Abstract
An analysis of more than 500 liver transplants has demonstrated that HLA compatibility is associated with diminished allograft survival. Liver transplants with zero mismatches for class I and/or class II HLA antigens have shown significantly lower actuarial survival rates than transplants with one or more mismatches for these loci. In a group of 119 failed liver allografts from patients undergoing retransplantation, a higher incidence of failure due to rejection correlated with a lower degree of HLA compatibility especially for HLA-DR. In contrast, the incidence of liver transplant failures due to primary nonfunction was relatively higher with HLA-DR compatible transplants. Considering the role of HLA as a restriction element in cellular interactions during the immune response, these findings suggest that HLA compatibility may have a dualistic effect on liver transplant outcome. On one hand, HLA compatibility reduced transplant rejection—and on the other hand, it may enhance other immunological mechanisms leading to allograft dysfunction, particularly in patients at risk of developing recurrent autoimmune diseases or infection.
HLA compatibility has been widely recognized to improve the outcome of kidney (1–3) and, probably, heart transplants (4), but no beneficial effect has been reported for liver transplants (5, 6). Additionally, humoral sensitization to HLA antigens prior to transplantation and a positive donor-specific crossmatch does not seem to influence liver allograft survival (7–9). In combined liver-kidney transplants, we have demonstrated that removal of circulating donor-specific antibodies by the liver transplant is without adverse effect on the graft itself and, that subsequent kidney transplants show good function and no hyperacute rejection (10, 11). In certain instances, the liver allograft may undergo antibody-mediated hyperacute rejection (12). Presensitization and positive crossmatches have been interpreted by some investigators to be associated with an increased incidence of vanishing bile duct syndrome in liver transplant recipients (13). This syndrome may occur more often in liver transplants from class I HLA-incompatible donors with a partial or complete match for class II DR antigens (14). HLA-specific alloreactive T cells have recently been demonstrated in lymphocyte cultures grown from hepatic allografts, providing evidence that HLA antigens are involved in cellular immune mechanisms leading to rejection of liver allografts (15, 16).
In view of the role of HLA in transplant immunity, we have recently examined the question of HLA compatibility and liver transplant survival. Our analysis of more than 500 liver allografts has confirmed previous findings that HLA compatibility does not improve overall survival of liver allografts. Here we present data that actually suggest that compatibility for both class I and class II HLA antigens is associated with a significant decrease in liver transplant survival. These surprising findings can be explained by considering a dualistic role of HLA, whereby, on the one hand, HLA operates as a system of transplantation antigens important in allograft rejection and, on the other hand, it functions as restriction element (self-recognition) important in cellular processes leading to cell-mediated immunological damage to the liver transplant.
MATERIALS AND METHODS
Between March 1980 and December 1986, 1053 orthotopic liver transplants were performed and 821 patients received first allografts, while second and third transplants were done in 232 patients. Of these, 527 adults were given 654 grafts and 294 children were given 399 grafts. All patients have been followed through July 1, 1987 and received cyclosporine and steroids as immunosuppressive drugs. As of December 1984 OKT3 monoclonal antibody therapy has been added to treat acute rejection episodes (17).
HLA typing was performed using the Amos modified (class I HLA antigens) and two-color fluorochromasia (class II HLA antigens) techniques. The tissue typing results were generally obtained shortly following transplantation and therefore played no role in recipient selection. Data on HLA-A, B phenotypes were available for 574 donor-recipient pairs, 458 of which had primary transplants. In this group, 78% were adult recipients (18 years or older) and 22% were pediatric patients. For 507 donor-recipient combinations, we had data for HLA-DR antigens, 405 of whom were primary transplants. HLA-DQ phenotyping was done on insufficient numbers of patients and donors; therefore matching for DQ was not evaluated.
This analysis also included liver transplants performed at Baylor Medical Center in Dallas. Donor and recipient HLA A,B phenotypes were obtained for 64 cases (11.1% of the total group) and 55 (10.8%) of the donor-recipient pairs were typed for HLA-DR.
The age of patients in this study group ranged from 0.6 to 67.9 years (mean 33.3±17.8 years). The most common primary indications for liver replacement in these patients included cirrhosis (35.8%), primary biliary cirrhosis (21.0%), biliary atresia (10.9%), sclerosing cholangitis (9.8%), inborn errors of metabolism (9.2%), and primary liver tumors (4.4%).
