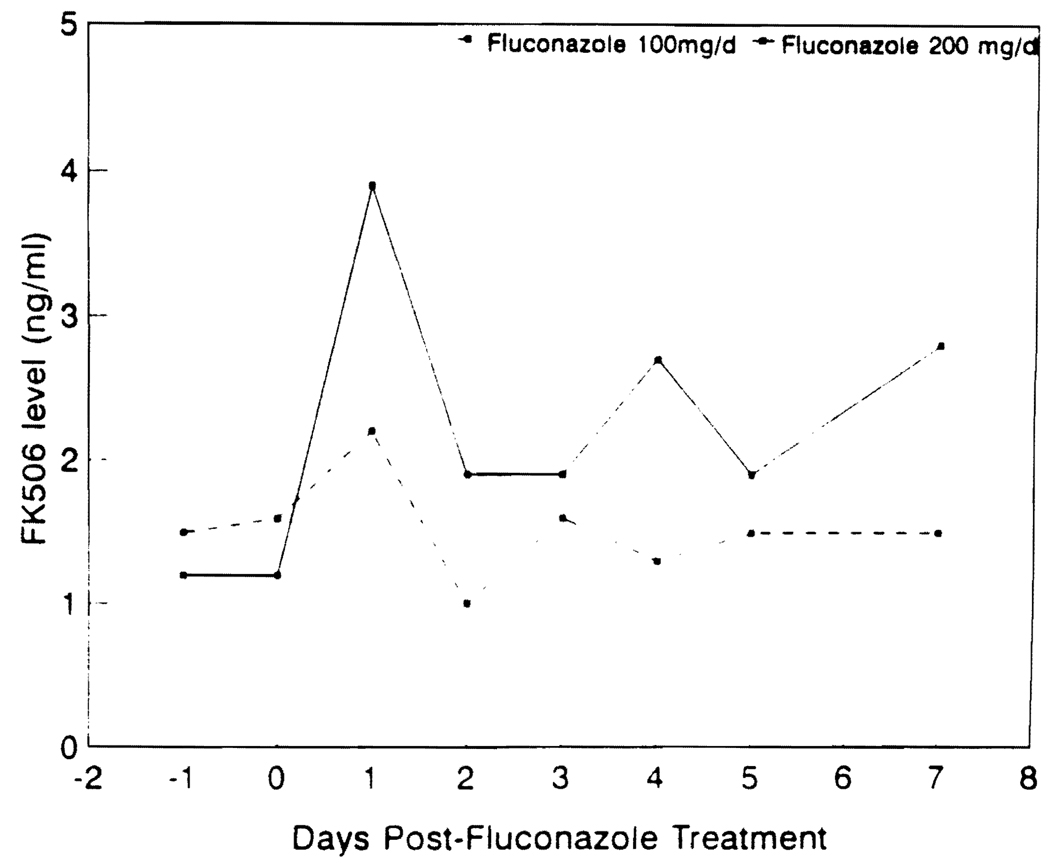
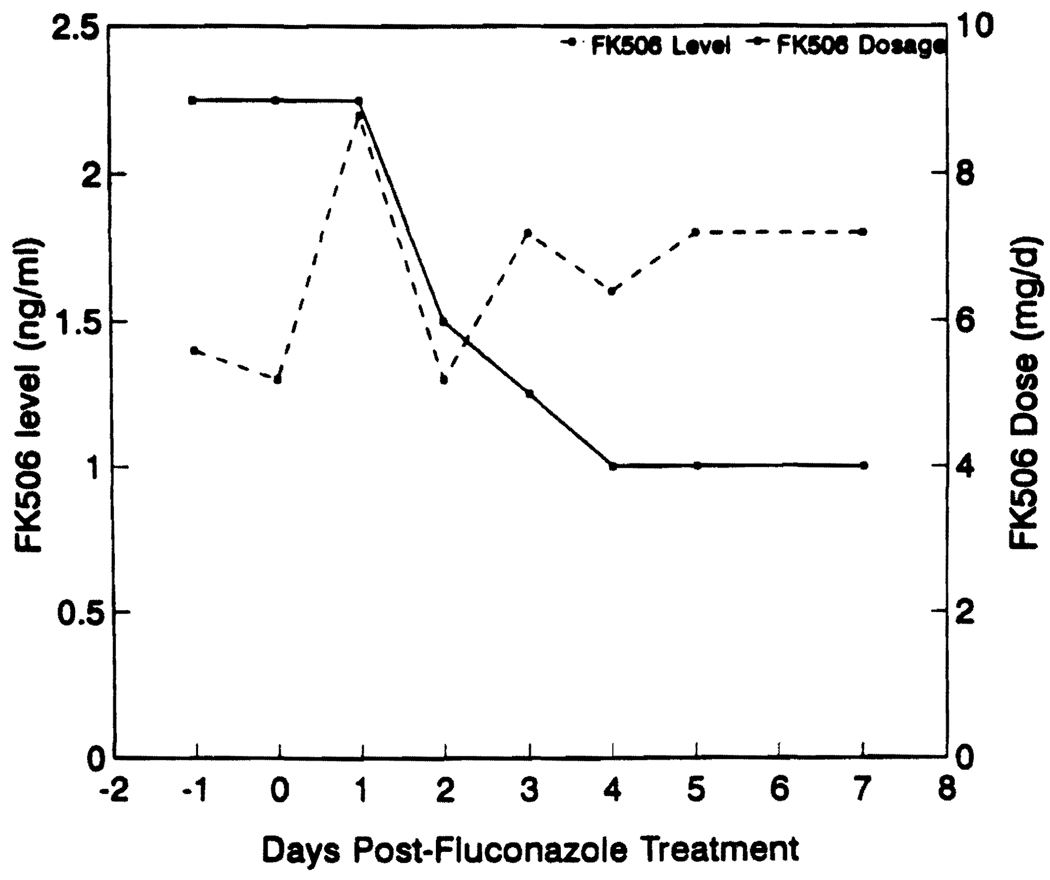
Sixteen of the patients studied had documented fungal infection (7 urinary tract infections, 4 esophagitis, 2 pyelonephritis, 2 tracheobronchitis, and 1 cholangitis). Candida albicans was the most common pathogen isolated (14 patients), whereas Candida tropicalis and Cryptococcus neoformans were isolated in one patient each. Four additional patients were considered high-risk patients and were treated with prophylactic fluconazole. The median time from transplantation to the initiation of fluconazole therapy was 60 days (range 2–1680 days). In patients with documented fungal infection, fluconazole was given for a median of 14 days (range 7–100 days). Fourteen of the 16 patients were successfully treated and became culture-negative. Two failures were noted in two kidney transplant patients who required transplant nephrectomy for persistent fungal invasion of the graft. Four patients received prophylactic fluconazole for a median of 12 days (range 6–30 days), and all remained fungal culture negative. Fluconazole was used at a dose of 200 mg/day in 8 patients and 100 mg/day in 12 patients. FK506 plasma concentrations were measured by an ELISA (9). The intraday coefficient of variation for this assay ranged from 4.2 to 5.5% and the interday coefficient of variation ranged from 14.4 to 