Abstract
Anti-cancer therapy drug development is an arduous task, taking 10-15 years to complete, requiring approximately one billion dollars, and rarely leads to Food and Drug Administration approval. Methods to predict unacceptable drug-induced toxicity, such as a prolonged QTc interval/risk of torsade de pointes, should be highly informative to quickly and accurately determine if further resources should be allocated in the continued development of an agent. Expert consensus has established guidelines to ascertain the ability of a new drug to prolong the QTc interval. While QTc measurement is the best way to assess arrhythmic risk, it is imprecise for a variety of reasons. Additionally, oncology patients have multiple risk factors for QTc prolongation at baseline. Competing interests involved in assessing arrhythmic risk of a new oncology agent include inability to precisely follow published guidelines for QTc assessment, patients' concomitant medical problems interfering with drug assessment and therefore clinical trial enrollment, patient safety concerns, general public safety concerns regarding toxicity assessment, need for discovery of more curative drug therapies, and individual patient perception of therapeutic risk versus benefit. Oncology patients are concerned about access to experimental agents, as well as early abandonment of a potentially beneficial agent due to a low estimated risk of toxicity, even if the event is catastrophic. We review the issues involved in evaluating the QTc interval-prolonging risk in new anti-cancer agents.
Advances in biological science have permitted the development of molecularly targeted anticancer drugs with the hope of improving survival and reducing toxicities from oncologic treatments. Some oncologic drugs have been previously shown to alter cardiac performance; these novel targeted drugs can also influence cardiac repolarization, as reflected in the QTc interval. As development of these oncologic interventions proceeds, often taking years and requiring an investment of approximately a billion dollars to achieve Food and Drug Administration (FDA) approval, a particularly complex issue has emerged: defining the risk of drug-induced, catastrophic ventricular arrhythmia. Most concerning is torsade de pointes (TdP), a polymorphic arrhythmia that can lead to sudden death. Post-approval surveillance demonstrating an increased risk of TdP has resulted in withdrawal of medications (terfenadine) or changes in labeling (cisapride) with voluntary withdrawal from the U.S. market. Because of the low incidence of TdP (a high incidence would have precluded approved for general use), as well as its association with other drugs, arrhythmias may require multiple drug exposures to be manifest. In the case of specific chemotherapeutics, these findings may not be noted for years, perhaps not until the drug is more widely prescribed for other malignancies or after approval for adjuvant use (additional treatments given after definitive local cancer therapy such as surgery). Therefore, uncovering a proarrhythmic predilection in a drug under development as early as possible is likely to prevent intellectual and financial investment spent on an agent that cannot meet approval, as well as the consequences of discontinuing a drug that some patients and providers may have perceived as having a therapeutic benefit. For example, the development of the aurora kinase inhibitor VX-680 was canceled after QTc prolongation was uncovered during phase I clinical trials (1). International guidelines for the screening of new agents for QTc interval prolongation, a possible marker for the development of TdP, have been published after an International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICHTR) (2). These guidelines apply for all medicinal agents seeking formal approval, as it is generally agreed that TdP is an unacceptable risk and patient safety in drug development is the overarching concern. However, with the number of targeted agents being tested and the nuances of the cancer patients themselves, medical oncologists and industry are now reevaluating the best approach for developing oncologic drugs. The unique aspect in new oncology drug development is that early therapies can only be tested in patients with advanced cancer, who have a poor prognosis, but who may be more willing to risk treatment-induced toxicities such as TdP in hopes of achieving longer survival. By excluding some patients with cardiac diagnoses cardiac in these early studies, TdP may only surface after the drug is used in a broader population of cancer patients, an experience similar to that of cisapride. In addition, cancer agents found to be efficacious are often later assessed as adjuvant therapy in patients with a good to excellent prognosis. These patients are less accepting of life-threatening toxicities. Complicating issues include frequent drug interactions which have important influences on the QTc interval and the variability in the measurement of the QTc interval, which may falsely indicate an arrhythmic risk and lead to abandonment of important cancer therapeutics. The task of novel cancer drug development, and the topic to be discussed here, is to define arrhythmic potential of agents prior to expending resources for their development, but without abandoning a potentially beneficial therapy or denying access to cancer patients based on an unwarranted estimated excessive risk of TdP.
What is the Incidence of Drug-Induced TdP?
