Capsule Summary
Pet-ownership, which has been shown to be protective against allergic disease development, is associated with increased house dust bacterial diversity and fewer fungal species, suggesting a potentially microbial-based mechanism for this protective effect.
Keywords: Pet ownership, allergic disease, house dust, 16S rRNA PhyloChip, F-ARISA
To the Editor
The prevalence of asthma amongst children has been steadily climbing in Westernized nations. Although host genotype likely plays a role in predisposition to development of allergic disease, the rate at which asthma has increased and the geographically distinct location of this phenomenon, implicates environmental factors as being important. Epidemiological studies have suggested that contact with animals provides protection against allergic disease development1; childhood farm exposure, specifically to livestock, is associated with a significant decrease in risk of atopic sensitization a protective effect that persists into early adulthood. More recently, in our own birth cohort, maternal pre-natal exposure to household pets, particularly dogs, has been suggested to impact fetal immune response development2. Higher concentrations of cord blood Immunoglobulin E (IgE) were associated with mothers unexposed to pets2 a notable finding given that previous studies have demonstrated a link between elevated cord blood IgE and risk for subsequent development of allergic disorders3
In this study, we examined whether the presence of dogs or cats or the absence of a furred pet impacted the microbial composition of house dust. Using a high-density phylogenetic microarray, the 16S rRNA PhyloChip4 and fungal automated rRNA intergenic spacer analysis [F-ARISA;], we examined sixteen dust samples collected from households with ≥1 dog (D; n = 6), ≥1 cat (C; n = 5) or no furred pets (NP; n = 5). Five (83.3%), five (100%) and three (60%) samples from D, C and NP households respectively, had sufficient material for microbial analysis.
Bacterial community richness (number of bacterial taxa detected), evenness (relative distribution of taxa in communities) and diversity (calculated using richness and evenness indices) were notably increased in all dog- and a subset of cat-owning households. Dust from households with dogs was significantly richer (p=0.04) and more diverse (p=0.04) compared with those without pets (Fig. 1A and B); community evenness was not significantly different between these two groups (p=0.14; Fig 1C; Table E1). These data suggest that dog ownership increases house dust diversity, driven largely by introduction of additional types of bacteria.
Fig. 1.
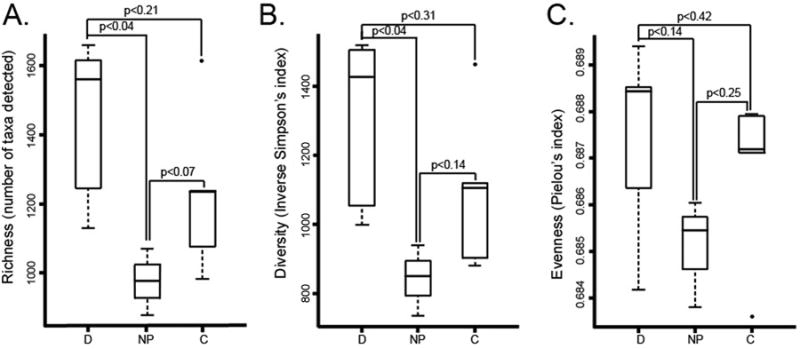
Comparison of bacterial community A. Richness, B. Diversity and C. Evenness, in dog, cat and no pet samples.
Significant differences in taxon relative abundance between D or NP groups identified 337 taxa significantly increased in abundance in dog-owning houses (Table E2, Appendix). They primarily belonged to the Proteobacteria (112 taxa), Actinobacteria (63 taxa), Firmicutes (47 taxa), Verrucomicrobia (7 taxa), Bacteroidetes (41 taxa) and Spirochaetes (22 taxa). Although some taxa were significantly increased in abundance in C compared to NP samples, they did not meet our minimum false discovery rate cut-off and no statistically significant differences in house dust richness, evenness or diversity existed between C and NP samples (Fig. 1).