17% at concentrations measured in this study. Figure 1 shows the median plasma trough concentration of FK506 on days -1, 0, 1, 2, 3, 4, 5, and 7 after fluconazole therapy as a function of the fluconazole dose administered. On day 1, the median plasma trough concentration of FK506 increased 1.4-fold in patients receiving 100 mg/day of fluconazole and 3.1-fold in patients receiving 200 mg/day. Thereafter the FK506 plasma concentration decreased as the FK506 dose was reduced to prevent higher FK506 concentrations. Figure 2 shows the median dose of FK506 and the plasma concentration of FK506 on days -1, 0, 1, 2, 3, 4, 5, and 7 of fluconazole therapy. A median FK506 dose reduction of 56% (range 0% to 88%) was required in order to maintain the FK506 concentrations below 2 ng/ml. There was no change in the route of FK506 administration during the period studied. The initial increase in FK506 plasma concentration during fluconazole therapy was associated with acute renal dysfunction in 3 patients (hemodialysis required in one) and acute changes in the mental status in 2 patients.
FIGURE 1.
Median plasma trough concentration of FK506 in patients receiving 100 mg (continuous line) and 200 mg (interrupted line) of fluconazole.
FIGURE 2.
Median dose (mg/day) (continuous line) and plasma trough concentration (ng/ml) (interrupted line) of FK506 during fluconazole therapy.
