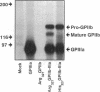
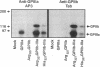
Abstract
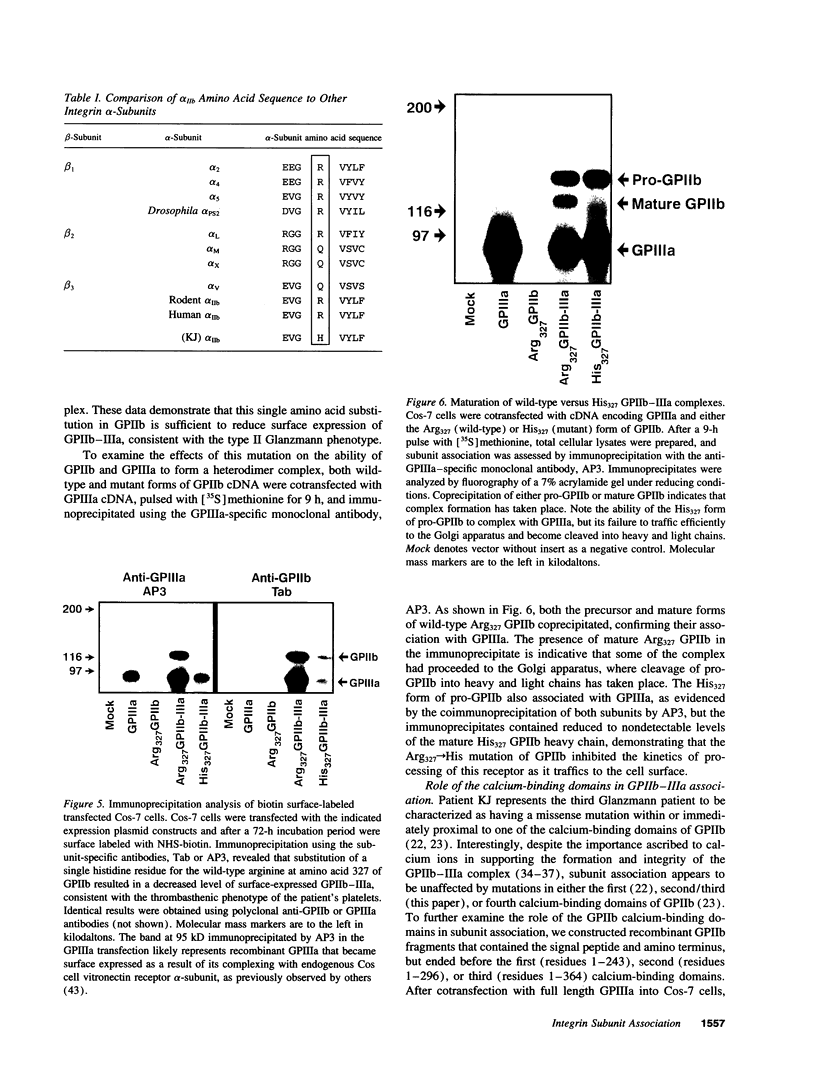
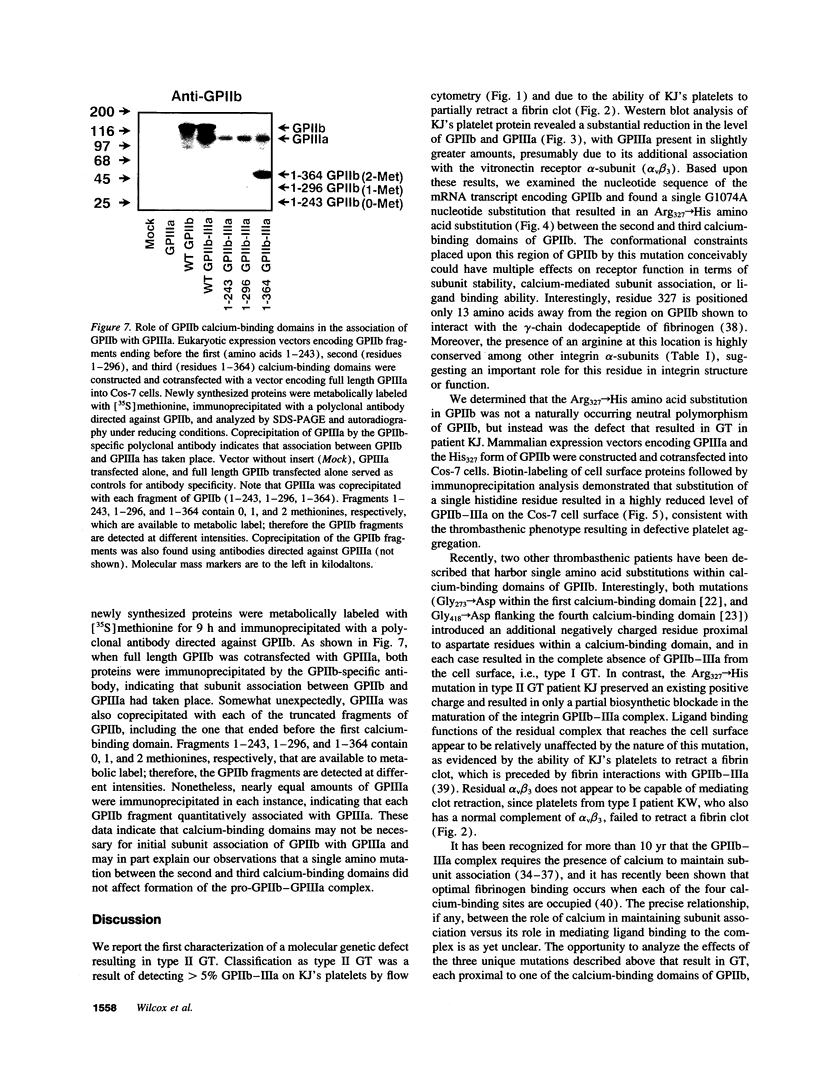
To gain insight into region of the platelet GPIIb-IIIa complex involved in receptor biogenesis and function, we examined the biochemical properties of a defective GPIIb-IIIa complex from patient suffering from type II Glanzmann thrombasthenia. Flow cytometric as well as immunoblot analysis of patient platelets showed significantly reduced levels of GPIIb and GPIIIa compared with a normal control. Patient platelets, however, retained the ability to retract a fibrin clot. Sequence analysis of PCR-amplified platelet GPIIb mRNA revealed an Arg327-->His amino acid substitution between the second and third calcium-binding domains of the GPIIb heavy chain, a residue that is highly conserved among integrin alpha-subunits. The recombinant His327 form of GPIIb was found to be fully capable of associating with GPIIIa, therefore the role of the calcium-binding domains in intersubunit association was further examined by constructing amino-terminal segments of GPIIb that ended before the first, second, and third calcium-binding domains. All three fragments were found to associate with GPIIIa, demonstrating that the calcium-binding domains of GPIIb are not necessary for initial complex formation. Regions amino-terminal to the calcium-binding domains of GPIIb may play a heretofore unappreciated role in integrin subunit association.