Defining the risk of TdP is challenging. In one study of consecutive patients on continuous monitoring receiving any proarrhythmic drug in Sweden, the incidence of TdP was estimated at approximately 4 cases per 100,000 patients (3). Other estimates, especially for non-cardiac medications, have ranged from < 1 in 10,000 to 1 in 100,000 cases (4, 5). When adjusted for TdP risk factors, the range could be as frequent as 1 in 2000 to > 20,000 (6). It is necessary to know that the risk of TdP for cisapride was considered to be 1 in 120,000 (7). Caution must be used when predicting the degree of risk based on a prolonged corrected QT interval (pQTc) as this does not necessarily correlate with a ventricular arrhythmia, lead to TdP, or result in sudden death. Nonsustained TdP with spontaneous resolution is known to occur, but the frequency cannot be quantified. In this situation, the incidence of an event depends on how it is recognized and reported. Consider two instances of syncope outside of a monitored setting. One patient may have experienced TdP, but then spontaneously converts to normal sinus rhythm. The other may have had TdP, but it progresses to ventricular fibrillation and sudden death occurs. The presentation for both patients is the exactly same: syncope with sudden loss of consciousness and no prodrome as a warning. The survivor may be diagnosed with TdP if there is a pQTc on the ECG and the unlucky victim may have an autopsy with no other discernible cause of death. Neither patient can be truly diagnosed with TdP. Therefore, the incidence is perhaps higher than currently identified, especially in advanced cancer patients where a cardiac cause of death is not anticipated and another cause of sudden death, e.g. pulmonary embolism, is common. These patients are often though to expire from the general consequences of their cancer, frequently without investigation into the exact cause of death. Thus, defining the risk of TdP or sudden cardiac death for patients exposed to potentially proarrhythmic agents may theoretically not be possible at an early stage in drug development.
The Importance of the QT Interval and Measurement Problems
The QTc interval is monitored meticulously by drug regulatory agencies and its prolongation is the most common reason for FDA withdrawal, non-approvals, or delayed approval for all categories of non-cardiology drugs (8). The QTc interval is at this time the best, albeit very imprecise, way to measure cardiac repolarization. It is considered a marker for the risk of TdP (4, 9), even though the actual clinical relationship between drug-induced QTc interval prolongation and TdP is likely only with very long intervals (> 500 msec) and is less defined with smaller interval changes (10). At this time, there is no method to predict who will develop TdP with a short to moderately prolonged QTc interval on electrocardiogram.
Multiple factors interfere with true QTc interval measures, confounding the reliability of results. Normal intervals have been defined as less than 450 msec for men and less than 460 msec for women in healthy, young volunteers. Correcting the QT interval for bradycardia and tachycardia is considered the standard approach, but at least five methods have been proposed without any one method showing consistent superiority (11). In one small study of healthy volunteers with continuous ECG monitoring, women displayed a mean maximum QTc interval of 495 msec; in all subjects the QTc interval was longer at night and above 450 msec approximately 42% of the time (12). There is consensus that inter- and intra- person variability is high, with a mean 36 msec variation in QTc intervals (range 8 -112 msec) in normal controls (13). QT assessment studies monitor drugs for a change of 10 msec or greater in the QTc, well within this normal variation. Other related factors include position of ECG leads, body posture, pre-existing cardiac disease, T wave and other ECG abnormalities, automated versus physician readings, and variability between cardiologist readings. This lack of precision in a measurement would usually preclude such emphasis placed on the results, but at the present time, despite the limitations, this appears to be the accepted approach to defining a risk for TdP.
Much of the data on treatment-induced TdP is from the congenital Long QT Syndrome (LQTS), where the risk of TdP appears to be greater if the QTc interval is > 500 msec (14). Many ion channels are involved in the production of the action potential; the most commonly implicated is the alpha-subunit of the rapid component of the delayed rectifying potassium current IKr, controlled by the human ether-a-go-go related gene (hERG) (15). Mutations in the hERG gene have been identified in the LQTS (16) and drug-related or acquired LQTS can inhibit the IKr current (17). However, not every drug causing hERG dysfunction or blockade of current leads to TdP (18). Other channel mutations have been recorded, but their clinical significance may remain unknown until a QTc prolonging agent or a condition., such as hypokalemia, is introduced (17). The physiologic inhibition of the IKr current is influenced by many factors (drug-induced, mutation-induced, metabolically driven, etc) but the mechanism does not indicate which agents will cause clinical arrhythmias and which will cause only a prolonged QTc interval.