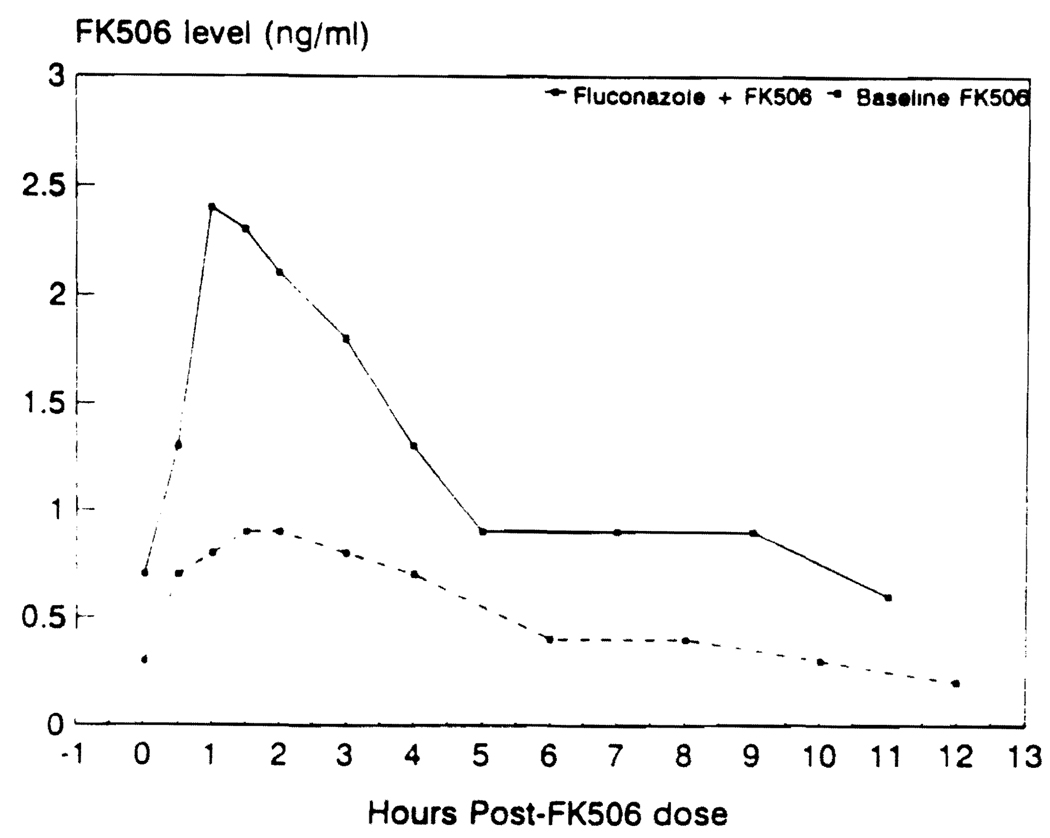
The pharmacokinetics of FK506 was evaluated in one patient on two occasions, once while on fluconazole therapy (100 mg oral dose, 1 hr prior to FK506 dosing of 3 mg) and again eight days after stopping fluconazole therapy. Figure 3 shows FK506 the plasma concentration vs. time profile over a dosing interval in this patient with and without fluconazole therapy. Discontinuation of fluconazole therapy resulted in a significant decrease in the area under the plasma concentration–versus–time curve of FK506 (from 13 ng/ml/hr to 5.4 ng/ml/hr). The half-life maximum plasma concentration and the trough plasma concentration of FK506 also decreased during this period.
FIGURE 3.
FK506 pharmacokinetic profile of one patient with (continuous line) and without (interrupted line) fluconazole therapy.
FK506 is a new immunosuppressive drug with a proven beneficial effect over currently available agents (10–12). Its elimination through hepatic metabolism by cytochrome P-450 enzyme makes it susceptible to possible interactions with other drugs as that are known to induce or inhibit these enzymes, such as phenobarbital, phenytoin, rifampin, clotrimazole, ketoconazole, itraconazole, fluconazole, cimetidine, and erythromycin (7). In rats, FK506 blood concentrations are reported to increase in the presence of fluconazole, erythromycin, ketoconazole, and diltiazam (8). Erythromycin and methylprednisolone have been reported to increase FK506 plasma concentrations (7). Clotrimazole treatment also increases FK506 plasma concentrations in liver transplant patients (13). The data reported here show an increase in trough and maximal plasma concentration of FK506, along with an increase in the half-life of FK506 in the presence of fluconazole. This suggests that fluconazole inhibits FK506 metabolism. It is not possible to predict whether fluconazole decreases FK506 metabolism in the gut and therefore increases the extent of absorption or whether it affects only the hepatic metabolism of FK506. Additional studies are required to understand the extent to which this interaction takes place in the small bowel and/or the liver. The increases in the plasma concentration of FK506 were higher at a 200 mg/day fluconazole dose than at a 100 mg/day dose. This dose-related effect, also reported with CsA (6), may have important implications when fluconazole is used in the treatment of severe fungal infections. Recently, a new fluconazole dosing regimen (200–400 mg/day) has been suggested for the treatment of invasive candidiasis (14). FK506 concentrations should be carefully monitored when using these dosing regimens. Despite the similarities between fluconazole’s interaction with CsA and FK506, some differences were observed. In renal transplant recipients, fluconazole slowly increased cyclosporine trough concentrations over a two-week period, reaching a maximum of twice the baseline level (6). In our patients the highest concentration of FK506 was seen within three days after the introduction of fluconazole therapy.