Full text
PDF

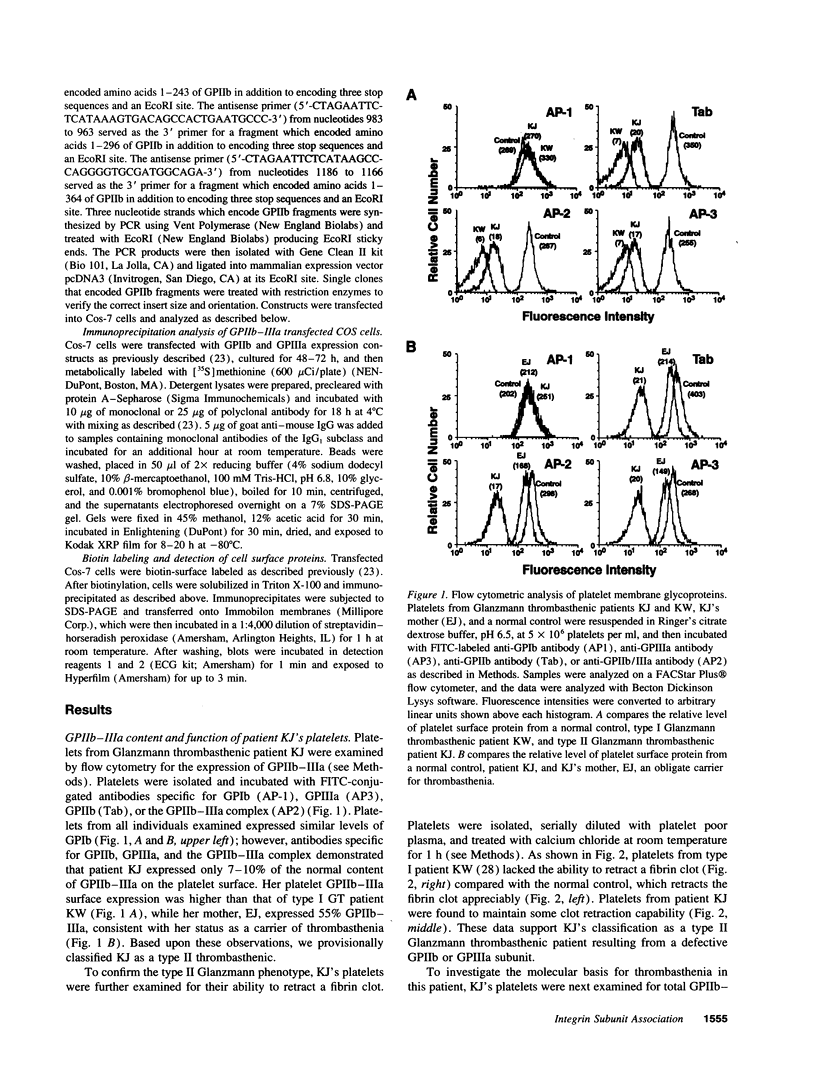
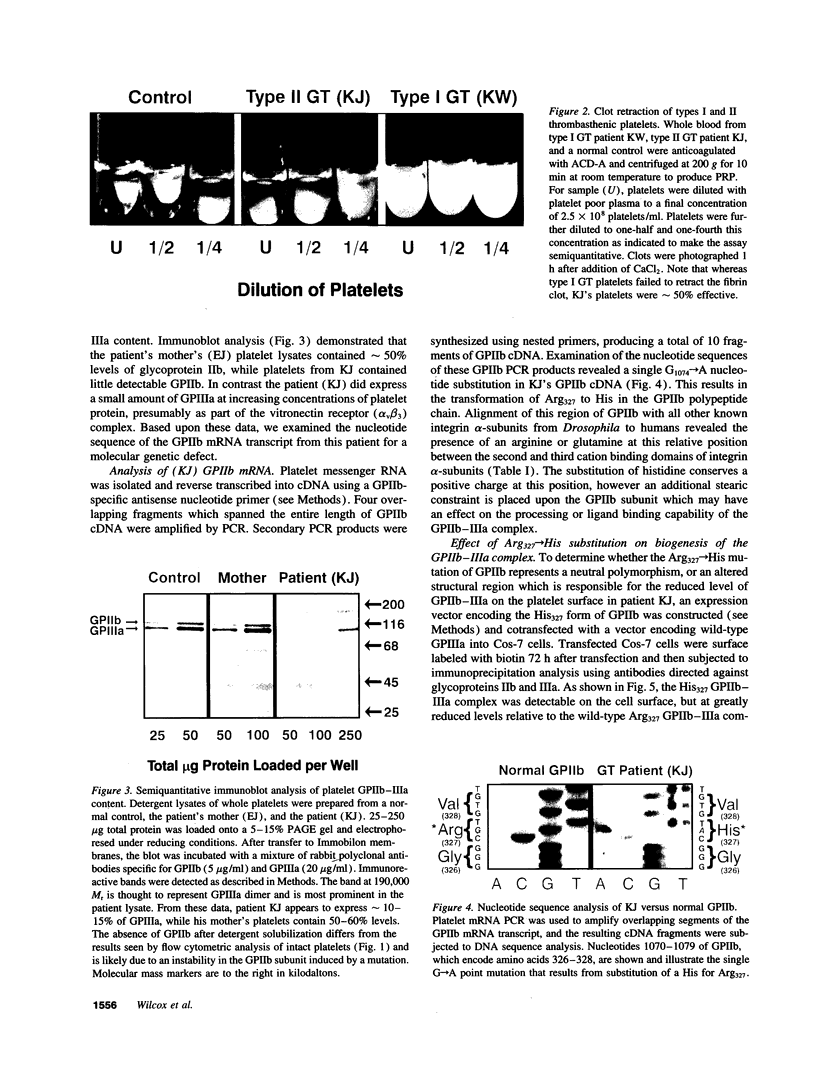


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- BRAUNSTEINER H., PAKESCH F. Thrombocytoasthenia and thrombocytopathia-old names and new diseases. Blood. 1956 Nov;11(11):965–976. [PubMed] [Google Scholar]
- Bajt M. L., Ginsberg M. H., Frelinger A. L., 3rd, Berndt M. C., Loftus J. C. A spontaneous mutation of integrin alpha IIb beta 3 (platelet glycoprotein IIb-IIIa) helps define a ligand binding site. J Biol Chem. 1992 Feb 25;267(6):3789–3794. [PubMed] [Google Scholar]
- Bennett J. S., Vilaire G. Exposure of platelet fibrinogen receptors by ADP and epinephrine. J Clin Invest. 1979 Nov;64(5):1393–1401. doi: 10.1172/JCI109597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass L. F., Shattil S. J., Kunicki T. J., Bennett J. S. Effect of calcium on the stability of the platelet membrane glycoprotein IIb-IIIa complex. J Biol Chem. 1985 Jul 5;260(13):7875–7881. [PubMed] [Google Scholar]
- Bray P. F., Rosa J. P., Lingappa V. R., Kan Y. W., McEver R. P., Shuman M. A. Biogenesis of the platelet receptor for fibrinogen: evidence for separate precursors for glycoproteins IIb and IIIa. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1480–1484. doi: 10.1073/pnas.83.5.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk C. D., Newman P. J., Lyman S., Gill J., Coller B. S., Poncz M. A deletion in the gene for glycoprotein IIb associated with Glanzmann's thrombasthenia. J Clin Invest. 1991 Jan;87(1):270–276. doi: 10.1172/JCI114982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvete J. J., Henschen A., González-Rodríguez J. Complete localization of the intrachain disulphide bonds and the N-glycosylation points in the alpha-subunit of human platelet glycoprotein IIb. Biochem J. 1989 Jul 15;261(2):561–568. doi: 10.1042/bj2610561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvete J. J., Mann K., Alvarez M. V., López M. M., González-Rodríguez J. Proteolytic dissection of the isolated platelet fibrinogen receptor, integrin GPIIb/IIIa. Localization of GPIIb and GPIIIa sequences putatively involved in the subunit interface and in intrasubunit and intrachain contacts. Biochem J. 1992 Mar 1;282(Pt 2):523–532. doi: 10.1042/bj2820523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. P., Djaffar I., Pidard D., Steiner B., Cieutat A. M., Caen J. P., Rosa J. P. Ser-752-->Pro mutation in the cytoplasmic domain of integrin beta 3 subunit and defective activation of platelet integrin alpha IIb beta 3 (glycoprotein IIb-IIIa) in a variant of Glanzmann thrombasthenia. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10169–10173. doi: 10.1073/pnas.89.21.10169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza S. E., Ginsberg M. H., Burke T. A., Plow E. F. The ligand binding site of the platelet integrin receptor GPIIb-IIIa is proximal to the second calcium binding domain of its alpha subunit. J Biol Chem. 1990 Feb 25;265(6):3440–3446. [PubMed] [Google Scholar]