Actuarial survival of liver allografts with various degrees of HLA compatibility was calculated by the life-table method. Criteria for transplant failures included patient death and allograft removal regardless of graft function. Statistical analysis of transplant survival rates was done by the Breslow (generalized Wilcoxon) and Mantel-Cox (generalized Savage) tests using the 1L program of the BMDP software package (BMDP Statistical Software, Inc., Los Angeles, CA) (18). The Breslow test is weighted toward earlier events and the Mantel-Cox test emphasizes differences later during the posttransplant period.
Statistical analysis of differences between the match-groups was done with the 4F program of the BMDP software package and Pearson chi-square statistics.
RESULTS
The analysis of the effect of HLA compatibility on liver transplant survival was based on the number of donor antigens mismatched at the HLA-A, HLA-B, and HLA-DR loci. The results considered both primary grafts and retransplants. Overall actuarial survival for the 574 liver allografts included in this analysis was 59.2% for the one-year and 55.2% for the two-year period. Figure 1 shows the survival rates of liver allografts with zero, one, and two HLA-A mismatches. Lower survival rates were observed for transplants with zero mismatches as compared with transplants with one or two HLA-A mismatches. The differences between the zero versus one and two mismatches were statistically significant as determined by Breslow analysis (early events, P=0.057) and Mantel-Cox (late events, P=0.029). The one-year survival rates were 41.1% of the zero (n=42) and 60.6% of the one- and two- (n=532) HLA-A antigen mismatch groups. After two years, the survival rates were 36.5% and 56.7%, respectively. Survival rates of one and two HLA-A mismatched liver transplants were approximately the same. The numbers of patients in the group of zero mismatches at HLA-B was too small (n=12) to permit a meaningful statistical analysis of the effect of HLA-B compatibility.
Figure 1.
Actuarial survival of 574 liver transplants with different degrees of HLA-A incompatibility. The differences between the zero versus one and two mismatches for HLA-A were statistically significant as determined by Breslow analysis (early events): P=0.057; and by Mantel-Cox analysis (late events): P=0.029.
The effect of compatibility for HLA-DR on liver transplant outcome is illustrated in Figure 2. The group of zero HLA-DR mismatches showed longer survival rates than transplants with one and two HLA-DR mismatches (Breslow: P=0.054; Mantel-Cox: P=0.087). Transplant survivals after one year were 51.9% for zero HLA-DR mismatches (n=52) and 60.3% for one or two HLA-DR mismatches (n=455). After two years, the survival rates were 45.0% and 56.9%, respectively.
Figure 2.
Actuarial survival of 507 liver transplants with different degrees of HLA-DR incompatibility. The group of zero HLA-DR mismatches showed significantly lower survival rates than transplants with one and two HLA-DR mismatches (Breslow: P=0.054; Mantel-Cox: P=0.087).
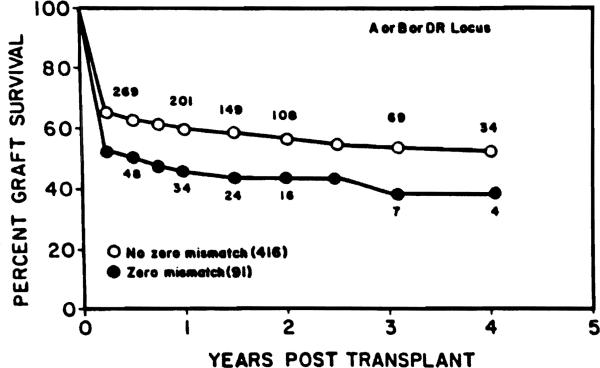
In 507 transplants with complete typing information for all HLA-A, B and DR loci, we identified 91 patients for whom there was a zero mismatch for at least one of these loci. Highly significantly lower survival rates were observed with this group as compared with the remaining group of 416 patients receiving transplants without zero mismatches at any of the HLA-A, B or DR loci (Breslow: P=0.008; Mantel-Cox: P=0.008) (Fig. 3). Transplant survivals of the two groups were after one year 47.7% and 61.9%, and after two years 44.1% and 58.3%, respectively.
Figure 3.
In 507 transplants with complete typing information for all HLA-A, B, and DR loci, 91 patients were identified for whom there was a zero mismatch for at leaat one of these loci. Highly significant lower survival rates were observed with this group as compared with the remaining group of 416 patients receiving tranaplants without zero mismatches at any of the HLA-A, B, or DR loci (Breslow: P=0.008; Mantel-Cox: P=0.008).
This study also considered a group of patients, who were retransplanted after their initial allograft had failed. A total of 119 failures were classified into three diagnostic categories based on clinical and pathological assessment as previously described (19). A diagnosis of rejection was made for 53 failed allografts (44.5%), whereas in 31 cases (26.1%) the cause of failure was primary nonfunction. A third group of 35 failures (29.4%) included vascular thrombosis, “technical” complications, and 4 cases of infection.