Hierarchical cluster analysis (HCA) of all samples demonstrated that they fell into two clear clusters, (G1 and G2; Figure 2). All of the NP samples belonged to the G2 group, while the majority of D and some C samples resided in G1. G1 samples were significantly richer (p=0.008), more even (p=0.002), and more diverse (p<0.001) compared with G2 samples and exhibited the presence of 757 taxa in significantly higher abundance (Table E3), representing the phyla Acidobacteria, Actinobacteria, Bacteriodetes, Firmicutes, Proteobacteria, Spirochaetes and Verrucomicrobia. The majority of high diversity samples originated from homes with pets permitted both in- and out-doors, low diversity samples were typically from NP homes or those that possessed either an exclusively indoor or outdoor animal, suggesting that pet access to both in- and outdoor environments may influence house dust bacterial diversity. Exploratory examination of other sample data indicated that variables such as the number of children or individuals living in the home, mean humidity or a housekeeping index (a Likert scale assessing overall cleanliness) did not different between high and low diversity samples, although a study substantially larger than this investigation is necessary to consider these variables.
Fig. 2.
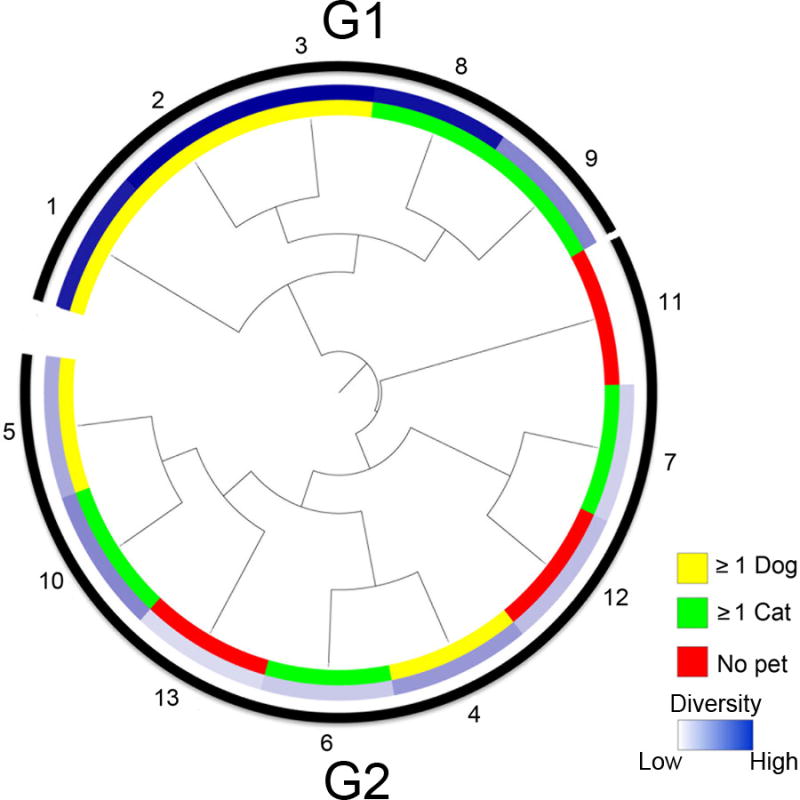
Two groups of house dust samples (G1 and G2) are distinguished by hierarchical cluster analysis. D = households with dogs, C = households with cats, NP = households with no pets.
Fungal richness was significantly lower in D compared with NP samples (p=0.049), suggestive for C vs D (p=0.051), and not significant between C vs NP (p=0.34; Table E1). Fungal richness of G1 samples was significantly lower than G2 samples (p=0.02) and a strong and significant negative relationship existed between bacterial diversity and fungal richness (r = -0.65; p=0.02; 95% CI: -0.88 to -0.15). Fungal ribotypes were identified by sequencing; the majority belonged to the Dothideomycetidaceae and Eurotiomycetidae and included many known allergenic fungi including Alternaria alternata, Mucor plumbeus and Mucor hiemalis, Cladosporium cladosporioides and Malassezia restricta (formerly Pityrosporum restricta; Table E4).
Our findings support the hypothesis that dog ownership may act as a surrogate marker for particular microbe-associated exposures. No significant differences between C and NP samples were detected, likely due to a small sample size. However, cats, relative to dogs, have been inconsistently associated with protection against allergic disease development5. Cats are more likely to be exclusively indoor pets, a characteristic that if accounted for, may explain reported discordance in their protective effect. Our data show that while pet-ownership was necessary for the presence of high bacterial diversity it was not sufficient and we hypothesize that animal behavior may play a key role in defining the microbial composition in the home. Presumably indoor/outdoor pets facilitate introduction of environmental air-, water- or soil-borne microbes into the home, thus increasing exposure to species typically encountered in these environments.
