Abstract
Alzheimer’s disease (AD), the most common cause of dementia among the elderly, may either represent the far end of a continuum that begins with age-related memory decline or a distinct pathobiological process. Although mice that faithfully model all aspects of AD do not yet exist, current mouse models have provided valuable insights into specific aspects of AD pathogenesis. We will argue that transgenic mice expressing amyloid precursor protein should be considered models of accelerated brain aging or asymptomatic AD, and the results of interventional studies in these mice should be considered in the context of primary prevention. Studies in mice have pointed to the roles of soluble beta amyloid (Aβ) oligomers and soluble tau in disease pathogenesis, and support a model in which soluble Aβ oligomers trigger synaptic dysfunction, but formation of abnormal tau species leads to neuron death and cognitive decline severe enough to warrant a dementia diagnosis.
1. Introduction
Alzheimer’s disease (AD) is the most common cause of dementia among the elderly. As life expectancy increases, the number of AD cases worldwide is expected to increase from 35 million today to more than 115 million by 2050 (http://www.alz.org/national/documents/report_full_2009worldalzheimerreport.pdf). The most effective approach to lessening the personal and financial costs of AD is to prevent the disease, but in order to develop prevention strategies, it is necessary to know when and how the disease begins. Molecular studies of the brain at the earliest stages of memory loss associated with AD have been difficult in humans. There is not even consensus among neurologists and neuroscientists as to whether AD is at the far end of a continuum that begins with age-related memory decline or whether AD is a distinct process.
There are still no mice harboring a single Alzheimer’s-linked gene variant that develop the progressive cognitive deficits, plaques, tangles and neuronal loss characteristic of the human disease. Although there are not yet mice that faithfully model AD, existing mouse models have provided valuable insights into specific aspects of AD pathogenesis.
2. The definition of AD is evolving
The nosology of AD keeps shifting, the consequence of not knowing its etiology. This situation makes it difficult to place mouse models precisely into human context and demands an adaptive framework for utilizing mice as models of the human disease.
2.1 AD has a long asymptomatic phase
Past and current criteria for a diagnosis of AD rely upon the presence at autopsy of characteristic neuropathological lesions, amyloid plaques formed from aggregated beta-amyloid protein (Aβ) and neurofibrillary tangles (NFTs) formed from abnormally processed tau protein. Recent advances in imaging technology now permit the observation of these lesions in living subjects and confirm the findings from neuropathological studies that up to 40% of non-demented older adults have sufficient amyloid deposition to meet neuropathological criteria for AD (Bennett et al., 2006; Hulette et al., 1998; Price et al., 2009). The presence of substantial neuropathology in the brains of apparently normal individuals prompted the hypothesis there is a long asymptomatic phase of AD, during which cognitive function is largely sustained in the presence of amyloid pathology (Price and Morris, 1999). Consistent with this hypothesis, a recent longitudinal study found that elevated cortical amyloid predicted decline in memory function (Storandt et al., 2009) and the development of symptomatic AD (Morris et al., 2009) in elderly adults.
Structural and functional neuroimaging studies provide further support for the existence of a prolonged asymptomatic phase in AD, which might precede the onset of clinical symptoms by decades. Individuals carrying mutations associated with familial autosomal dominant AD are destined to develop symptoms at a predictable age for each family; abnormal patterns of brain activity and subtle cognitive deficits have been observed in mutation carriers more than 25 years before their expected conversion to dementia (Mondadori et al., 2006; Mosconi et al., 2006). Accelerated brain atrophy appears to follow functional changes, with regional differences between carriers and non-carriers apparent ~5 years prior to the onset of clinical symptoms in the mutation carriers (Knight et al., 2009; Ridha et al., 2006). The ε4 allele of apolipoprotein E (APOE4) is the major genetic risk factor for sporadic Alzheimer’s disease (Corder et al., 1993), and hypometabolism in brain regions affected in AD (de Leon et al., 1983; Kumar et al., 1991) has been observed in young adult (Reiman et al., 2004) as well as middle-aged (Caselli et al., 2008; Reiman et al., 2001; Reiman et al., 1996) APOE4 carriers. In longitudinal studies, cognitively normal subjects exhibiting regional hypometabolism during baseline examinations were more likely to suffer future cognitive decline (de Leon et al., 2001; Small et al., 2000) and to convert to AD (Mosconi et al., 2008) than were subjects with normal metabolic patterns. Volume loss in medial temporal regions in non-demented elderly individuals increases the risk for later conversion to mild cognitive impairment, considered by many to be a prodrome of AD (Petersen and Negash, 2008), or to AD (de Toledo-Morrell et al., 2000; den Heijer et al., 2006; Rusinek et al., 2003).
It is now possible to envision detecting AD long before the onset of clinical symptoms. An international consortium has attempted to identify AD at earlier stages using pathologically relevant biochemical, imaging, genetic and neuropsychological tests (Dubois et al., 2007). However, the revised criteria are not yet ready for clinical use, because the proposed new biomarkers require further validation and are not available in most community clinical settings (Foster, 2007).
2.2 “Pure” AD is uncommon
Complicating our ability to define AD is the observation that amyloid plaques and NFTs coexist with other pathologies in 1/3–1/2 of patients with clinically diagnosed AD (Kovacs et al., 2008; Schneider et al., 2009a; Schneider et al., 2009b). Infarcts are the most commonly-occurring co-pathology in AD, and two theories have been proposed to explain the observed relationships between vascular pathology and AD: one posits an interaction between the pathogenic factors causing the two conditions (Korczyn, 2002); the other that vascular pathology, specifically cerebral amyloid angiopathy and the physiological effects of Aβ on blood-flow regulation, constitutes an integral pathogenic feature of AD (Iadecola, 2004). Additional co-pathologies include cortical Lewy bodies formed from aggregated α-synuclein (Schneider et al., 2009a; Schneider et al., 2007; Schneider et al., 2009b), and TDP-43-immunoreactive inclusions (Amador-Ortiz et al., 2007; Higashi et al., 2007; Josephs et al., 2008; Kadokura et al., 2009; Uryu et al., 2008), characteristic neuropathological lesions of Parkinson’s disease and ALS, respectively. It is not known whether the co-existence of these lesions with amyloid plaques and NFTs represents a pathological state that promotes protein misaggregation or the coincidental occurrence of independent pathological processes. Regardless of etiology, the likelihood of clinical dementia is significantly greater in persons who have mixed pathology than in individuals with plaques and NFTs alone (Langa et al., 2004; Schneider et al., 2007; Schneider et al., 2009b; Snowdon et al., 1997). There is therefore concern that the effects of new Alzheimer therapies that are initially tested in mouse models and humans with pure AD may differ when they are dispensed to the general population.
3. Modeling AD in APP- and tau- transgenic mice
Pioneering investigations of amyloid plaques and the neurofibrillary tangles led to the identification of the molecules comprising these lesions, the amyloid-β (Aβ) and tau proteins, respectively. Whether or not these lesions are themselves pathogenic, their presence reflects aberrant processing of Aβ and tau that leads to protein aggregation. Studies of the mechanisms of synapto- and neuro-toxicity in transgenic mice have focused on toxic gains of function of these abnormal aggregates, although we cannot a priori exclude the possibility that loss of function of normal forms of these molecules also contributes to disease progression. Potential mechanisms of Aβ- and tau-induced toxicity will be discussed in Section 4 below.
Aβ is generated from the proteolytic processing of the amyloid precursor protein (APP) by β- and γ-secretases (Fig. 1). Genetic studies have revealed AD-linked mutations in APP and the presenilins, which form part of the γ-secretase complex (reviewed by Hardy (Hardy, 2006)). Although not found in AD, mutations in tau are linked to another neurodegenerative disorder, frontotemporal dementia.
Figure 1.
APP and its major proteolytic products. Cleavage of APP by α-, β-, γ-, and ε-secretases produces secreted amino-terminal fragments, carboxyl-terminal polypeptides, and Aβ. (Ma et al. (2007) Involvement of beta-site APP cleaving enzyme 1 (BACE1) in amyloid precursor protein-mediated enhancement of memory and activity-dependent synaptic plasticity. Proc Nat’l Acad Sci U S A. 104(19):8167–72. “Copyright (2007) National Academy of Sciences, U.S.A.”)
Three scientific breakthroughs made possible the creation of the first transgenic mouse models of AD: 1) the isolation and sequencing of the Aβ peptide in 1984 (Glenner and Wong, 1984); 2) the cloning of APP and the elucidation of its role in generating the Aβ peptide (Goldgaber et al., 1987; Kang et al., 1987; Robakis et al., 1987; Tanzi et al., 1987); and 3) the discovery of the first mutation in APP in autosomal dominant AD (Goate et al., 1991).
3.1 Genetic linkage studies highlight APP and tau
More than 20 autosomal dominant APP mutations linked to AD or cerebral amyloid angiopathy have been discovered (http://www.molgen.ua.ac.be/ADMutations). These mutations appear to enhance the aggregation of Aβ, which occurs by one of four mechanisms. The Swedish mutation facilitating APP cleavage near the β-secretase site (Mullan et al., 1992) increases the overall production of all forms of Aβ. A mutation within Aβ, called the Arctic mutation, enhances protofibril formation (Nilsberth et al., 2001). Several mutations near the γ-secretase site increase the relative production of the more amyloidogenic Aβ42 (Chartier-Harlin et al., 1991; Goate et al., 1991; Murrell et al., 1991; Murrell et al., 2000). Duplications of the APP gene increase production of all forms of Aβ (Rovelet-Lecrux et al., 2006; Sleegers et al., 2006). Recently, an autosomal recessive mutation involving the deletion of glutamate at Aβ residue 22 was found in a woman with dementia who apparently lacks amyloid plaques imaged with PiB (Tomiyama et al., 2008). This discovery raises the intriguing possibility that amyloid plaques may not be required to cause AD.
Mutations in genes encoding the presenilins (~80 mutations in presenilin-1 and ~10 mutations in presenilin-2) cause the remaining cases of early onset familial AD (http://www.alzforum.org/res/com/mut/pre/default.asp). Presenilin variants shift APP cleavage at the γ-secretase site, resulting in higher levels of the more amyloidogenic Aβ42 peptide.
Familial AD caused by autosomal dominant mutations in APP or the presenilins account for less than 10% of AD cases (Brouwers et al., 2008; Cruts and Van Broeckhoven, 1998), while the vast majority of cases arise sporadically. Aside from the earlier age of onset in familial AD cases, the neuropathological and cognitive abnormalities are generally similar in familial and sporadic AD (Lleo et al., 2004; Rossor et al., 1996; Swearer et al., 1992). The view thus has emerged that pathological processing of APP, whether initiated by age-related factors or mutations, triggers the downstream processes that mediate disease progression (Hardy and Selkoe, 2002).