Table 1 shows the incidence of different causes of liver transplant failure in the various HLA match categories. Complete HLA-DR typing data were available for 108 failed allografts. In the group of 13 zero mismatches for HLA-DR, primary nonfunction was the most frequently observed cause of transplant failure (61.5%). A diagnosis of rejection was made for only 2 zero-HLA-DR mismatches (15.4%). An increasing degree of HLA-DR incompatibility was associated with a higher incidence of rejection but a lower frequency of graft failures caused by primary nonfunction. The differences in HLA-DR effects on rejection and primary nonfunction were statistically significant (P=0.007), whereas no HLA-DR influence was noted on liver allograft failures from other causes.
Table 1.
Frequencies of causes of failures of HLA-matched and - mismatched liver transplants in patients requiring retransplantation
| No. HLA mismatches | No. transplants | Frequency of cause of failures |
||
|---|---|---|---|---|
| Rejection | Primary nonfunction | Othera | ||
| 0 HLA-DR | 13 | 15.4% | 61.5% | 23.1% |
| 1 HLA-DR | 31 | 35.5% | 29.0% | 35.5% |
| 2 HLA-DR | 64 | 50.0%b | 18.8%b | 31.3% |
| 0 HLA-A | 12 | 33.3% | 25.0% | 41.7% |
| 1 HLA-A | 46 | 43.5% | 28.3% | 28.3% |
| 2 HLA-A | 61 | 47.5% | 24.6% | 27.9% |
| 0 HLA-B | 6 | 16.7% | 16.7% | 66.7% |
| 1 HLA-B | 22 | 36.4% | 36.4% | 27.3% |
| 2 HLA-B | 91 | 48.4% | 24.2% | 27.5% |
| 0 HLA-A or 0 HLA-B | 17 | 29.4% | 23.5% | 47.1% |
| No 0 HLA-A and no 0 HLA-B | 102 | 47.1%c | 26.5% | 26.5%c |
Other causes of failure include vascular thrombosis, technical complications, and 4 cases of infection.
The differences in HLA-DR effects on rejection and primary nonfunction were statistically significant (P = 0.007).
The combination of zero mismatches for either HLA-A or HLA-B was associated with a lower incidence of rejection, but a higher frequency of other causes of graft failures as compared with transplants with one or more HLA-A and HLA-B mismatches. The differences between these groups were of borderline statistical significance (P = 0.082).
The data in Table 1 also showed a similar trend toward an association of HLA-A and HLA-B incompatibility with an increased incidence of rejection, but the effect in comparison with other groups was not statistically significant (P<0.10). The lack of statistical significance might be due to the relatively low numbers of observations in each group. It was also noted that the incidence of transplant failures from other causes (i.e., vascular thrombosis, technical complications, and infections) was higher among allografts from donors with zero mismatches for HLA-A and HLA-B. However, the differences were statistically insignificant (P>0.10). We also observed that a combination of zero mismatches for either HLA-A or HLA-B was associated with a lower incidence of rejection, but a higher frequency of other causes of graft failures as compared with transplants with one or more HLA-A and HLA-B mismatches. The differences between these groups were of borderline statistical significance (P=0.082).
DISCUSSION
These data suggest that HLA compatibility is associated with a decreased survival of liver transplants. The effect is evident for both class I antigens of HLA-A and class II antigens of the HLA-DR locus. The number of cases available was not sufficient to evaluate the influence of the highly polymorphic HLA-B locus. The present findings are in contrast to the widely reported beneficial effect of HLA compatibility on kidney transplant outcome (1–3).
Our inability to demonstrate a favorable effect of matching for HLA on liver transplant survival does not necessarily conflict with the concept that HLA influences transplant rejection of liver allografts. This is apparent from our observations that the frequency of liver transplant failures caused by rejection correlated with the degree of HLA mismatching, especially for HLA-DR. Additional evidence for the role of HLA as transplantation antigens in liver allograft immunity has been obtained with studies of transplant biopsy-grown lymphocytes (15, 16). Such graft infiltrating cells may exhibit alloreactivity specific for class I and/or class II HLA antigens of the donor. The infiltration by class II-specific cells is associated with increased serum levels of gamma glutamyl transpeptidase and alkaline phosphatase (20). During rejection, the biliary epithelium shows strong expression of class II HLA antigens and constitutes a preferred target for graft-infiltrating lymphocytes (21). Class I–specific cells seem primarily involved in the early events of allograft rejection when the vascular endothelium expresses mostly class I HLA antigens (22).