Increased bacterial diversity was due primarily to increased abundance of taxa belonging to relatively few phyla, that represent key lineages in the human GI tract6. This study thus stimulates speculation that the protective effect of pet ownership against allergic disease may be due to a number of distinct mechanisms. First, exposure to a larger diversity of bacterial species, may permit maturation of a balanced immune response. Secondly, these exposures may serve as an inoculum for the developing infant GI microbiome. Lack of microbial exposures and failure to inoculate the GI tract with appropriate microbes, may permit outgrowth of certain opportunistic colonizers, which has been reported for infants who subsequently develop childhood allergic disease7.
Samples with lower bacterial diversity possessed greater fungal richness, a large majority of which were known allergenic fungi known to induce potent IgE-based responses, lung inflammation and hyperreactivity, even in non-allergic mice8. Fungal exposure has long been associated with asthma and a recent study demonstrated that chitin, a key component of fungal cell walls, induces an airway eosinophil and basophil influx in challenged mice similar to that observed in asthmatic airways9. Increased exposure to fungal species, could directly lead to bronchial inflammation, which may be compounded by a parallel lack of bacterial exposure necessary to shape the developing immune response.
These data suggest that specific house dust microbial communities are associated with pet-keeping and is consistent with the theory that a lack of exposure to a broadly diverse bacterial community, coupled with an increase in exposure to chitin-containing microorganisms could be related to the subsequent development of an allergic phenotype. We believe that this provocative data even with a limited number of samples merits further systematic exploration in a larger population with linked immunological and disease outcomes, to determine the potentially microbial-based mechanisms by which pet exposure appears to reduce the prevalence of allergic disease development.
Supplementary Material
Acknowledgments
Funding sources: This study was supported by a NIH/NIAID award A150681 to C.C.J. and a NIH/NIAID award A159415 to D.R.O. SVL is supported by the Rainin Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.von Mutius E, Radon K. Living on a farm: impact on asthma induction and clinical course. Immunol Allergy Clin North Am. 2008;28:631–47. ix–x. doi: 10.1016/j.iac.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Aichbhaumik N, Zoratti EM, Strickler R, Wegienka G, Ownby DR, Havstad S, et al. Prenatal exposure to household pets influences fetal immunoglobulin E production. Clin Exp Allergy. 2008;38:1787–94. doi: 10.1111/j.1365-2222.2008.03079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergmann RL, Edenharter G, Bergmann KE, Guggenmoos-Holzmann I, Forster J, Bauer CP, et al. Predictability of early atopy by cord blood-IgE and parental history. Clin Exp Allergy. 1997;27:752–60. [PubMed] [Google Scholar]
- 4.Brodie EL, Desantis TZ, Joyner DC, Baek SM, Larsen JT, Andersen GL, et al. Application of a high-density oligonucleotide microarray approach to study bacterial population dynamics during uranium reduction and reoxidation. Appl Environ Microbiol. 2006;72:6288–98. doi: 10.1128/AEM.00246-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ownby DR, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. JAMA. 2002;288:963–72. doi: 10.1001/jama.288.8.963. [DOI] [PubMed] [Google Scholar]
- 6.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–20. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 7.Penders J, Stobberingh EE, Thijs C, Adams H, Vink C, van Ree R, et al. Molecular fingerprinting of the intestinal microbiota of infants in whom atopic eczema was or was not developing. Clin Exp Allergy. 2006;36:1602–8. doi: 10.1111/j.1365-2222.2006.02599.x. [DOI] [PubMed] [Google Scholar]
- 8.Havaux X, Zeine A, Dits A, Denis O. A new mouse model of lung allergy induced by the spores of Alternaria alternata and Cladosporium herbarum molds. Clin Exp Immunol. 2005;139:179–88. doi: 10.1111/j.1365-2249.2004.02679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, et al. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature. 2007;447:92–6. doi: 10.1038/nature05746. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