Variants of tau (~40 mutations) are linked to frontotemporal dementia (FTD), but no mutations in tau have been found in AD families (see http://www.molgen.ua.ac.be/ADMutations/default.cfm?MT=0&ML=1&Page=FTD for a regularly updated list of FTD-linked mutations).
3.2 APP and tau transgenic mice are incomplete models of AD
Mutations in APP or one of the presenilins are sufficient to cause the complete AD phenotype of progressive cognitive impairment, plaques, tangles and neuron loss in the human brain. However, when expressed in mouse brain, human transgenes with these same mutations replicate some, but not all, aspects of AD. APP transgenic mice develop memory loss and plaques, with no NFTs and little or no neuron loss. Although APP transgenic mice fail to replicate the full human disease, they appear to faithfully simulate the pre-dementia phase of AD. (A few dozen APP transgenic models have been published; a partial list can be found on-line http://www.alzforum.org/res/com/tra/app/default.asp). Presenilin variants produce no neuropathology, but potentiate plaque deposition in APP transgenic mice. Despite the absence of linkage to AD, transgenic mice expressing human tau variants have been used to study neurofibrillary pathology, because, unlike in humans, the expression of APP and presenilin variants in mice is insufficient to induce neurofibrillary changes. In support of this approach, AD-related post-translational modifications in tau, revealed by phosphorylation- and conformation-specific monoclonal antibodies, are promoted by FTD-linked tau mutations (Ramsden et al., 2005; Yoshiyama et al., 2007). Duyckaerts and colleagues provide an extensive review of the neuropathology of the most important lines and crosses that have been generated (Duyckaerts et al., 2008).
Several hypotheses have been put forward to explain why APP transgenic mice do not fully recapitulate AD pathology, including differences in neuroinflammatory responses of mice and humans that might influence disease progression (Schwab et al., 2004; Vitek et al., 1997; Webster et al., 1999) and differences in brain aging between mice and humans (Loerch et al., 2008). Genetic ablation of endogenous tau in mice expressing human tau enhances tangle formation (Andorfer et al., 2003), suggesting that endogenous mouse tau may interfere with the ability of human tau to form tangles.
In addition to mice that express transgenes with disease-linked mutations in APP, the presenilins or tau, other models have been developed to study specific aspects of AD pathology. Among these are mice with targeted replacement of ApoE that have been used to study the influence of the different human ApoE alleles on amyloid pathology (recently reviewed by (Kim et al., 2009)); transgenic mice expressing an anti-NGF antibody that exhibit an AD-like phenotype, including cognitive deficits, neurodegeneration, accumulation of insoluble tau, and amyloid deposits composed of endogenous mouse Aβ (Capsoni and Cattaneo, 2006); and mice that specifically express N-terminally truncated pyroglutamate-modified Aβ3–42, a major Aβ species in AD brains (Wirths et al., 2009).
Despite that fact that there are still no mice harboring a single Alzheimer’s-linked gene variant that develop the complete disease phenotype, existing mouse models have provided valuable insights into specific aspects of AD pathogenesis.
3.3 APP transgenic mice model accelerated aging
Two vantages have guided the interpretation of phenotypes caused by expressing APP: an intraspecific viewpoint and an extraspecific viewpoint. Studies that have used an intraspecific reference compare phenotypes obtained in transgenic mice to those naturally occurring in wild-type mice. Investigators that have used an extraspecific reference focus analyses on comparisons between transgenic mouse phenotypes and disease features in humans.
In humans, the assembly of Aβ into one or more abnormal species initiates a process that often, but not always, leads to a progressive neurodegenerative condition. In mice, the process initiated by Aβ develops into a cognitive disorder that falls short of becoming a full blown neurodegenerative condition. Instead, APP transgenic mice share many features with naturally aged, non-transgenic rodents (Table 1). That the disorder resembles natural aging distinguishes the expression of APP variants in mice from the expression of genes linked to other neurodegenerative conditions, such as Parkinson’s disease, amyotrophic lateral sclerosis, prion diseases, and Huntington’s disease, and may provide a tantalizing clue about why AD is the most common neurodegenerative condition in man.
TABLE 1. Comparison of memory loss in aging rodents and APP rodents.
Shaded boxes indicate studies that directly compared phenotypes of transgenic mice and age-matched and older non-transgenic mice of the same background strain.
| Similarities | Aged rats & mice | Citations | Transgenic APP mice and Aβ- infused rats |
Citations |
|---|---|---|---|---|
| Neurological deficits |
Neophobia in aged FVB/N mice |
Hsiao et al. (1995) Neuron. 15(5):1203–18. |
Neophobia in FVB/N mice with APP transgenes |
Hsiao et al. (1995) Neuron. 15(5):1203–18. |
| Hypometabolism in specific brain regions |
Hypometabolism in brain regions linked to memory function in aged FVB/N mice |
Hsiao et al. (1995) Neuron. 15(5):1203–18. |
Hypometabolism in brain regions linked to memory function in FVB/N mice with APP transgenes |
Hsiao et al. (1995) Neuron. 15(5):1203–18. |
| Deficits in LTP likely due to increased GABA inhibition |
Significantly greater enhancement of LTP by bicuculline in aged mice than in young adult mice |
Yoshiike et. al (2008) PLoS One. 3(8): e3029. |
Significantly greater enhancement of LTP by bicuculline in young adult APP/PS1 transgenic mice than in young adult non-transgenic mice |
Yoshiike et. al (2008) PLoS One. 3(8): e3029. |
|
In vivo LTP maintenance impaired |
Impaired maintenance, but not induction, of hippocampal LTP in aged rats |
Barnes et al. (1979) J Comp Physiol Psychol. 93(1): 74–104. |
Impaired maintenance, but not induction, of hippocampal LTP in transgenic mice (Tg2576, APP/PS1) and in Aβ-infused rats |
Chapman, et al. (1999) Nat Neurosci 2(3): 271–6 Gureviciene et al. (2004) Neurobiol Dis. 15(2): 188–95. Walsh et al. (2002) Nature 416(6880): 535–9. |
| Hippocampal hyperexcitability |
Increased firing rates of CA3 place cells in aged rats |
Wilson et al. (2005) J Neurosci. 25(29):6877–86. |
Spontaneous seizure activity in hippocampal networks |
Palop et al. (2007) Neuron. 55(5):697–711. |
| Impaired Ca2+ homeostasis in neurons |
Ca2+ elevated in neurons of aged rats; Ca2+ elevated in pre-synaptic terminals of aged rats; altered sources of Ca2+ influx during induction of LTP in aged rats; Ca2+ chelation restores LTP in slices from aged rats but reduces LTP in slices from young rats and reverses memory deficits in aged rats; |
Hajieva et al. (2009) Neurosci Lett. 451(2): 119–23. Tonkikh et al. (2006) Exp Neurol. 197(2): 291–300. Shankar et al. (1998) J Neurophysiol. 79(1): 334–41. Boric et al. (2008) J Neurosci. 28(32): 8034–9. Reviewed in Foster TC (2007) Aging Cell. 6(3): 319–25. |
No studies yet reported in pre-plaque APP transgenic mice that do not also contain a mutant PS1 transgene, which itself disrupts Ca2+ dynamics |
Reviewed in Green & LaFerla (2008) Neuron. 59(2): 190–4. Stutzman GE (2007) Neuroscientist. 13(5): 546–59. |
| Hypothalamic- pituitary-adrenal (HPA) stress response defect |
Impaired HPA response to restraint- induced stress |
Bizon et al. (2001) Eur J Neurosci. 14(10): 1739–51 |
Aberrant HPA stress response to hypoglycemia in Tg2576 mice |
Pedersen et al. (1999) J Mol Neurosci. 13:(1–2): 159–65. |
| Preservation of neuron number in hippocampus |
No neuronal loss in hippocampus of aged rats with spatial memory deficits |
Rapp & Gallagher (1996) Proc Natl Acad Sci U S A. 93(18): 9926– 30. |
No neuronal loss in hippocampus of memory-impaired Tg2576 mice or in aged PDAPP mice |
Irizarry et al. (1997) J Neuropathol Exp Neurol. 56(9): 965–73. Irizarry et al. (1997) J Neurosci. 17(18): 7053–9. |
| Cholinergic system changes |
Shrinkage of basal forebrain neurons | Reviewed in Finch CE (1993) Trends Neurosci. 16(3): 104–10. |
Cholinergic terminal reorganization in Tg2576 × PS1 mice; basal forebrain neuron shrinkage in TgAPP mice with London mutation |
Wong et al. (1999) J Neurosci. 19(7): 2706–16. Bronfman et al. (2000) Neurobiol Dis. 7(3): 152–68. |
| Oxidative stress markers increased |
Increased signatures of oxidative stress in aged rats with spatial memory deficits |
Nicolle et al. (2001) Neuroscience. 107(3): 415–31. |
Increased oxidative stress markers in aged Tg2576 mice |
Smith et al. (1998) J Neurochem. 70(5): 2212–5. Pappolla et al. (1999) J Pineal Res. 27(4): 226–9. Pratico et al. (2001) J Neurosci. 21(12): 4183–7 |
| Caloric deprivation |
Caloric deprivation prevents memory loss in rats |
Pitsikas & Algeri (1992) Neurobiol Aging. 13(3): 369–73. |
Caloric restriction decreases amyloid deposition in APP and APP/PS1 mice; preserves spatial memory in Tg2576 mice |
Patel et al. (2005) Neurobiol Aging. 26(7): 995–1000. Mouton et al. (2009) Neurosci Lett. 464(3): 184–7. Qin et al. (2008) Ann N Y Acad Sci. 1147: 335–47. |
| Beneficial effects of COX inhibition |
Nimesulide (COX-2 inhibitor) attenuates memory deficits in aged mice; NSAIDs (nimesulide, rofecoxib, naproxen) reverse memory deficits in aged mice, no effect in young mice |
Bishnoi et al. (2005) Methods Find Exp Clin Pharmacol. 27(7): 465–70. Jain et al. (2002) Behav Brain Res. 133(2): 369–76. |