In summary, fluconazole therapy in patients under FK506 immunosuppression appears to be beneficial for both prophylaxis and treatment of fungal infections. Fluconazole doses of up to 200 mg/day can be safely and effectively administered to transplant patients on FK506 therapy provided that the FK506 dose is reduced by half. Use of higher doses of fluconazole would require careful monitoring of trough FK506 plasma concentrations and corresponding reduction in FK506 doses to avoid nephrotoxicity.
Footnotes
This work was supported by research grants from the Veterans Administration and by Project Grant 29961 from the National Institutes of Health, Bethesda, MD.
REFERENCES
- 1.Kusne S, Dummer JS, Singh N, et al. Fungal infections after liver transplantation. Transplant Proc. 1988;20:650. [PMC free article] [PubMed] [Google Scholar]
- 2.Wajszczuk CP, Dummer JS, Ho M, et al. Fungal infections in liver transplant recipients. Transplantation. 1985;40:347. doi: 10.1097/00007890-198510000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castaldo P, Stratta RJ, Wood RP, et al. Clinical spectrum of fungal infections after orthotopic liver transplantation. Arch Surg. 1991;126:149. doi: 10.1001/archsurg.1991.01410260033005. [DOI] [PubMed] [Google Scholar]
- 4.Flannery MT, Simmons DB, Saba H, Altus P, Wallach PM, Adelman HM. Fluconazole in the treatment of hepatosplenic candidiasis. Arch Intern Med. 1992;152:406. [PubMed] [Google Scholar]
- 5.Goodman JL, Winston DJ, Greenfield RA, et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med. 1992;326:845. doi: 10.1056/NEJM199203263261301. [DOI] [PubMed] [Google Scholar]
- 6.Canafax DM, Graves NM, Hilligoss DM, Carleton BC, Gardner MJ, Matas AJ. Interaction between cyclosporine and fluconazole in renal allograft recipients. Transplantation. 1991;51:1014. doi: 10.1097/00007890-199105000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Venkataramanan R, Jain A, Warty VS, et al. Pharmacokinetics of FK506 in transplant patients. Transplant Proc. 1991;23:2736. [PMC free article] [PubMed] [Google Scholar]
- 8.Rui X, Flowers J, Warty VS, Venkataramanan R. Drug interactions with FK 506. Pharm Res. 1992;9:S314. [Google Scholar]
- 9.Warty VS, Venkataramanan R, Zendehrouh P, et al. Practical aspects of FK506 analysis. Transplant Proc. 1991;23:2730. [PMC free article] [PubMed] [Google Scholar]
- 10.Fung J, Abu-Elmagd K, Jain A, et al. A randomized trial of primary liver transplantation under immunosuppression with FK506 vs cyclosporine. Transplant Proc. 1991;23:2977. [PMC free article] [PubMed] [Google Scholar]
- 11.Armitage JM, Kormos RL, Fung J, Starzl TE. The clinical trial of FK506 and rescue immunosuppression in adult cardiac transplantation. Transplant Proc. 1991;23:3054. [PMC free article] [PubMed] [Google Scholar]
- 12.Shapiro R, Jordan M, Scantlebury V, et al. FK506 in clinical kidney transplantation. Transplant Proc. 1991;23:3065. [PMC free article] [PubMed] [Google Scholar]
- 13.Mieles L, Venkataramanan R, Yokoyama I, Warty VJ, Starzl TE. Interaction between FK506 and clotrimazole in a liver transplant recipient. Transplantation. 1991;52:1086. doi: 10.1097/00007890-199112000-00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milatovic D, Voss A. Efficacy of fluconazole in the treatment of systemic fungal infections. Eur J Clin Microbiol Infect Dis. 1992;11:395. doi: 10.1007/BF01961853. [DOI] [PubMed] [Google Scholar]