Besides transplant rejection induced by HLA incompatibility, other immunological mechanisms might induce liver transplant failure. These mechanisms could be specific for a variety of antigens including viruses, autoantigens, and tissue-specific components. An important consideration is the immunological etiology and many end-stage liver diseases, including primary biliary cirrhosis (23), sclerosing cholangitis (24), autoimmune chronic active hepatitis, and viral hepatitis (25). Following transplantation, a persistence of disease-associated immunological mechanisms may lead to a recurrence of liver failure. An important consideration is the influence of HLA—especially in view of our findings that HLA compatibility is associated with decreased liver transplant survival and a higher incidence of non–rejection-related transplant failures.
One of the most distinctive features of HLA is its role in cellular interactions during the immune response. This phenomenon is referred to as major histocompatibility complex restriction and has been observed in several animal species (26, 27). Many cellular interactions are HLA restricted—that is, they are efficient only if the cells involved express shared HLA antigens. This MHC restriction (or self-recognition) has been demonstrated at several levels during the immune response, especially in interactions between antigen-presenting cells and T lymphocytes (28) and in cytotoxic T cell–induced lysis of virus-infected and other antigen-expressing target cells (29). HLA restriction has been demonstrated for cellular immunity to clinically relevant viral antigens, such as cytomegalovirus (30–32), Epstein-Barr virus (33, 34) and herpes simplex (35). During infection, cytotoxic lymphocyte–mediated damage would probably be more efficient if infected target cells in the allograft expressed compatible HLA antigens.
The phenomenon of HLA restriction has not been extensively studied in autoimmune liver diseases, because the antigens involved are largely undefined. However, HLA has been indirectly implemented through its association with several liver diseases (36, 37). Associations have been reported for chronic active hepatitis with DR3 (38), sclerosing cholangitis with B8 (39), and alcoholic cirrhosis with DR2 (40). Thus far, published reports show no association of HLA-A or -B antigens with primary biliary cirrhosis (36, 41, 42), although there appears to be a genetic predisposition from the substantial number of intrafamilial cases of primary biliary cirrhosis (43). Relatives of patients have a higher-than-expected frequency of other autoimmune diseases (44). Our analysis of 74 female primary biliary cirrhosis patients has demonstrated an increased frequency of HLA-DR7 (manuscript in preparation). The HLA associations with these diseases can be explained with the presence of HLA-linked immune response genes that influence immunological mechanisms relevant to disease processes (27). The products of these genes could operate through MHC restriction in various cellular interactions during the immune response. These processes would not only affect the original liver, but also contribute to recurrent disease of the transplanted liver, especially from an HLA compatible donor.
Disease recurrence in liver transplant patients has been documented in patients with chronic active hepatitis B (45, 46), hepatic malignancies (47, 48) and Budd-Chiari syndrome (49–51). However, the diagnosis of recurrent disease is much more difficult in transplant patients with primary biliary cirrhosis (50, 52), non-A non-B hepatitis, and sclerosing cholangitis (50). The difficulties arise from apparent pathophysiologic similarities between primary biliary sclerosis and chronic rejection; the lack of a specific marker for non-A, non-B hepatitis; and the problem with postoperative biliary strictures for sclerosing cholangitis. Functional studies of lymphocytes propagated from liver allografts may enable a better differentiation between transplant rejection and other immunological mechanisms leading to hepatic dysfunction.
It has recently been reported, that pancreas transplants from HLA-identical donors show a high incidence of isletitis and recurrent diabetes (53). The occurrence of isletitis, associated with a T cell and macrophage infiltrate, was more pronounced in HLA-identical grafts, suggesting that this isletitis might be initiated by the recognition of identical MHC antigens shared between donor and recipient. It was suggested that the selective destruction of beta cells in HLA-identical grafts represents an anamnestic cytotoxic T lymphocyte–mediated autoimmune response (54), whereas isletitis in nonidentical grafts is caused by rejection due to class I and class II HLA antigen disparity.
Recurrent disease may also be a complication in renal transplantation. This particularly applies to patients with focal segmental glomerulosclerosis (55, 56), IgA nephropathy (57), and membranous glomerulopathy (55, 58) who receive kidney transplants from HLA compatible donors. Interestingly, several of these renal diseases have an immunological basis and show HLA associations (36). For instance, IgA nephropathy possibly associates with DR4 (59) and membranous glomerulopathy with DR3 and B18 (60). No studies have been reported thus far on the HLA association with focal segmented glomerulosclerosis. The HLA associations with certain renal diseases and the increased disease recurrence in HLA-compatible kidney transplants suggests that HLA is involved in these two related phenomena. The most likely mechanism is that HLA functions as a restriction element during immunological processes involved in the pathogenesis of these diseases. Nevertheless, disease recurrence is relatively uncommon in kidney transplantation. Thus the potentially adverse effect of HLA compatibility in promoting disease recurrence is negated by the large number of cases wherein HLA compatibility enhances renal transplant survival through decreasing allograft rejection. On the other hand, disease recurrence is more common in liver transplantation, and the possibility that HLA compatibility may increase disease recurrence may explain the lower survival rates of HLA-compatible liver allografts.