NSAIDs prevent (ibuprofen) or reverse (ibuprofen, naproxen, MF- tricyclic) memory deficits in Tg2576 mice, no effect in non-tg |
Kotilinek et al. (2008) Brain. 131(Pt 3): 651–64. |
| PKC-gamma elevation |
Increased in aged rats with spatial memory deficits |
Colombo et al. (1997) Proc Natl Acad Sci U S A. 94(25): 14195–9. Colombo & Gallagher (2002) Hippocampus. 12(2): 285–9. |
Increased in aged Tg2576 mice | Rossner et al. (2001) Eur J Neurosci. 13(2): 269–78. |
| Environmental enrichment |
Environmental enrichment improves memory performance in aged but not young male mice; exercise improves memory in younger female mice, but exercise + cognitive enrichment needed to improve memory in older female mice |
Harburger et al. (2007) Behav Brain Res. 185(1): 43–8. Harburger et al. (2007) Behav Neurosci. 121(4): 679–88. |
Exercise, social interactions and cognitive enrichment improve cognitive function in APP and APP/PS1 transgenic mice, but also improve cognitive function in non- transgenic controls |
Costa et al. (2007) Neurobiol Aging 28: 831–844. Jankowsky et al. (2005) J Neurosci. 25(21):5217–5224. |
| Brain Aβ elevation |
Murine Aβ rises with age in mice | Fukumoto et al. (2004) Am J Pathol. 164(2): 719–25. |
Human Aβ rises with age in APP transgenic mice |
Games et al. (1995) Nature. 373(6514): 523–7. Hsiao et al. (1996) Science. 274(5284): 99–102. Sturchler-Pierrat et al. (1997) Proc Natl Acad Sci U S A. 94(24): 13287–92. Chishti et al. (2001) J Biol Chem. 276(24): 21562–21570. |
| Differences | Aged rats & mice | Citations |
Transgenic APP mice and Aβ- infused rats |
Citations |
| Amyloid deposits | None (rodent Aβ) | Present (human Aβ) | ||
| AMPA binding in hippocampus |
No change in aged rats; no relationship to memory |
Nicolle et al. (1996) Neuroscience. 74(3): 741–56. |
Increased in aged Tg2576 mice | Cha et al. (2001) Neurobiol Dis. 8(1): 90–102. |
| NMDA binding in hippocampus |
Decreased in aged rats; inverse relationship to memory |
Nicolle et al. (1996) Neuroscience. 74(3): 741–56. Adams et al. (2001) J Comp Neurol. 432(2): 230–43. |
No change in aged Tg2576 mice | Cha et al. (2001) Neurobiol Dis. 8(1): 90–102. |
| Effect of SOD2 overexpression |
SOD2 over expression has no effect on age-associated memory impairment or LTP |
Hu et al. (2007) Neurobiol Learn Mem. 87(3): 372–84. |
SOD2 over expression prevents memory impairments in Tg19959 mice |
Dumont et al. (2009) FASEB J. 23(8): 2459–66. |
Studies using the intraspecific approach support the idea that natural aging and aberrant APP metabolism may be inextricably linked. A comprehensive study in the FVB/N mouse strain exemplifies this approach (Hsiao et al., 1995). In ~20% of FVB/N mice >150 days of age, a neurological syndrome develops that is characterized by neophobia, cerebral astrogliosis without amyloid plaques, and diminished cerebral glucose utilization in the hippocampus, amygdala, entorhinal cortex, temporal cortex and parietal cortex (association neocortex), but not in the somatosensory, frontal or occipital cortices (primary neocortex). The regional brain glucose hypo-utilization occurs in regions associated with learning, memory and affective behavior and overlaps strikingly with brain regions that are affected in people with AD or at risk for AD (Fig. 2). The expression of human APP in mice accelerates this syndrome in a dose-dependent fashion. Importantly no amyloid plaques form in the impaired mice, suggesting that the APP metabolite(s) responsible for initiating brain dysfunction are soluble Aβ species that are generated prior to amyloid deposition.
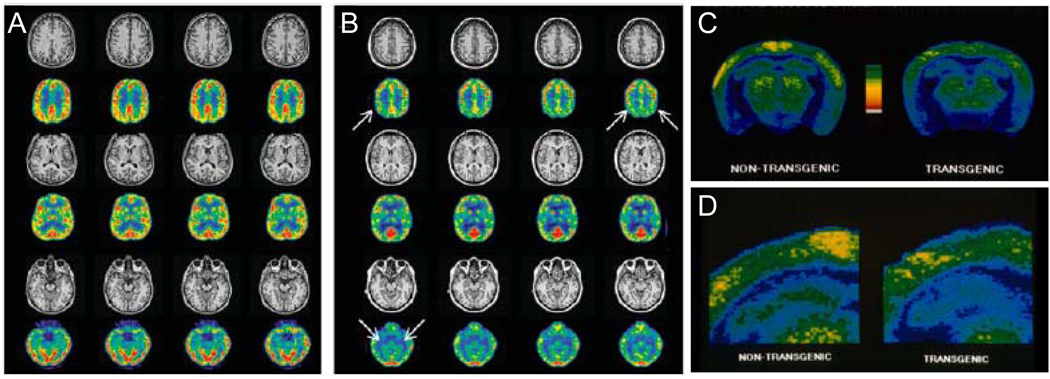
Figure 2.
Regional hypometabolism in APP transgenic mice and people at risk for AD. MRI and [18F]-FDG-PET scans of (A) a control subject and (B) a carrier of a presenilin-1 mutation associated with familial autosomal dominant AD, 27 years before mean age of onset of dementia in her family. Bilateral hypometabolism of parietal cortex and medial temporal lobe is evident on PET in the absence of atrophy on MRI. C) Regional cerebral glucose utilization, monitored with [14C]2-deoxyglucose, in the cortex and hippocampus of a neophobic transgenic FVB/N mouse expressing human APP with a disease-linked mutation. Compared to its non-transgenic littermate, the transgenic mouse shows decreases in glucose utilization in cerebral cortex and hippocampus. Pseudocolored images of autoradiograms of coronal sections through cerebral cortex, hippocampus, and anterior brainstem; warmer colors represent greater glucose uptake. D) Magnified view of (C) showing hippocampus and overlying cerebral cortex. (A,B) reprinted by permission of the Society of Nuclear Medicine from: Mosconi L, Sorbi S, de Leon MJ, et al. Hypometabolism exceeds atrophy in presymptomatic early-onset familial Alzheimer's disease. J Nucl Med. 2006; 47(11): 1778–86. Figure 2. (C,D) reprinted from Neuron 15(5), Hsiao KK, Borchelt DR, Olson K, Johannsdottir R, Kitt C, Yunis W, Xu S, Eckman C, Younkin S, Price D, et al., Age-related CNS disorder and early death in transgenic FVB/N mice overexpressing Alzheimer amyloid precursor proteins, Pages 1203–18, Copyright (1995), with permission from Elsevier.
The prediction that aberrant APP metabolism will accelerate age-related neural dysfunction was borne out in studies of senescence-accelerated prone mice (SAMP), which were derived from a parent strain of AKR/J mice (Takeda et al., 1981). Fourteen sub-strains of SAMP mice have been generated (Takeda, 1999), five of which develop deficits in learning and memory within the first year. One of these sub-strains, SAMP8, has elevated levels of APP and Aβ compared to the parent strain (Okuma and Nomura, 1998). The cognitive deficits in SAMP8 mice improve following administration of Aβ antibodies or antisense oligonucleotides that lower APP mRNA, indicating a role for the aberrant metabolism of mouse APP and the overproduction of mouse Aβ (Banks et al., 2001; Kumar et al., 2000; Morley et al., 2000). No amyloid plaques form in SAMP8 mice. These studies in AKR/J mice confirm the finding in FVB/N mice that abnormal APP metabolism can accelerate naturally occurring age-related brain disorders, independently of amyloid plaque deposition.
The conclusion that aberrant APP processing accelerates age-related neural dysfunction was drawn from studies using an intraspecific frame of reference, comparing the phenotypes of transgenic or selectively-inbred mice to the parent strain. Using an extraspecific frame of reference, comparing patterns of brain hypometabolism observed in APP transgenic mice (Hsiao et al., 1995) with those observed in humans with AD or at risk for AD (de Leon et al., 2007; Mosconi et al., 2008; Petrie et al., 2009), we suggest that APP transgenic mice model asymptomatic AD (Fig. 3). The factors that cause the transition from asymptomatic AD to full-blown AD are unknown and there are not yet transgenic mouse models suitable for addressing this question.
Figure 3.
APP transgenic mice model asymptomatic Alzheimer’s disease. APP transgenic mice exhibit functional abnormalities similar to those observed in people at risk for Alzheimer’s disease, but do not display the loss of neurons seen in people clinically diagnosed with Mild Cognitive Impairment or Alzheimer’s disease.
3.4 Initiation versus mediation of cognitive dysfunction
In mice the amount and type of Aβ generated govern the formation of amyloid plaques. Amyloid plaques will form in the cerebrum within the first year of life when Aβ levels exceed a threshold, which is ~30 pmoles/gm Aβ40 and ~10 pmoles/gm Aβ42 (Hsiao et al., 1996). Modifier genes that reduce Aβ40 and Aβ42 below this threshold by only ~20% cause a near doubling of the time to amyloid deposition (Lehman et al., 2003), and further reductions in Aβ levels preclude amyloid plaque formation altogether. Increasing the ratio of Aβ42: Aβ40 or introducing mutations into Aβ that increase its propensity to aggregate lowers the threshold required for plaques to form (Cheng et al., 2004; Mucke et al., 2000). Neurofibrillary tangles form in the brains of mice expressing either wild-type or mutant human tau. Neurofibrillary tangles form in cortical neurons of mice by 18 months of age when wild-type tau expression occurs at ~5-fold that of endogenous mouse tau (Ishihara et al., 2001). In general, mutant tau induces neurofibrillary tangle formation at lower levels than wild-type tau, and tangles develop earlier, often within the first year of life. The effective levels for generating tangles of mutant tau range from ~0.7-fold to ~5-fold endogenous mouse tau levels (Gotz et al., 2001a; Schindowski et al., 2006; Yoshiyama et al., 2007).
Within a given APP or tau transgenic line, different mice succumb at different ages, even when the mice are inbred, indicating that environmental or stochastic processes, about which we know nothing, interact with the transgenic proteins to induce disease. Once disease has begun, all mice in plaque- and tangle-bearing APP and tau transgenic lines will eventually develop these neuropathological lesions. However, APP and tau transgenic mice differ in the proportion of mice that develop behavioral abnormalities. Not all APP transgenic mice become impaired; a small number of very old APP transgenic mice will possess abundant plaques but minimal cognitive deficits (Westerman et al., 2002), reminiscent of cognitively intact humans with abundant plaques at death (Schneider et al., 2007). In contrast, all tau mice that exhibit neuropathology eventually develop neurological deficits.