- Duperray A., Troesch A., Berthier R., Chagnon E., Frachet P., Uzan G., Marguerie G. Biosynthesis and assembly of platelet GPIIb-IIIa in human megakaryocytes: evidence that assembly between pro-GPIIb and GPIIIa is a prerequisite for expression of the complex on the cell surface. Blood. 1989 Oct;74(5):1603–1611. [PubMed] [Google Scholar]
- Fournier D. J., Kabral A., Castaldi P. A., Berndt M. C. A variant of Glanzmann's thrombasthenia characterized by abnormal glycoprotein IIb/IIIa complex formation. Thromb Haemost. 1989 Nov 24;62(3):977–983. [PubMed] [Google Scholar]
- Fujimura K., Phillips D. R. Calcium cation regulation of glycoprotein IIb-IIIa complex formation in platelet plasma membranes. J Biol Chem. 1983 Sep 10;258(17):10247–10252. [PubMed] [Google Scholar]
- George J. N., Caen J. P., Nurden A. T. Glanzmann's thrombasthenia: the spectrum of clinical disease. Blood. 1990 Apr 1;75(7):1383–1395. [PubMed] [Google Scholar]
- Ginsberg M. H., Lightsey A., Kunicki T. J., Kaufmann A., Marguerie G., Plow E. F. Divalent cation regulation of the surface orientation of platelet membrane glycoprotein IIb. Correlation with fibrinogen binding function and definition of a novel variant of Glanzmann's thrombasthenia. J Clin Invest. 1986 Oct;78(4):1103–1111. doi: 10.1172/JCI112667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulino D., Boudignon C., Zhang L. Y., Concord E., Rabiet M. J., Marguerie G. Ca(2+)-binding properties of the platelet glycoprotein IIb ligand-interacting domain. J Biol Chem. 1992 Jan 15;267(2):1001–1007. [PubMed] [Google Scholar]
- HARDISTY R. M., DORMANDY K. M., HUTTON R. A. THROMBASTHENIA. STUDIES ON THREE CASES. Br J Haematol. 1964 Jul;10:371–387. doi: 10.1111/j.1365-2141.1964.tb00714.x. [DOI] [PubMed] [Google Scholar]
- Kolodziej M. A., Vilaire G., Gonder D., Poncz M., Bennett J. S. Study of the endoproteolytic cleavage of platelet glycoprotein IIb using oligonucleotide-mediated mutagenesis. J Biol Chem. 1991 Dec 5;266(34):23499–23504. [PubMed] [Google Scholar]
- Kunicki T. J., Pidard D., Rosa J. P., Nurden A. T. The formation of Ca++-dependent complexes of platelet membrane glycoproteins IIb and IIIa in solution as determined by crossed immunoelectrophoresis. Blood. 1981 Aug;58(2):268–278. [PubMed] [Google Scholar]