Another explanation for the association of HLA compatibility with decreased liver transplant survival is based on the concept that MHC restriction may also operate in transplant immunity. This has been demonstrated using in vitro assays (61, 62) and in several allograft models in mice, wherein transplant rejection across minor histocompatibility barriers was shown to be restricted by H-2 (63). In the human situation, HLA restriction of transplant immunity to the male-specific H-Y antigen has been observed in bone marrow transplantation (64) and following rejection of a kidney transplant from an HLA-identical male sibling donor (65). It has recently been reported that liver transplants with a complete mismatch for class I antigens, together with a partial or complete match for class II HLA antigens, experience a high incidence of vanishing bile duct syndrome, a manifestation of chronic rejection involving biliary epithelium (14). An hypothesis has been forwarded that cytotoxic T cells sensitized to class I antigens would recognize bile duct epithelium in the context of class II compatibility between target and effector cells (14). Thus it is possible that MHC-restricted transplant rejection mechanisms may influence liver transplant outcome.
The data presented in Figure 3 suggest that compatibility for only one of the HLA loci (i.e., HLA-A, -B, or -DR) is already sufficient to cause a significant decrease in liver allograft survival. This could mean that compatibility for a single HLA locus may significantly enhance MHC-restricted immunological mechanisms of liver allograft injury associated with viral infection and recurrent autoimmune disease.
In summary, our present findings suggest that HLA compatibility has a dualistic effect on liver transplant outcome: on the one hand it may reduce the rejection process, whereas, on the other hand, it may enhance other immunological mechanisms leading to allograft dysfunction. The practical implication of this concept is that the degree of HLA compatibility of liver allografts might be considered in the selection and management of transplant recipients, especially those at risk of developing recurrent disease or immunologically mediated complications other than rejection.
Acknowledgments
We appreciate the help of James E. Strawn of the South Eastern Organ Procurement Foundation (SEOPF) in providing some donor information.
REFERENCES
- 1.Cecka JM. The changing role of HLA matching. In: Terasaki PI, editor. Clinical kidney transplants 1986. UCLA Tissue Typing Laboratory; Los Angeles: 1986. p. 141. [Google Scholar]
- 2.Opelz G. Effect of HLA matching in 10,000 cyclosporine-treated cadaver kidney transplants. Transplant Proc. 1987;19:641. [PubMed] [Google Scholar]
- 3.Sanfilippo F, Vaughn WK, Spees EK, Light JA, LeFor WM. Benefits of HLA-A and HLA-B matching on graft and patient outcome after cadaveric-donor renal transplantation. N Engl J Med. 1984;311:358. doi: 10.1056/NEJM198408093110603. [DOI] [PubMed] [Google Scholar]
- 4.Yacoub M, Festenstein p, Doyle P, et al. The influence of HLA matching in cardiac allograft recipients receiving cyclosporine and azathioprine. Transplant Proc. 1987;19:2487. [PubMed] [Google Scholar]
- 5.Starzl TE, Iwatsuki S, Van Thiel TE, et al. Evolution of liver transplantation. Hepatology. 1982;2:614. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malatack JJ, Zitelli BJ, Gartner JC, Jr, Shaw BW, Jr, Iwatsuki S, Starzl TE. Pediatric liver transplantation under therapy with cyclosporine-A and steroids. Transplant Proc. 1983;15:1292. [PMC free article] [PubMed] [Google Scholar]
- 7.Starzl TE, Ishikawa M, Putnam CW, et al. Progress in and deterrents to orthotopic liver transplantation, with special reference to survival, resistance to hyperacute rejection, and biliary duct reconstruction. Transplant Proc. 1974;6:129. [PMC free article] [PubMed] [Google Scholar]