In APP transgenic mice, the incomplete penetrance of the neurological deficits contrasts with the complete penetrance of the neuropathology. This suggests that processes downstream or parallel to the generation of observable lesions, present in some but not all mice, must be engaged to impair cognitive function. Conversely, as yet unknown compensatory mechanisms might exist that protect some, but not all, mice from suffering cognitive decline. Compensatory mechanisms appear unable to protect tau transgenic mice from brain dysfunction, however. These observations are consistent with the hypothesis that Aβ initiates a disease process that might progress to a stage of cognitive impairment, but that tau mediates cognitive dysfunction (Fig. 4). Several lines of evidence from transgenic mice and observations of human pathology support this hypothesis.
Figure 4.
Ab initiates a disease process that might progress to a stage of cognitive impairment, but tau mediates cognitive dysfunction. In this hypothetical scheme, Aβ*56 (see Section 4.1) and perhaps other Aβ oligomers, cause synaptic dysfunction that manifests as subtle cognitive deficits in APP transgenic mice and in people in the asymptomatic phase of AD. Toxic Aβ oligomers surrounding plaques locally damage neurons, but their effects on cognition are unknown (see Section 4.2). Abnormal tau processing triggered by pathological forms of Aβ in AD, and by mutations in tau transgenic mice, cause loss of synapses and neurons and cognitive deficits severe enough to warrant a diagnosis of Mild Cognitive Impairment (MCI) or AD. The effects of NFTs, rather than soluble pathological forms of tau, are unknown (see Section 4.4). It is also not known whether the same effector molecules/mechanisms responsible for Aβ-induced synaptic and cognitive dysfunction trigger tau abnormalities or whether a separate pathway is involved.
Studies in mice from at least four independent laboratories have shown that Aβ can potentiate tau pathology (Bolmont et al., 2007; Gotz et al., 2001b; Lewis et al., 2001; Oddo et al., 2004), consistent with the hypothesis that tau abnormalities develop downstream of Aβ in AD. An increase in NFTs was observed in the brains of transgenic mice expressing human tauP301L, a tau variant linked to frontotemporal dementia, when these mice were crossed with mice expressing human APP with a mutation linked to familial AD (Lewis et al., 2001). Intracerebral injection of fibrillar synthetic Aβ42 (Gotz et al., 2001b), or brain lysates from APP transgenic mice (Bolmont et al., 2007) induced a significant increase in the numbers of NFTs in the brains of transgenic mice that express human tauP301L. In the 3×Tg-AD mouse, subtle memory deficits presenting prior to tau pathology give way to severe deficits that are accompanied by tau abnormalities (Billings et al., 2005). The reduction of soluble Aβ and tau, but not soluble Aβ alone, abrogated the severe deficits (Oddo et al., 2006). Thus, it appears that pathologically altered tau is a major factor in late-stage cognitive deficits.
4. Physiological basis of memory loss in Alzheimer mouse models
Memory loss in AD historically was explained by the disruption of neuronal architecture, connectivity and viability due to the accumulation of NFTs and amyloid plaques. However, while studies in humans have thus far focused on the correlation between neuropathological lesions and cognitive decline, recent studies in transgenic mice suggest that attention also should be paid to soluble species of Aβ and tau that appear before amyloid plaques and NFTs and cause synaptic dysfunction and neurodegeneration.
4.1 Soluble Aβ oligomers are associated with memory deficits in APP transgenic mice
In elderly individuals, amyloid burden, considered independently of NFTs, is a poor predictor of cognitive function (Bennett et al., 2004; Giannakopoulos et al., 2003; Grober et al., 1999). Similarly, APP and APP/PS1 transgenic mice exhibit a range of correlations between memory dysfunction and plaques (Gordon et al., 2001; Holcomb et al., 1999; Westerman et al., 2002). The inconsistent relationship between plaque load and degree of cognitive impairment led to the hypothesis that soluble oligomeric forms of Aβ are the pathogenic species in AD (Westerman et al., 2002). Lesné et al. (Lesne et al., 2006) isolated from the brains of APP transgenic mice a specific Aβ oligomer, Aβ*56, that correlates with impaired memory in these mice and induces memory dysfunction when injected into the brains of normal rats. Interestingly, a transient dip in the levels of Aβ*56 coincides with an interlude of normal memory function occurring at a time when the rate, not the amount, of amyloid deposition peaks (Lesne et al., 2008). Cheng et al. (Cheng et al., 2007) compared memory function in plaque-forming APP transgenic mice possessing Aβ*56 to plaque-forming mice lacking Aβ*56, and found deficits only in the mice with Aβ*56.
4.2 Plaque-associated abnormalities
The studies of Cheng et al. and Lesné et al. indicate that when the plaque load is modest (<1% of the cross-sectional area of brain sections), Aβ*56 appears to be the cause of memory dysfunction, but do not address whether abundant amyloid plaques contribute to cognitive decline. There is plentiful evidence that the microenvironment surrounding amyloid plaques is toxic to neurons. Neurons immediately adjacent (within ~10 µm of the edge of the fibrillar Aβ) to plaques die (Urbanc et al., 2002), and those located within an ~50 µm halo exhibit altered electrophysiological properties (Busche et al., 2008; Stern et al., 2004), distorted neurites (Knowles et al., 1999; Meyer-Luehmann et al., 2008), and loss of dendritic spines (Spires-Jones et al., 2007; Spires et al., 2005)and excitatory synapses (Koffie et al., 2009). Passive immunotherapy with monoclonal antibodies directed against Aβ decreases the number of dystrophic neurites without altering plaque burden (Rozkalne et al., 2009), suggesting that soluble Aβ in the vicinity of plaques mediates the effects on neurite architecture. Interestingly, antibodies directed against oligomeric Aβ stain profiles within and surrounding plaques; although it cannot be certain that the locations of these profiles in fixed tissue precisely reflects their locations in vivo, excitatory post-synaptic densities that co-localize with these profiles are ~40% smaller than normal (Koffie et al., 2009). Notwithstanding the robust neuronal abnormalities associated with plaques, their contribution to memory and cognitive deficits remains unknown.
The contributions to cognitive dysfunction of specific Aβ oligomers and fibrillar Aβ deposited in plaques are clinically significant, as this will determine the success of new methods for reducing and imaging plaque load in affecting and monitoring the neurological status of Alzheimer patients. The relevance of Aβ*56 to human disease has yet to be established, although synaptotoxic Aβ dimers have been isolated from AD brains (Shankar et al., 2008). Finally, the issue has been raised as to whether particular Aβ oligomers exist in vivo or whether they are created artifactually during the extraction process. Pending the development of oligomer-specific reagents capable of detecting their targets in vivo, this will remain a question for debate.
4.3 Neurophysiological abnormalities in APP transgenic mice
Studies in APP transgenic mice, which exhibit cognitive deficits in the absence of neurodegeneration, have led to the conclusion that AD begins as a disease of synaptic dysfunction. Given that hippocampal long-term potentiation (LTP) arguably represents an electrophysiological correlate of learning and memory (Martin et al., 2000), several laboratories have attempted to determine whether APP transgenic mice exhibit deficits in hippocampal LTP. Unfortunately, a consistent story has not emerged from these studies, with results ranging from deficits in LTP to enhanced LTP (summarized in Supplementary Table 1). These discrepancies might be explained by several factors, including transgene, background strain, age, anesthetic state of mice in studies in vivo, health of in vitro preparations, and the precise stimulus protocols used to evoke LTP.
Based on their immunohistochemical and electrophysiological findings, Mucke and colleagues have put forth the intriguing suggestion that hyperexcitability within the hippocampus triggers a compensatory remodeling of inhibitory circuits, either or both of which could contribute to deficits in hippocampal-based memory tasks (Palop et al., 2007). In support of this hypothesis, non-epileptic doses of the GABAA antagonist picrotoxin rescued cognitive deficits in mice expressing transgenes for both mutant human APP and mutant human PS1 (Yoshiike et al., 2008). Cognitive deficits in APP transgenic mice can be rapidly reversed by passive immunization with monoclonal antibodies directed against Aβ (Dodart et al., 2002; Kotilinek et al., 2002). If immunization as rapidly reverses the remodeling of inhibitory circuits, this would provide further support for the hypothesis of Mucke and Palop; otherwise, it is more likely that Aβ-induced memory dysfunction in these mice is due to acute effects of Aβ at synapses.
If the hippocampus is indeed hyperexcitable in APP transgenic mice, these mice might be expected to have an increased susceptibility to epileptic seizures compared to non-transgenic mice of the same age and background. Lower seizure thresholds or more severe seizures in response to excitotoxic challenge (Del Vecchio et al., 2004; Jolas et al., 2002; Palop et al., 2007; Westmark et al., 2008) and spontaneous behavioral or electrographic seizures (Chin et al., 2007; Minkeviciene et al., 2009; Palop et al., 2007) have been reported in several lines. Epilepsy does occur more commonly among elderly with AD than in cognitively intact individuals (recently reviewed by Palop and Mucke (Palop and Mucke, 2009)), suggesting that the relevance of seizure susceptibility to the mechanism of memory dysfunction in AD deserves further study.
It is quite possible that APP transgenic mice undergo more complex changes in activity-dependent plasticity or metaplasticity than those revealed in conventional studies of LTP. Electrophysiological studies in APP transgenic mice appear to have fallen out of favor, probably due to the discouraging lack of consistency in existing studies. Correlative behavioral, electrophysiological, and biochemical studies of mice at different ages within single lines are needed to determine how abnormal Aβ species impact the function of specific neural circuits.
4.4 Targets of Aβ
The molecular targets of specific, naturally-derived Aβ oligomers are largely unknown, although their interactions with particular pathways have been inferred from physiological, biochemical and pharmacological studies. Supplementary Table 2 lists the several receptors and processes shown to be affected by various Aβ species. As mentioned above, the determination of which Aβ species are actually important in disrupting memory function in vivo awaits the development of reagents that target specific Aβ oligomers.
The intracellular effector molecules and pathways modulated by Aβ are also largely unknown, although P21-activated kinases (Ma et al., 2008; Zhao et al., 2006), the Src family tyrosine kinase Fyn (Chin et al., 2005; Lambert et al., 1998), protein phosphatase 2B and the tyrosine phosphatase STEP (Snyder et al., 2005), cyclooxygenase-2 (Kotilinek et al., 2008), cAMP (Shrestha et al., 2006) and phospholipase A2 (Sanchez-Mejia et al., 2008) have all been implicated in Aβ-induced synaptic deficits and cognitive dysfunction. In addition to the neurodegenerative changes thought to underlie the clinical phase of AD, tau might also mediate the more subtle synaptic and cognitive deficits induced by Aβ (Roberson et al., 2007).