- Lam S. C., Plow E. F., Ginsberg M. H. Platelet membrane glycoprotein IIb heavy chain forms a complex with glycoprotein IIIa that binds Arg-Gly-Asp peptides. Blood. 1989 May 1;73(6):1513–1518. [PubMed] [Google Scholar]
- Lanza F., Stierlé A., Fournier D., Morales M., André G., Nurden A. T., Cazenave J. P. A new variant of Glanzmann's thrombasthenia (Strasbourg I). Platelets with functionally defective glycoprotein IIb-IIIa complexes and a glycoprotein IIIa 214Arg----214Trp mutation. J Clin Invest. 1992 Jun;89(6):1995–2004. doi: 10.1172/JCI115808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus J. C., O'Toole T. E., Plow E. F., Glass A., Frelinger A. L., 3rd, Ginsberg M. H. A beta 3 integrin mutation abolishes ligand binding and alters divalent cation-dependent conformation. Science. 1990 Aug 24;249(4971):915–918. doi: 10.1126/science.2392682. [DOI] [PubMed] [Google Scholar]
- Lyman S., Aster R. H., Visentin G. P., Newman P. J. Polymorphism of human platelet membrane glycoprotein IIb associated with the Baka/Bakb alloantigen system. Blood. 1990 Jun 15;75(12):2343–2348. [PubMed] [Google Scholar]
- McEver R. P., Baenziger J. U., Majerus P. W. Isolation and structural characterization of the polypeptide subunits of membrane glycoprotein IIb-IIIa from human platelets. Blood. 1982 Jan;59(1):80–85. [PubMed] [Google Scholar]
- McEver R. P., Bennett E. M., Martin M. N. Identification of two structurally and functionally distinct sites on human platelet membrane glycoprotein IIb-IIIa using monoclonal antibodies. J Biol Chem. 1983 Apr 25;258(8):5269–5275. [PubMed] [Google Scholar]
- Newman P. J., Allen R. W., Kahn R. A., Kunicki T. J. Quantitation of membrane glycoprotein IIIa on intact human platelets using the monoclonal antibody, AP-3. Blood. 1985 Jan;65(1):227–232. [PubMed] [Google Scholar]
- Newman P. J., Gorski J., White G. C., 2nd, Gidwitz S., Cretney C. J., Aster R. H. Enzymatic amplification of platelet-specific messenger RNA using the polymerase chain reaction. J Clin Invest. 1988 Aug;82(2):739–743. doi: 10.1172/JCI113656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurden A. T., Rosa J. P., Fournier D., Legrand C., Didry D., Parquet A., Pidard D. A variant of Glanzmann's thrombasthenia with abnormal glycoprotein IIb-IIIa complexes in the platelet membrane. J Clin Invest. 1987 Mar;79(3):962–969. doi: 10.1172/JCI112907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Platelet membrane defects in Glanzmann's thrombasthenia. Evidence for decreased amounts of two major glycoproteins. J Clin Invest. 1977 Sep;60(3):535–545. doi: 10.1172/JCI108805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidard D., Montgomery R. R., Bennett J. S., Kunicki T. J. Interaction of AP-2, a monoclonal antibody specific for the human platelet glycoprotein IIb-IIIa complex, with intact platelets. J Biol Chem. 1983 Oct 25;258(20):12582–12586. [PubMed] [Google Scholar]