- 8.Iwatsuki S, Rabin BS, Shaw BW, Jr, Starzl TE. Liver transplantation against T cell-positive crossmatches. Transplant Proc. 1984;16:1427. [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon RD, Fung JJ, Markus BM, et al. The antibody crossmatch in liver transplantation. Surgery. 1986;100:705. [PMC free article] [PubMed] [Google Scholar]
- 10.Fung JJ, Griffin M, Duquesnoy RJ, Shaw BW, Starzl TE. Successful sequential liver-kidney transplantation in a patient with preformed lymphocytotoxic antibodies. Transplant Proc. 1987;19:767. [PMC free article] [PubMed] [Google Scholar]
- 11.Starzl TE, Tzakis A, Makowka L, et al. Combined liver and kidney transplantation: with particular reference to positive cytotoxic crossmatches. Kidney Int. in press. [Google Scholar]
- 12.Gubernatis G, Lauchart W, Jonker M, et al. Signs of hyperacute rejection of liver grafts in rhesus monkeys after donor-specific presensitization. Transplant Proc. 1987;19:1082. [PubMed] [Google Scholar]
- 13.Batts KP, Moore SB, Perkins JD, Wiesner RH, Krom RAF. Influence of positive lymphocyte crossmatch and HLA mismatching on vanishing bile duct syndrome in human liver allografts. Transplantation. doi: 10.1097/00007890-198802000-00026. in press. [DOI] [PubMed] [Google Scholar]
- 14.Donaldson PT, Alexander GJM, O'Grady JG, et al. Evidence for an immune response to HLA class I antigens in the vanishing-bile duct syndrome after liver transplantation. Lancet. 1987;1:945. doi: 10.1016/s0140-6736(87)90293-5. [DOI] [PubMed] [Google Scholar]
- 15.Fung JJ, Zeevi A, Starzl TE, Demetris J, Iwatsuki S, Duquesnoy RJ. Functional characterization of infiltrating T lymphocytes in human hepatic allografts. Hum Immunol. 1986;16:182. doi: 10.1016/0198-8859(86)90047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markus BH, Fung JJ, Zeevi A, Starzl TE, Demetris AJ, Duquesnoy RJ. Analysis of T lymphocytes infiltrating human hepatic allografts. Transplant Proc. 1987;19:2470. [PMC free article] [PubMed] [Google Scholar]
- 17.Fung JJ, Markus BH, Gordon RD, et al. Impact of Orthoclone OKT3 on liver transplantation. Transplant Proc. 1987;19(suppl 1):37. [PMC free article] [PubMed] [Google Scholar]
- 18.Dixon WJ. BMDP statistical software manual. University of California Press; Los Angeles, CA: 1985. [Google Scholar]
- 19.Shaw BW, Jr, Gordon RG, Iwatsuki S, Starzl TE. Retransplantation of the liver. Semin Liver Dis. 1985;5:394. doi: 10.1055/s-2008-1040638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markus BH, Demetria AJ, Saidmans S, et al. Alloreactive T lymphocytes cultured from liver transplant biopsies: associations of HLA specificity with clinicopathological finding. Clin Transplant. 1988;2:70. [PMC free article] [PubMed] [Google Scholar]
- 21.Demetris AJ, Lasky S, Van Thiel DH, Starzl TE, Whiteside T. Induction of DR/IA antigens in human liver allografts. Transplantation. 1985;40:504. doi: 10.1097/00007890-198511000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fung JJ, Zeevi A, Markus B, Zerbe TR, Duquesnoy RJ. Dynamics of allospecific T lymphocyte infiltration in vascularized human allografts. Immunol Res. 1986;5:149. doi: 10.1007/BF02917589. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan MM. Primary biliary cirrhosis. N Engl J Med. 1984;316:521. doi: 10.1056/NEJM198702263160907. [DOI] [PubMed] [Google Scholar]
- 24.LaRusso NF, Wiesner RH, Ludwig J, MacCarty RL. Primary sclerosing cholangitis. N Engl J Med. 1984;310:899. doi: 10.1056/NEJM198404053101407. [DOI] [PubMed] [Google Scholar]
- 25.Thomas HC. Immunologic aspects of liver disease. In: Schiff L, Schiff ER, editors. Diseases of the liver. Lippincott; Philadelphia: 1987. p. 163. [Google Scholar]
- 26.Zinkernagel RM, Doherty PC. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]
- 27.Klein J. Natural history of the major histocompatibility complex. Wiley; New York: 1986. [Google Scholar]
- 28.Qvigstad E, Bruserud O, Thorsby E. The role of human class II molecules in activation of T4 lymphocytes. In: Solheim BG, Moller E, Ferrone S, editors. HLA class II antigens. Springer; Berlin: 1986. p. 473. [Google Scholar]