4.5 Do soluble tau species mediate cognitive dysfunction/neuronal damage?
When transgenic tau is suppressed in mice expressing a regulatable tau transgene (rTg4510 mice), NFTs continue to accumulate, but neuron loss is arrested (SantaCruz et al., 2005; Spires et al., 2006) and memory function recovers (SantaCruz et al., 2005). This dissociation between neurofibrillary pathology and neuron death is supported by observations that neuron loss far exceeds NFT number in AD brains (Gomez-Isla et al., 1997) and dying neurons in the brains of tau transgenic mice frequently do not contain NFTs (Andorfer et al., 2005; Spires et al., 2006). These observations led to the hypothesis that an as yet unknown post-translationally modified form of the tau protein, and not NFTs, is responsible for cognitive dysfunction and neuron loss. It has been reported that a 170 kDa tau multimer correlates with memory dysfunction in rTg4510 mice and with the appearance of motor deficits in JNPL3 tau transgenic mice that express tauP301L in the spinal cord and that ~170-kDa tau is found in brains of FTD and AD patients (Berger et al., 2007), but determining whether this species causes memory dysfunction will be challenging.
While much of the study of tau-induced pathology has focused on neuron loss, tau-induced synaptic dysfunction might also have a role in memory impairment. In transgenic mice expressing tauP301S, decreases in the pre-synaptic markers synaptophysin and α-synuclein become apparent at 3 months of age, and synaptic dysfunction is well advanced by the time NFTs appear at 6 months (Yoshiyama et al., 2007), suggesting the involvement of soluble tau. Additional studies have demonstrated synaptic dysfunction in transgenic tau mice, but these studies did not address whether functional deficits precede the appearance of NFTs (Rosenmann et al., 2008; Schindowski et al., 2006).
The mechanisms through which tau kills neurons or impairs synaptic function remain unknown. It has been suggested that impaired axon transport might prevent the anterograde delivery of necessary cellular components, including mitochondria, from the soma to the axon terminal or retrograde delivery of neurotrophic factors from post-synaptic targets to the nucleus (Mandelkow et al., 2003; Stoothoff et al., 2009).
4.6 Physiological roles of APP metabolites and tau
The majority of studies in transgenic mice have focused on toxic gains of function of abnormally processed APP and tau. However, it is also possible that loss of function of normal forms of these molecules or their metabolites contributes to disease progression.
APP moves via fast anterograde axonal transport from central and peripheral neuronal cell bodies to nerve terminals (Buxbaum et al., 1998; Koo et al., 1990). In nerve terminals APP undergoes proteolysis at α-, β-, γ-, and ε-secretase sites to release amino-terminal, internal and carboxyl-terminal polypeptides, including APP intracellular domain (AICD), Aβ, and secreted APPα (sAPPα). Because APP trafficking and cleavage can be regulated by neuronal activity (Cirrito et al., 2005; Kamenetz et al., 2003; Tampellini et al., 2009), a physiological role for APP and its cleavage products has been proposed. The observation that genetic ablation of APP impairs memory is consistent with this idea (Dawson et al., 1999; Senechal et al., 2007; Senechal et al., 2008). sAPPα enhances memory when injected into the cerebral ventricles of mice (Meziane et al., 1998) and AICD has been suggested to improve memory in transgenic mice (Laird et al., 2005; Ma et al., 2007). Cleavage of APP might also have a role in axon pruning during development; trophic factor deprivation stimulates β-secretase-dependent cleavage of an N-terminal fragment of APP, which binds to death receptor-6 and triggers axon degeneration (Nikolaev et al., 2009). The production of sAPPα via α-secretase cleavage of APP and of AICD and Aβ via the β– and γ– secretase pathways are mutually exclusive. Further studies are required to understand the types of neural processes that regulate and are in turn regulated by these two pathways.
Tau binds and promotes the assembly of tubulin (Weingarten et al., 1975) and is essential for normal neuronal maturation in culture and microtubule organization in mice (Dawson et al., 2001; Harada et al., 1994). Biochemical methods detect tau in all neuronal compartments, but immunohistochemistry shows it primarily in axons, where tau is enriched in axonal microtubules. Studies addressing the role of tau in the brain suggest involvement in development, learning and plasticity. The over expression of tau improved memory and long-lasting synaptic plasticity, before the onset of tau hyperphosphorylation and tauopathy (Boekhoorn et al., 2006). Its absence produced hyperactivity and impaired fear-conditioning in one tau knockout line (Ikegami et al., 2000), but not in another (Roberson et al., 2007). No explanation for the discrepancy between phenotypes in the two tau knockout lines has been offered. Genetic ablation of tau in adult mice, by conditional knockout or siRNA methods, has not been reported but may reveal additional functions of tau in the brain.
5. Overall conclusions
Despite being incomplete disease models, transgenic mice have provided important insights into the pathobiology of AD. Studies using the intraspecific approach have demonstrated that abnormal APP metabolism can accelerate naturally occurring age-related brain disorders, independently of amyloid plaque deposition. These results blur the distinction between age-related cognitive decline and asymptomatic AD. Further studies in mice have pointed to the roles of soluble Aβ oligomers and soluble tau in disease pathogenesis and support a model in which 1) soluble Aβ oligomers trigger synaptic dysfunction; 2) subsequently, Aβ oligomers shed from plaques might contribute to additional synaptic dysfunction but at this point, in humans, the disease is still pre-symptomatic; 3) formation of abnormal tau species leads to neuron death and cognitive decline severe enough to warrant a dementia diagnosis. The identity of soluble toxic tau species has yet to be confirmed, but several lines of evidence in mice lead to the conclusion that NFTs themselves are not required for neuron death or dysfunction.
Researchers urgently need more complete mouse models of AD, although it is far from clear how to achieve these. The ability of Aβ to induce NFTs comprised of wild-type human tau has never been demonstrated in transgenic mice. Since tau mutations are not required for NFT formation in AD, this failure of current mouse models seriously compromises our ability to study the nexus of interactions between Aβ and tau in AD, and also generates potentially misleading results in mice used for pre-clinical testing of AD therapies. At present, APP transgenic mice should be considered models of accelerated brain aging or asymptomatic AD, and the results of interventional studies in these mice should be considered in the context of primary prevention.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Citations
- Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann Neurol. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andorfer C, Acker CM, Kress Y, Hof PR, Duff K, Davies P. Cell-cycle reentry and cell death in transgenic mice expressing nonmutant human tau isoforms. J Neurosci. 2005;25:5446–5454. doi: 10.1523/JNEUROSCI.4637-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andorfer C, Kress Y, Espinoza M, de Silva R, Tucker KL, Barde YA, Duff K, Davies P. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem. 2003;86:582–590. doi: 10.1046/j.1471-4159.2003.01879.x. [DOI] [PubMed] [Google Scholar]
- Banks WA, Farr SA, Butt W, Kumar VB, Franko MW, Morley JE. Delivery across the blood-brain barrier of antisense directed against amyloid beta: reversal of learning and memory deficits in mice overexpressing amyloid precursor protein. J Pharmacol Exp Ther. 2001;297:1113–1121. [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol. 2004;61:378–384. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- Berger Z, Roder H, Hanna A, Carlson A, Rangachari V, Yue M, Wszolek Z, Ashe K, Knight J, Dickson D, et al. Accumulation of pathological tau species and memory loss in a conditional model of tauopathy. J Neurosci. 2007;27:3650–3662. doi: 10.1523/JNEUROSCI.0587-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings LM, Oddo S, Green KN, McGaugh JL, Laferla FM. Intraneuronal Abeta causes the onset of early Alzheimer' disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Boekhoorn K, Terwel D, Biemans B, Borghgraef P, Wiegert O, Ramakers GJ, de Vos K, Krugers H, Tomiyama T, Mori H, et al. Improved long-term potentiation and memory in young tau-P301L transgenic mice before onset of hyperphosphorylation and tauopathy. J Neurosci. 2006;26:3514–3523. doi: 10.1523/JNEUROSCI.5425-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolmont T, Clavaguera F, Meyer-Luehmann M, Herzig MC, Radde R, Staufenbiel M, Lewis J, Hutton M, Tolnay M, Jucker M. Induction of tau pathology by intracerebral infusion of amyloid-beta -containing brain extract and by amyloid-beta deposition in APP × Tau transgenic mice. Am J Pathol. 2007;171:2012–2020. doi: 10.2353/ajpath.2007.070403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers N, Sleegers K, Van Broeckhoven C. Molecular genetics of Alzheimer's disease: an update. Ann Med. 2008;40:562–583. doi: 10.1080/07853890802186905. [DOI] [PubMed] [Google Scholar]
- Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold KH, Haass C, Staufenbiel M, Konnerth A, Garaschuk O. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer's disease. Science. 2008;321:1686–1689. doi: 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD, Thinakaran G, Koliatsos V, O'Callahan J, Slunt HH, Price DL, Sisodia SS. Alzheimer amyloid protein precursor in the rat hippocampus: transport and processing through the perforant path. J Neurosci. 1998;18:9629–9637. doi: 10.1523/JNEUROSCI.18-23-09629.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capsoni S, Cattaneo A. On the molecular basis linking Nerve Growth Factor (NGF) to Alzheimer's disease. Cell Mol Neurobiol. 2006;26:619–633. doi: 10.1007/s10571-006-9112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Chen K, Lee W, Alexander GE, Reiman EM. Correlating cerebral hypometabolism with future memory decline in subsequent converters to amnestic pre-mild cognitive impairment. Arch Neurol. 2008;65:1231–1236. doi: 10.1001/archneurol.2008.1. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin M-C, Crawford F, Houlden H, Warren A, Hughes D, Fidani L, Goate A, Rossor M, Roques P, Hardy J, Mullan M. Early-onset Alzheimer's disease caused by mutation at codon 717 of the β-amyloid precursor protein gene. Nature. 1991;353:844–846. doi: 10.1038/353844a0. [DOI] [PubMed] [Google Scholar]