- Poncz M., Rifat S., Coller B. S., Newman P. J., Shattil S. J., Parrella T., Fortina P., Bennett J. S. Glanzmann thrombasthenia secondary to a Gly273-->Asp mutation adjacent to the first calcium-binding domain of platelet glycoprotein IIb. J Clin Invest. 1994 Jan;93(1):172–179. doi: 10.1172/JCI116942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsamooj P., Doellgast G. J., Hantgan R. R. Inhibition of fibrin(ogen) binding to stimulated platelets by a monoclonal antibody specific for a conformational determinant of GPIIIa. Thromb Res. 1990 Jun 15;58(6):577–592. doi: 10.1016/0049-3848(90)90304-u. [DOI] [PubMed] [Google Scholar]
- Rosa J. P., McEver R. P. Processing and assembly of the integrin, glycoprotein IIb-IIIa, in HEL cells. J Biol Chem. 1989 Jul 25;264(21):12596–12603. [PubMed] [Google Scholar]
- Ruggeri Z. M., Bader R., de Marco L. Glanzmann thrombasthenia: deficient binding of von Willebrand factor to thrombin-stimulated platelets. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6038–6041. doi: 10.1073/pnas.79.19.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri Z. M., De Marco L., Gatti L., Bader R., Montgomery R. R. Platelets have more than one binding site for von Willebrand factor. J Clin Invest. 1983 Jul;72(1):1–12. doi: 10.1172/JCI110946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattil S. J., Brass L. F., Bennett J. S., Pandhi P. Biochemical and functional consequences of dissociation of the platelet membrane glycoprotein IIb-IIIa complex. Blood. 1985 Jul;66(1):92–98. [PubMed] [Google Scholar]
- Wilcox D. A., Wautier J. L., Pidard D., Newman P. J. A single amino acid substitution flanking the fourth calcium binding domain of alpha IIb prevents maturation of the alpha IIb beta 3 integrin complex. J Biol Chem. 1994 Feb 11;269(6):4450–4457. [PubMed] [Google Scholar]
- Wippler J., Kouns W. C., Schlaeger E. J., Kuhn H., Hadvary P., Steiner B. The integrin alpha IIb-beta 3, platelet glycoprotein IIb-IIIa, can form a functionally active heterodimer complex without the cysteine-rich repeats of the beta 3 subunit. J Biol Chem. 1994 Mar 25;269(12):8754–8761. [PubMed] [Google Scholar]
- Zucker M. B., Pert J. H., Hilgartner M. W. Platelet function in a patient with thrombasthenia. Blood. 1966 Oct;28(4):524–534. [PubMed] [Google Scholar]