- 29.McMichael AJ, Ting A, Zweerink HJ, Askonal BA. HLA restriction of cell-mediated lysis of influenza virus-infected human cells. Nature. 1977;270:524. doi: 10.1038/270524a0. [DOI] [PubMed] [Google Scholar]
- 30.Sethi KK, Stroehmann I, Brandis H. Human T-cell cultures from virus-sensitized donors can mediate virus-specific and HLA-restricted cell lysis. Nature. 1980;286:718. doi: 10.1038/286718a0. [DOI] [PubMed] [Google Scholar]
- 31.Quinnan GV, Jr, Kirmani N, Esber E, et al. HLA-restricted cytotoxic T lymphocyte and nonthymic cytotoxic lymphocyte responses to cytomegalovirus infection of bone marrow transplant recipients. J Immunol. 1981;126:2036. [PubMed] [Google Scholar]
- 32.Lindsley MD, Torpey DJ, III, Rinaldo CR., Jr HLA-DR-restricted cytotoxicity of cytomegalovirus-infected monocytes mediated by Leu-3-positive cells. J Immunol. 1986;136:3045. [PubMed] [Google Scholar]
- 33.Misko IS, Moss DJ, Pope JH. HLA antigen restriction of T lymphocyte cytotoxicity to Epstein Barr Virus. Proc Natl Acad Sci USA. 1980;77:4247. doi: 10.1073/pnas.77.7.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka Y, Sugamura K, Hinuma Y. Heterogeneity of allogeneic restriction of human Cytotoxic T cell clones for Epstein Barr Virus. J Immunol. 1982;128:1241. [PubMed] [Google Scholar]
- 35.Yasukawa M, Zarling JM. Human cytotoxic T cell clones directed against herpes simplex virus-infected cella: I. Lysis restricted by HLA class II MB and DR antigens. J Immunol. 1984;133:422. [PubMed] [Google Scholar]
- 36.Tiwari JL, Terasaki PI. HLA and disease associations. Springer; New York: 1985. [Google Scholar]
- 37.Stastny P, Ball EJ, Dry PJ, Nunez G. The human immune response region (HLA-D) and disease susceptibility. Immunol Rev. 1983;70:113. doi: 10.1111/j.1600-065x.1983.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 38.Opelz G, Vogten AJM, Summerskill WHJ, Schalm SW, Terasaki PI. HLA determinants in chronic active liver disease: possible relation of HLA-DW3 to prognosis. Tissue Antigens. 1977;10:63. doi: 10.1111/j.1399-0039.1977.tb01077.x. [DOI] [PubMed] [Google Scholar]
- 39.Chapmann RW, Varghese Z, Gaul R, Patel G, Kokinon N, Sherlock S. Association of primary sclerosing cholangitis with HLA-B8. Gut. 1983;24:38. doi: 10.1136/gut.24.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tait BD, Mackay IR. HLA and alcoholic cirrhosis. Tissue Antigens. 1982;19:6. doi: 10.1111/j.1399-0039.1982.tb01410.x. [DOI] [PubMed] [Google Scholar]
- 41.Galbraith RM, Eddleaton ALWF, Smith MGM, et al. Histocompatibility antigens in active chronic hepatitis and primary biliary cirrhosis. Br Med J. 1974;3:604. doi: 10.1136/bmj.3.5931.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamlyn AN, Adams D, Sherlock S. Primary or secondary sicca complex? investigation in primary biliary cirrhosis by histocompatibility testing. Br Med J. 1980;2:425. doi: 10.1136/bmj.281.6237.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaup BH, Zettergren LSW. Familial occurrence of primary biliary cirrhosis associated with hypergammaglobulinemia in descendants: a family study. Gastroenterology. 1980;78:549. [PubMed] [Google Scholar]
- 44.Galbraith RM, Smith M, MacKenzie RM, et al. High prevalence of seroimmunologic abnormalities in relatives of patients with active chronic hepatitis or primary biliary cirrhosis. N Engl J Med. 1974;290:63. doi: 10.1056/NEJM197401102900201. [DOI] [PubMed] [Google Scholar]
- 45.Demetris AJ, Jaffe R, Sheahan DG, et al. Recurrent hepatitis B in liver allograft recipients: differentiation between viral hepatitis B and rejection. Am J Pathol. 1986;125:161. [PMC free article] [PubMed] [Google Scholar]