- Cheng IH, Palop JJ, Esposito LA, Bien-Ly N, Yan F, Mucke L. Aggressive amyloidosis in mice expressing human amyloid peptides with the Arctic mutation. Nat Med. 2004;10:1190–1192. doi: 10.1038/nm1123. [DOI] [PubMed] [Google Scholar]
- Cheng IH, Scearce-Levie K, Legleiter J, Palop JJ, Gerstein H, Bien-Ly N, Puolivali J, Lesne S, Ashe KH, Muchowski PJ, Mucke L. Accelerating amyloid-beta fibrillization reduces oligomer levels and functional deficits in Alzheimer disease mouse models. J Biol Chem. 2007;282:23818–23828. doi: 10.1074/jbc.M701078200. [DOI] [PubMed] [Google Scholar]
- Chin J, Ling H-P, Comery T, Pangalos M, Reinhart P, Wood A. Chronic imbalance in neuronal activity and compensatory plasticity are associated with cognitive impairment in Tg2576 and PS1/APP mouse models of Alzheimer’s disease. International Conference on Alzheimer’s Disease; Chicago, IL. 2007. [Google Scholar]
- Chin J, Palop JJ, Puolivali J, Massaro C, Bien-Ly N, Gerstein H, Scearce-Levie K, Masliah E, Mucke L. Fyn kinase induces synaptic and cognitive impairments in a transgenic mouse model of Alzheimer's disease. J Neurosci. 2005;25:9694–9703. doi: 10.1523/JNEUROSCI.2980-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak VMA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Cruts M, Van Broeckhoven C. Molecular genetics of Alzheimer's disease. Ann Med. 1998;30:560–565. doi: 10.3109/07853899809002605. [DOI] [PubMed] [Google Scholar]
- Dawson GR, Seabrook GR, Zheng H, Smith DW, Graham S, O'Dowd G, Bowery BJ, Boyce S, Trumbauer ME, Chen HY, et al. Age-related cognitive deficits, impaired long-term potentiation and reduction in synaptic marker density in mice lacking the beta-amyloid precursor protein. Neuroscience. 1999;90:1–13. doi: 10.1016/s0306-4522(98)00410-2. [DOI] [PubMed] [Google Scholar]
- Dawson HN, Ferreira A, Eyster MV, Ghoshal N, Binder LI, Vitek MP. Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. J Cell Sci. 2001;114:1179–1187. doi: 10.1242/jcs.114.6.1179. [DOI] [PubMed] [Google Scholar]
- de Leon MJ, Convit A, Wolf OT, Tarshish CY, DeSanti S, Rusinek H, Tsui W, Kandil E, Scherer AJ, Roche A, et al. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-D-glucose/poitron-emission tomography (FDG/PET) Proc Natl Acad Sci U S A. 2001;98:10966–10971. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon MJ, Ferris SH, George AE, Christman DR, Fowler JS, Gentes C, Reisberg B, Gee B, Emmerich M, Yonekura Y, et al. Positron emission tomography studies of aging and Alzheimer's disease. American Journal of Neuroradiology. 1983;4:568–571. [PMC free article] [PubMed] [Google Scholar]
- de Leon MJ, Mosconi L, Blennow K, DeSanti S, Zinkowski R, Mehta PD, Pratico D, Tsui W, Saint Louis LA, Sobanska L, et al. Imaging and CSF studies in the preclinical diagnosis of Alzheimer's disease. Ann N Y Acad Sci. 2007;1097:114–145. doi: 10.1196/annals.1379.012. [DOI] [PubMed] [Google Scholar]
- de Toledo-Morrell L, Goncharova I, Dickerson B, Wilson RS, Bennett DA. From healthy aging to early Alzheimer's disease: in vivo detection of entorhinal cortex atrophy. Ann N Y Acad Sci. 2000;911:240–253. doi: 10.1111/j.1749-6632.2000.tb06730.x. [DOI] [PubMed] [Google Scholar]
- Del Vecchio RA, Gold LH, Novick SJ, Wong G, Hyde LA. Increased seizure threshold and severity in young transgenic CRND8 mice. Neurosci Lett. 2004;367:164–167. doi: 10.1016/j.neulet.2004.05.107. [DOI] [PubMed] [Google Scholar]
- den Heijer T, Geerlings MI, Hoebeek FE, Hofman A, Koudstaal PJ, Breteler MM. Use of hippocampal and amygdalar volumes on magnetic resonance imaging to predict dementia in cognitively intact elderly people. Arch Gen Psychiatry. 2006;63:57–62. doi: 10.1001/archpsyc.63.1.57. [DOI] [PubMed] [Google Scholar]
- Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holtzman DM, Paul SM. Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer's disease model. Nat Neurosci. 2002;5:452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- Duyckaerts C, Potier MC, Delatour B. Alzheimer disease models and human neuropathology: similarities and differences. Acta Neuropathol. 2008;115:5–38. doi: 10.1007/s00401-007-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster NL. A new framework for the diagnosis of Alzheimer's disease. Lancet Neurol. 2007;6:667–669. doi: 10.1016/S1474-4422(07)70179-5. [DOI] [PubMed] [Google Scholar]
- Giannakopoulos P, Herrmann FR, Bussiere T, Bouras C, Kovari E, Perl DP, Morrison JH, Gold G, Hof PR. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer's disease. Neurology. 2003;60:1495–1500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984;122:1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- Goate AM, Chartier-Harlin CM, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Goldgaber D, Lerman MI, McBride OW, Saffiotti U, Gajdusek DC. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer's disease. Science. 1987;235:877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, Parisi JE, Hyman BT. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer's disease. Ann Neurol. 1997;41:17–24. doi: 10.1002/ana.410410106. [DOI] [PubMed] [Google Scholar]
- Gordon MN, King DL, Diamond DM, Jantzen PT, Boyett KV, Hope CE, Hatcher JM, DiCarlo G, Gottschall WP, Morgan D, Arendash GW. Correlation between cognitive deficits and Abeta deposits in transgenic APP+PS1 mice. Neurobiol Aging. 2001;22:377–385. doi: 10.1016/s0197-4580(00)00249-9. [DOI] [PubMed] [Google Scholar]
- Gotz J, Chen F, Barmettler R, Nitsch RM. Tau filament formation in transgenic mice expressing P301L tau. J Biol Chem. 2001a;276:529–534. doi: 10.1074/jbc.M006531200. [DOI] [PubMed] [Google Scholar]
- Gotz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001b;293:1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- Grober E, Dickson D, Sliwinski MJ, Buschke H, Katz M, Crystal H, Lipton RB. Memory and mental status correlates of modified Braak staging. Neurobiol Aging. 1999;20:573–579. doi: 10.1016/s0197-4580(99)00063-9. [DOI] [PubMed] [Google Scholar]
- Harada A, Oguchi K, Okabe S, Kuno J, Terada S, Ohshima T, Sato-Yoshitake R, Takei Y, Noda T, Hirokawa N. Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature. 1994;369:488–491. doi: 10.1038/369488a0. [DOI] [PubMed] [Google Scholar]
- Hardy J. A hundred years of Alzheimer's disease research. Neuron. 2006;52:3–13. doi: 10.1016/j.neuron.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Higashi S, Iseki E, Yamamoto R, Minegishi M, Hino H, Fujisawa K, Togo T, Katsuse O, Uchikado H, Furukawa Y, et al. Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer's disease and dementia with Lewy bodies. Brain Res. 2007;1184:284–294. doi: 10.1016/j.brainres.2007.09.048. [DOI] [PubMed] [Google Scholar]
- Holcomb LA, Gordon MN, Jantzen P, Hsiao K, Duff K, Morgan D. Behavioral changes in transgenic mice expressing both amyloid precursor protein and presenilin-1 mutations: lack of association with amyloid deposits. Behav Genet. 1999;29:177–185. doi: 10.1023/a:1021691918517. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Hsiao KK, Borchelt DR, Olson K, Johannsdottir R, Kitt C, Yunis W, Xu S, Eckman C, Younkin S, Price D, et al. Age-related CNS disorder and early death in transgenic FVB/N mice overexpressing Alzheimer amyloid precursor proteins. Neuron. 1995;15:1203–1218. doi: 10.1016/0896-6273(95)90107-8. [DOI] [PubMed] [Google Scholar]
- Hulette CM, Welsh-Bohmer KA, Murray MG, Saunders AM, Mash DC, McIntyre LM. Neuropathological and neuropsychological changes in "normal" aging: evidence for preclinical Alzheimer disease in cognitively normal individuals. J Neuropathol Exp Neurol. 1998;57:1168–1174. doi: 10.1097/00005072-199812000-00009. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Ikegami S, Harada A, Hirokawa N. Muscle weakness, hyperactivity, and impairment in fear conditioning in tau-deficient mice. Neurosci Lett. 2000;279:129–132. doi: 10.1016/s0304-3940(99)00964-7. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Zhang B, Higuchi M, Yoshiyama Y, Trojanowski JQ, Lee VM. Age-dependent induction of congophilic neurofibrillary tau inclusions in tau transgenic mice. Am J Pathol. 2001;158:555–562. doi: 10.1016/S0002-9440(10)63997-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolas T, Zhang XS, Zhang Q, Wong G, Del Vecchio R, Gold L, Priestley T. Long-term potentiation is increased in the CA1 area of the hippocampus of APP(swe/ind) CRND8 mice. Neurobiol Dis. 2002;11:394–409. doi: 10.1006/nbdi.2002.0557. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Knopman DS, Hu WT, Stroh DA, Baker M, Rademakers R, Boeve BF, Parisi JE, Smith GE, et al. Abnormal TDP-43 21 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology. 2008;70:1850–1857. doi: 10.1212/01.wnl.0000304041.09418.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadokura A, Yamazaki T, Lemere CA, Takatama M, Okamoto K. Regional distribution of TDP-43 inclusions in Alzheimer disease (AD) brains: their relation to AD common pathology. Neuropathology. 2009;29:566–573. doi: 10.1111/j.1440-1789.2009.01017.x. [DOI] [PubMed] [Google Scholar]
- Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–937. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- Kang J, Lemaire H-G, Unterbeck A, Salbaum JM, Masters CL, Grzeschik K-H, Multhaup G, Beyreuther K. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer's disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight WD, Kim LG, Douiri A, Frost C, Rossor MN, Fox NC. Acceleration of cortical thinning in familial Alzheimer's disease. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Knowles RB, Wyart C, Buldyrev SV, Cruz L, Urbanc B, Hasselmo ME, Stanley HE, Hyman BT. Plaque-induced neurite abnormalities: implications for disruption of neural networks in Alzheimer's disease. Proc Natl Acad Sci U S A. 1999;96:5274–5279. doi: 10.1073/pnas.96.9.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, Garcia-Alloza M, Micheva KD, Smith SJ, Kim ML, Lee VM, et al. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0811698106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo EH, Sisodia SS, Archer DR, Martin LJ, Weidemann A, Beyreuther K, Fischer P, Masters CL, Price DL. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci U S A. 1990;87:1561–1565. doi: 10.1073/pnas.87.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korczyn AD. Mixed dementia--the most common cause of dementia. Ann N Y Acad Sci. 2002;977:129–134. doi: 10.1111/j.1749-6632.2002.tb04807.x. [DOI] [PubMed] [Google Scholar]
- Kotilinek LA, Bacskai B, Westerman M, Kawarabayashi T, Younkin L, Hyman BT, Younkin S, Ashe KH. Reversible memory loss in a mouse transgenic model of Alzheimer's disease. J Neurosci. 2002;22:6331–6335. doi: 10.1523/JNEUROSCI.22-15-06331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotilinek LA, Westerman MA, Wang Q, Panizzon K, Lim GP, Simonyi A, Lesne S, Falinska A, Younkin LH, Younkin SG, et al. Cyclooxygenase-2 inhibition improves amyloid-beta-mediated suppression of memory and synaptic plasticity. Brain. 2008;131:651–664. doi: 10.1093/brain/awn008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GG, Alafuzoff I, Al-Sarraj S, Arzberger T, Bogdanovic N, Capellari S, Ferrer I, Gelpi E, Kovari V, Kretzschmar H, et al. Mixed brain pathologies in dementia: the BrainNet Europe consortium experience. Dement Geriatr Cogn Disord. 2008;26:343–350. doi: 10.1159/000161560. [DOI] [PubMed] [Google Scholar]
- Kumar A, Schapiro MB, Grady C, Hasby JV, Wagner E, Salerno JA, Friedland RP, Rapoport SI. High resolution PET studies in Alzheimer's disease. Neuropsychopharmacology. 1991;4:35–45. [PubMed] [Google Scholar]
- Kumar VB, Farr SA, Flood JF, Kamlesh V, Franko M, Banks WA, Morley JE. Site-directed antisense oligonucleotide decreases the expression of amyloid precursor protein and reverses deficits in learning and memory in aged SAMP8 mice. Peptides. 2000;21:1769–1775. doi: 10.1016/s0196-9781(00)00339-9. [DOI] [PubMed] [Google Scholar]
- Laird FM, Cai H, Savonenko AV, Farah MH, He K, Melnikova T, Wen H, Chiang HC, Xu G, Koliatsos VE, et al. BACE1, a major determinant of selective vulnerability of the brain to amyloid-beta amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J Neurosci. 2005;25:11693–11709. doi: 10.1523/JNEUROSCI.2766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, et al. Diffusible, nonfibrillar ligands derived from Abeta1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa KM, Foster NL, Larson EB. Mixed dementia: emerging concepts and therapeutic implications. JAMA. 2004;292:2901–2908. doi: 10.1001/jama.292.23.2901. [DOI] [PubMed] [Google Scholar]
- Lehman EJ, Kulnane LS, Lamb BT. Alterations in beta-amyloid production and deposition in brain regions of two transgenic models. Neurobiol Aging. 2003;24:645–653. doi: 10.1016/s0197-4580(02)00153-7. [DOI] [PubMed] [Google Scholar]
- Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Lesne S, Kotilinek L, Ashe KH. Plaque-bearing mice with reduced levels of oligomeric amyloid-beta assemblies have intact memory function. Neuroscience. 2008;151:745–749. doi: 10.1016/j.neuroscience.2007.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- Lleo A, Berezovska O, Growdon JH, Hyman BT. Clinical, pathological, and biochemical spectrum of Alzheimer disease associated with PS-1 mutations. Am J Geriatr Psychiatry. 2004;12:146–156. doi: 10.1097/00019442-200403000-00006. [DOI] [PubMed] [Google Scholar]
- Loerch PM, Lu T, Dakin KA, Vann JM, Isaacs A, Geula C, Wang J, Pan Y, Gabuzda DH, Li C, et al. Evolution of the aging brain transcriptome and synaptic regulation. PLoS One. 2008;3:e3329. doi: 10.1371/journal.pone.0003329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Lesne S, Kotilinek L, Steidl-Nichols JV, Sherman M, Younkin L, Younkin S, Forster C, Sergeant N, Delacourte A, et al. Involvement of beta-site APP cleaving enzyme 1 (BACE1) in amyloid precursor protein-mediated enhancement of memory and activity-dependent synaptic plasticity. Proc Natl Acad Sci U S A. 2007;104:8167–8172. doi: 10.1073/pnas.0609521104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma QL, Yang F, Calon F, Ubeda OJ, Hansen JE, Weisbart RH, Beech W, Frautschy SA, Cole GM. p21-activated kinase-aberrant activation and translocation in Alzheimer disease pathogenesis. J Biol Chem. 2008;283:14132–14143. doi: 10.1074/jbc.M708034200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelkow EM, Stamer K, Vogel R, Thies E, Mandelkow E. Clogging of axons by tau, inhibition of axonal traffic and starvation of synapses. Neurobiol Aging. 2003;24:1079–1085. doi: 10.1016/j.neurobiolaging.2003.04.007. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer's disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meziane H, Dodart JC, Mathis C, Little S, Clemens J, Paul SM, Ungerer A. Memory-enhancing effects of secreted forms of the beta-amyloid precursor protein in normal and amnestic mice. Proc Natl Acad Sci U S A. 1998;95:12683–12688. doi: 10.1073/pnas.95.21.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkeviciene R, Rheims S, Dobszay MB, Zilberter M, Hartikainen J, Fulop L, Penke B, Zilberter Y, Harkany T, Pitkanen A, Tanila H. Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. J Neurosci. 2009;29:3453–3462. doi: 10.1523/JNEUROSCI.5215-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondadori CR, Buchmann A, Mustovic H, Schmidt CF, Boesiger P, Nitsch RM, Hock C, Streffer J, Henke K. Enhanced brain activity may precede the diagnosis of Alzheimer's disease by 30 years. Brain. 2006;129:2908–2922. doi: 10.1093/brain/awl266. [DOI] [PubMed] [Google Scholar]
- Morley JE, Kumar VB, Bernardo AE, Farr SA, Uezu K, Tumosa N, Flood JF. Beta-amyloid precursor polypeptide in SAMP8 mice affects learning and memory. Peptides. 2000;21:1761–1767. doi: 10.1016/s0196-9781(00)00342-9. [DOI] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Grant EA, Head D, Storandt M, Goate AM, Fagan AM, Holtzman DM, Mintun MA. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol. 2009;66:1469–1475. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, De Santi S, Li J, Tsui WH, Li Y, Boppana M, Laska E, Rusinek H, de Leon MJ. Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiol Aging. 2008;29:676–692. doi: 10.1016/j.neurobiolaging.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, Sorbi S, de Leon MJ, Li Y, Nacmias B, Myoung PS, Tsui W, Ginestroni A, Bessi V, Fayyazz M, et al. Hypometabolism exceeds atrophy in presymptomatic early-onset familial Alzheimer's disease. J Nucl Med. 2006;47:1778–1786. [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of abeta 1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullan M, Crawford F, Axelman K, Houlden H, Lilius L, Winblad B, Lannfelt L. A pathogenic mutation for probable Alzheimer's disease in the APP gene at the N-terminus of beta-amyloid. Nat Genet. 1992;1:345–347. doi: 10.1038/ng0892-345. [DOI] [PubMed] [Google Scholar]
- Murrell J, Farlow M, Ghetti B, Benson MD. A mutation in the amyloid precursor protein associated with hereditary Alzheimer's disease. Science. 1991;254:97–99. doi: 10.1126/science.1925564. [DOI] [PubMed] [Google Scholar]
- Murrell JR, Hake AM, Quaid KA, Farlow MR, Ghetti B. Early-onset Alzheimer disease caused by a new mutation (V717L) in the amyloid precursor protein gene. Arch Neurol. 2000;57:885–887. doi: 10.1001/archneur.57.6.885. [DOI] [PubMed] [Google Scholar]
- Nikolaev A, McLaughlin T, O'Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nilsberth C, Westlind-Danielsson A, Eckman CB, Condron MM, Axelman K, Forsell C, Stenh C, Luthman J, Teplow DB, Younkin SG, et al. The 'Arctic' APP mutation (E693G) causes Alzheimer's disease by enhanced Abeta protofibril formation. Nat Neurosci. 2001;4:887–893. doi: 10.1038/nn0901-887. [DOI] [PubMed] [Google Scholar]
- Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43:321–332. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Oddo S, Vasilevko V, Caccamo A, Kitazawa M, Cribbs DH, LaFerla FM. Reduction of soluble Abeta and tau, but not soluble Abeta alone, ameliorates cognitive decline in transgenic mice with plaques and tangles. J Biol Chem. 2006;281:39413–39423. doi: 10.1074/jbc.M608485200. [DOI] [PubMed] [Google Scholar]
- Okuma Y, Nomura Y. Senescence-accelerated mouse (SAM) as an animal model of senile dementia: pharmacological, neurochemical and molecular biological approach. Jpn J Pharmacol. 1998;78:399–404. doi: 10.1254/jjp.78.399. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer's disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop JJ, Mucke L. Epilepsy and cognitive impairments in Alzheimer disease. Arch Neurol. 2009;66:435–440. doi: 10.1001/archneurol.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Negash S. Mild cognitive impairment: an overview. CNS Spectr. 2008;13:45–53. doi: 10.1017/s1092852900016151. [DOI] [PubMed] [Google Scholar]
- Petrie EC, Cross DJ, Galasko D, Schellenberg GD, Raskind MA, Peskind ER, Minoshima S. Preclinical evidence of Alzheimer changes: convergent cerebrospinal fluid biomarker and fluorodeoxyglucose positron emission tomography findings. Arch Neurol. 2009;66:632–637. doi: 10.1001/archneurol.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, McKeel DW, Jr, Buckles VD, Roe CM, Xiong C, Grundman M, Hansen LA, Petersen RC, Parisi JE, Dickson DW, et al. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30:1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Morris JC. Tangles and plaques in nondemented aging and "preclinical" Alzheimer's disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Ramsden M, Kotilinek L, Forster C, Paulsen J, McGowan E, SantaCruz K, Guimaraes A, Yue M, Lewis J, Carlson G, et al. J Neurosci. Vol. 25. 2005. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L) pp. 10637–10647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Chen K, Alexander GE, Bandy D, Frost J. Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: A foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer's disease. Proc Natl Acad Sci U S A. 2001;98:3334–3339. doi: 10.1073/pnas.061509598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D. Preclinical evidence of Alzheimer's disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc Natl Acad Sci U S A. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridha BH, Barnes J, Bartlett JW, Godbolt A, Pepple T, Rossor MN, Fox NC. Tracking atrophy progression in familial Alzheimer's disease: a serial MRI study. Lancet Neurol. 2006;5:828–834. doi: 10.1016/S1474-4422(06)70550-6. [DOI] [PubMed] [Google Scholar]