- 46.Corman JL, Putnam CW, Iwatsuki S, et al. Liver allograft: its uses in chronic active hepatitis with macronodular cirrhosis, hepatitis B surface antigen. Arch Surg. 1979;114:75. doi: 10.1001/archsurg.1979.01370250077016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iwatsuki S, Gordon RD, Shaw B, Jr, Starzl TE. Role of liver transplantstion in cancer therapy. Ann Surg. 1985;202:401. doi: 10.1097/00000658-198510000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wight DGD. Pathology of liver transplantation (other than rejection). In: Caine RY, editor. Liver transplantation: the Cambridge/Kings College experience. Grune & Stratton; New York: 1983. p. 289. [Google Scholar]
- 49.Seltman HJ, Dekker A, Van Thiel DH, Boggs DR, Starzl TE. Budd-Chiari syndrome recurring in a transplanted liver. Gastroenterology. 1983;84:640. [PMC free article] [PubMed] [Google Scholar]
- 50.Demetris AJ, Jaffe R, Starzl TE. A review of adult and pediatric post-transplant liver pathology. Pathol Annu. in press. [PubMed] [Google Scholar]
- 51.Portmann B, O'Grady J, Williama R. Disease recurrence following orthotopic liver transplantation. Transplant Proc. 1986;18(suppl 4):136. [Google Scholar]
- 52.Neuberger J, Portmann B, Macdougall BRD, Caine RY, Williams R. Recurrence of primary biliary cirrhosis after liver transplantation. N Engl J Med. 1982;306:1. doi: 10.1056/NEJM198201073060101. [DOI] [PubMed] [Google Scholar]
- 53.Sibley RK, Sutherland DER. Pancreas transplantation: an immunohistologic and histopathologic examination of 100 grafts. Am J Pathol. 1987;128:151. [PMC free article] [PubMed] [Google Scholar]
- 54.Sibley RK, Sutherland DER, Goetz F, Michael AF. Recurrent diabetes mellitus in the pancreas iso- and allograft. Lab Invest. 1985;53:132. [PubMed] [Google Scholar]
- 55.Pinto J, Lacerda G, Cameron JS, et al. Recurrence of focal segmental glomerulosclerosis in renal allografts. Transplantation. 1981;32:83. doi: 10.1097/00007890-198108000-00001. [DOI] [PubMed] [Google Scholar]
- 56.Torres VE, Velosa JA, Holley KE, Frohnert PP. Meclofenamate treatment of recurrent idiopathic nephrotic syndrome with focal segmental glomerulosclerosis after renal transplantation. Mayo Clin Proc. 1984;59:146. doi: 10.1016/s0025-6196(12)60765-4. [DOI] [PubMed] [Google Scholar]
- 57.Morzycka M, Croker BP, Seigler H, Tisher CC. Evaluation of recurrent glomerulonephritis in kidney allografts. Am J Med. 1982;72:588. doi: 10.1016/0002-9343(82)90453-3. [DOI] [PubMed] [Google Scholar]
- 58.Berger BE, Vincenti F, Biava C, Amend WJ, Jr, Feduaka N, Salvatierra O., Jr De novo and recurrent membranous glomerulopathy following kidney transplantation. Transplantation. 1983;35:315. doi: 10.1097/00007890-198304000-00010. [DOI] [PubMed] [Google Scholar]
- 59.Fauchet R, Gueguen M, Genetet B, et al. HLA-DR antigen and IgA nephropathy (Berger's disease). N Engl J Med. 1980;302:1033. doi: 10.1056/nejm198005013021820. [DOI] [PubMed] [Google Scholar]
- 60.Klouda PT, Acheson EJ, Goldby FS, et al. Strong association between idiopathic membranous nephropathy and HLA-DRW3. Lancet. 1979;2:770. doi: 10.1016/s0140-6736(79)92118-4. [DOI] [PubMed] [Google Scholar]
- 61.Bevan MJ. The major histocompatibility complex determines susceptibility to cytotoxic T cella directed against minor histocompatibility antigens. J Exp Med. 1975;142:1349. doi: 10.1084/jem.142.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gordon RD, Simpson E, Samelson LE. In vitro cell-mediated response to the male specific (H-Y) antigen in mice. J Exp Med. 1975;142:1108. doi: 10.1084/jem.142.5.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Silvers WK, Bartlett ST, Chen HD, Fleming HL, Naji A, Barker CF. Major histocompatibility complex restriction and transplantation immunity. Transplantation. 1984;37:28. [PubMed] [Google Scholar]
- 64.Goulmy E, Termijtelen A, Bradley BA, Van Rood JJ. V-antigen killing by T cella of women is restricted by HLA. Nature. 1977;266:544. doi: 10.1038/266544a0. [DOI] [PubMed] [Google Scholar]
- 65.Pfeffer PF, Thorsby E. HLA restricted cytotoxicity against male-specific (H-Y) antigen after acute rejection of an HLA identical sibling kidney. Transplantation. 1982;33:52. doi: 10.1097/00007890-198201000-00011. [DOI] [PubMed] [Google Scholar]