- Robakis NK, Ramakrishna N, Wolfe G, Wisniewski HM. Molecular cloning and characterization of a cDNA encoding the cerebrovascular and the neuritic plaque amyloid peptides. Proc. Natl. Acad. Sci. 1987;84:4190–4194. doi: 10.1073/pnas.84.12.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- Rosenmann H, Grigoriadis N, Eldar-Levy H, Avital A, Rozenstein L, Touloumi O, Behar L, Ben-Hur T, Avraham Y, Berry E, et al. A novel transgenic mouse expressing double mutant tau driven by its natural promoter exhibits tauopathy characteristics. Exp Neurol. 2008;212:71–84. doi: 10.1016/j.expneurol.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Rossor MN, Fox NC, Freeborough PA, Harvey RJ. Clinical features of sporadic and familial Alzheimer's disease. Neurodegeneration. 1996;5:393–397. doi: 10.1006/neur.1996.0052. [DOI] [PubMed] [Google Scholar]
- Rovelet-Lecrux A, Hannequin D, Raux G, Le Meur N, Laquerriere A, Vital A, Dumanchin C, Feuillette S, Brice A, Vercelletto M, et al. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet. 2006;38:24–26. doi: 10.1038/ng1718. [DOI] [PubMed] [Google Scholar]
- Rozkalne A, Spires-Jones TL, Stern EA, Hyman BT. A single dose of passive immunotherapy has extended benefits on synapses and neurites in an Alzheimer's disease mouse model. Brain Res. 2009;1280:178–185. doi: 10.1016/j.brainres.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusinek H, De Santi S, Frid D, Tsui WH, Tarshish CY, Convit A, de Leon MJ. Regional brain atrophy rate predicts future cognitive decline: 6-year longitudinal MR imaging study of normal aging. Radiology. 2003;229:691–696. doi: 10.1148/radiol.2293021299. [DOI] [PubMed] [Google Scholar]
- Sanchez-Mejia RO, Newman JW, Toh S, Yu GQ, Zhou Y, Halabisky B, Cisse M, Scearce-Levie K, Cheng IH, Gan L, et al. Phospholipase A2 reduction ameliorates cognitive deficits in a mouse model of Alzheimer's disease. Nat Neurosci. 2008;11:1311–1318. doi: 10.1038/nn.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SantaCruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindowski K, Bretteville A, Leroy K, Begard S, Brion JP, Hamdane M, Buee L. Alzheimer's disease-like tau neuropathology leads to memory deficits and loss of functional synapses in a novel mutated tau transgenic mouse without any motor deficits. Am J Pathol. 2006;169:599–616. doi: 10.2353/ajpath.2006.060002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA. The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis. 2009a;18:691–701. doi: 10.3233/JAD-2009-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009b;66:200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab C, Hosokawa M, McGeer PL. Transgenic mice overexpressing amyloid beta protein are an incomplete model of Alzheimer disease. Exp Neurol. 2004;188:52–64. doi: 10.1016/j.expneurol.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Senechal Y, Kelly PH, Cryan JF, Natt F, Dev KK. Amyloid precursor protein knockdown by siRNA impairs spontaneous alternation in adult mice. J Neurochem. 2007;102:1928–1940. doi: 10.1111/j.1471-4159.2007.04672.x. [DOI] [PubMed] [Google Scholar]
- Senechal Y, Kelly PH, Dev KK. Amyloid precursor protein knockout mice show age-dependent deficits in passive avoidance learning. Behav Brain Res. 2008;186:126–132. doi: 10.1016/j.bbr.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha BR, Vitolo OV, Joshi P, Lordkipanidze T, Shelanski M, Dunaevsky A. Amyloid beta peptide adversely affects spine number and motility in hippocampal neurons. Mol Cell Neurosci. 2006;33:274–282. doi: 10.1016/j.mcn.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Sleegers K, Brouwers N, Gijselinck I, Theuns J, Goossens D, Wauters J, Del-Favero J, Cruts M, van Duijn CM, Van Broeckhoven C. APP duplication is sufficient to cause early onset Alzheimer's dementia with cerebral amyloid angiopathy. Brain. 2006;129:2977–2983. doi: 10.1093/brain/awl203. [DOI] [PubMed] [Google Scholar]
- Small GW, Ercoli LM, Silverman DH, Huang SC, Komo S, Bookheimer SY, Lavretsky H, Miller K, Siddarth P, Rasgon NL, et al. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer's disease. Proc Natl Acad Sci U S A. 2000;97:6037–6042. doi: 10.1073/pnas.090106797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. Jama. 1997;277:813–817. [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Spires-Jones TL, Meyer-Luehmann M, Osetek JD, Jones PB, Stern EA, Bacskai BJ, Hyman BT. Impaired spine stability underlies plaque-related spine loss in an Alzheimer's disease mouse model. Am J Pathol. 2007;171:1304–1311. doi: 10.2353/ajpath.2007.070055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires TL, Meyer-Luehmann M, Stern EA, McLean PJ, Skoch J, Nguyen PT, Bacskai BJ, Hyman BT. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J Neurosci. 2005;25:7278–7287. doi: 10.1523/JNEUROSCI.1879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires TL, Orne JD, SantaCruz K, Pitstick R, Carlson GA, Ashe KH, Hyman BT. Region-specific dissociation of neuronal loss and neurofibrillary pathology in a mouse model of tauopathy. Am J Pathol. 2006;168:1598–1607. doi: 10.2353/ajpath.2006.050840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern EA, Bacskai BJ, Hickey GA, Attenello FJ, Lombardo JA, Hyman BT. Cortical synaptic integration in vivo is disrupted by amyloid-beta plaques. J Neurosci. 2004;24:4535–4540. doi: 10.1523/JNEUROSCI.0462-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoothoff W, Jones PB, Spires-Jones TL, Joyner D, Chhabra E, Bercury K, Fan Z, Xie H, Bacskai B, Edd J, et al. Differential effect of three-repeat and four-repeat tau on mitochondrial axonal transport. J Neurochem. 2009;111:417–427. doi: 10.1111/j.1471-4159.2009.06316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Abeta deposition. Arch Neurol. 2009;66:1476–1481. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swearer JM, O'Donnell BF, Drachman DA, Woodward BM. Neuropsychological features of familial Alzheimer's disease. Ann Neurol. 1992;32:687–694. doi: 10.1002/ana.410320513. [DOI] [PubMed] [Google Scholar]
- Takeda T. Senescence-accelerated mouse (SAM): a biogerontological resource in aging research. Neurobiol Aging. 1999;20:105–110. doi: 10.1016/s0197-4580(99)00008-1. [DOI] [PubMed] [Google Scholar]
- Takeda T, Hosokawa M, Takeshita S, Irino M, Higuchi K, Matsushita T, Tomita Y, Yasuhira K, Hamamoto H, Shimizu K, et al. A new murine model of accelerated senescence. Mech Ageing Dev. 1981;17:183–194. doi: 10.1016/0047-6374(81)90084-1. [DOI] [PubMed] [Google Scholar]
- Tampellini D, Rahman N, Gallo EF, Huang Z, Dumont M, Capetillo-Zarate E, Ma T, Zheng R, Lu B, Nanus DM, et al. Synaptic activity reduces intraneuronal Abeta, promotes APP transport to synapses, and protects against Abeta-related synaptic alterations. J Neurosci. 2009;29:9704–9713. doi: 10.1523/JNEUROSCI.2292-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi RE, Gusella JF, Watkins PC, Bruns GAP, George-Hyslop PS, Keuren MLV, Patterson D, Pagan S, Kurnit DM, Neve RL. Amyloid β protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987;235:880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- Tomiyama T, Nagata T, Shimada H, Teraoka R, Fukushima A, Kanemitsu H, Takuma H, Kuwano R, Imagawa M, Ataka S, et al. A new amyloid beta variant favoring oligomerization in Alzheimer's-type dementia. Ann Neurol. 2008;63:377–387. doi: 10.1002/ana.21321. [DOI] [PubMed] [Google Scholar]
- Urbanc B, Cruz L, Le R, Sanders J, Ashe KH, Duff K, Stanley HE, Irizarry MC, Hyman BT. Neurotoxic effects of thioflavin S-positive amyloid deposits in transgenic mice and Alzheimer's disease. Proc Natl Acad Sci U S A. 2002;99:13990–13995. doi: 10.1073/pnas.222433299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uryu K, Nakashima-Yasuda H, Forman MS, Kwong LK, Clark CM, Grossman M, Miller BL, Kretzschmar HA, Lee VM, Trojanowski JQ, Neumann M. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol. 2008;67:555–564. doi: 10.1097/NEN.0b013e31817713b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitek MP, Snell J, Dawson H, Colton CA. Modulation of nitric oxide production in human macrophages by apolipoprotein-E and amyloid-beta peptide. Biochem Biophys Res Commun. 1997;240:391–394. doi: 10.1006/bbrc.1997.7408. [DOI] [PubMed] [Google Scholar]
- Webster SD, Tenner AJ, Poulos TL, Cribbs DH. The mouse C1q A-chain sequence alters beta-amyloid-induced complement activation. Neurobiol Aging. 1999;20:297–304. doi: 10.1016/s0197-4580(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975;72:1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, Carlson GA, Younkin SG, Ashe KH. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer's disease. J Neurosci. 2002;22:1858–1867. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmark CJ, Westmark PR, Beard AM, Hildebrandt SM, Malter JS. Seizure Susceptibility and Mortality in Mice that Over-Express Amyloid Precursor Protein. Int J Clin Exp Pathol. 2008;1:157–168. [PMC free article] [PubMed] [Google Scholar]
- Wirths O, Breyhan H, Cynis H, Schilling S, Demuth HU, Bayer TA. Intraneuronal pyroglutamate-Abeta 3–42 triggers neurodegeneration and lethal neurological deficits in a transgenic mouse model. Acta Neuropathol. 2009;118:487–496. doi: 10.1007/s00401-009-0557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiike Y, Kimura T, Yamashita S, Furudate H, Mizoroki T, Murayama M, Takashima A. GABA(A) receptor-mediated acceleration of aging-associated memory decline in APP/PS1 mice and its pharmacological treatment by picrotoxin. PLoS One. 2008;3:e3029. doi: 10.1371/journal.pone.0003029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski JQ, Lee VM. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Zhao L, Ma QL, Calon F, Harris-White ME, Yang F, Lim GP, Morihara T, Ubeda OJ, Ambegaokar S, Hansen JE, et al. Role of p21-activated kinase pathway defects in the cognitive deficits of Alzheimer disease. Nat Neurosci. 2006;9:234–242. doi: 10.1038/nn1630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.