Oncology Patients Present a High Risk for pQTc Interval
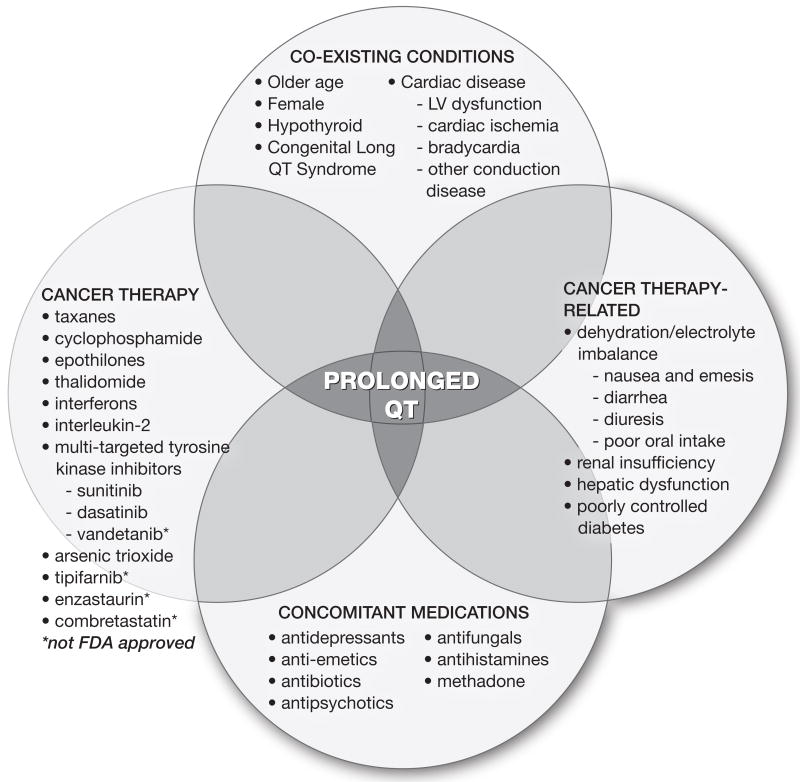
Appraising QTc intervals in oncology patients is confounded by several factors, including pre-existing cardiac perturbations. In a compilation of four early phase cancer trials, 36% of patients displayed some type of ECG abnormality at screening (19). A prolonged or borderline prolonged QTc interval was seen in 14-15% (19, 20). Cancer patients possess many of the risk factors for cardiac disease and pQTc interval, such as older age, under lying coronary disease or previous myocardial infarction. Even without medications, their baseline intervals may be longer than the younger volunteer population and thus may cross the threshold of abnormality with small drug-induced changes. Other cancer-related issues that prolong QTc intervals include treatment-induced hepatic and renal dysfunction, leading to interference with drug clearance and metabolism, and low electrolyte levels (from treatment-associated diarrhea, renal losses, diuretics, and poor oral intake). Multiple medications included in typical cancer therapy can also induce pQTc interval, such as 5-HT inhibitor anti-emetics, antihistamines, and anti-depressants, raising the possibility of additive effects (21). It is generally accepted that TdP does not occur in isolation, but happens in a host with these types of identifiable and sometimes manageable risk factors. Given these and other influences that can increase the QTc interval in oncology patients [figure 1], one can envision the fatal arrhythmias in this group to be multi-factorial and difficult to attribute solely to the cancer therapeutic.
Figure 1. Oncologic Factors Involved in QTc Prolongation.
Current International Recommendations for General QTc Interval Testing
Even with the limitations of QTc measurement, monitoring QT is an essential component of the drug development process. Essentially every drug is initially considered to be pro-arrhythmic. To form consensus on best practices and improve communication as industry moves drugs forward, the ICHTR published guidelines in 2005 on the “Clinical Evaluation of QT/QTc Prolongation and Pro-arrhythmic Potential” (2, 25). A preclinical document, ICH S7B, focuses on repolarization capacities of new drugs before first human dosing (25), with multiple possible models. The FDA does not rule out conduction abnormalities with a negative preclinical assessment and almost always waits for clinical testing results. However, this preclinical data can uncover compounds not to be developed further, or provide early identification of problems to guide clinical drug development. The clinical ICH E14 document focuses on the “thorough QT study” (TQTS), a concept to test even small (∼ 10 msec) drug-induced changes in the QT interval that could potentially be proarrhythmic (2, 26). In general, normal volunteers are given one or more doses of the new agent including a possibly supratherapeutic dose, a placebo, or a positive control, such as moxifloxicin, which reliably produces a QTc interval prolongation of 6-12 msec. The positive control is used for validation of test drug assay sensitivity and is somewhat controversial but assumed safe (3). Subjects are monitored by telemetry and drug plasma concentrations are usually obtained. These subjects, chosen for excellent overall health and lack of medication use, are supposedly the best human models to determine prolongation of QTc interval (3, 27, 28) by the candidate drugs while excluding other typical influences.
Issues in Testing the QTc Interval in Oncology Patients
Anti-cancer agents form a large category of drugs whose testing must deviate from the regular ICH E14 guidelines (2). Most importantly, complex advanced cancer patients must be utilized as the subjects in anti-cancer drug trials assessing prolonged QTc interval; administering more than limited dosing of an anti-cancer agent in subjects without malignancy would expose these healthy volunteers to unnecessary genetic and other adverse effects. Traditionally, phase I clinical trials are designed to find the maximally tolerated dose (MTD) based on observed toxicities without regard to efficacy, and this is where cardiotoxicity may first be manifested. With molecularly targeted agents, the optimum therapeutic dose is sought based on key tumor pathway inhibition; this dose may be lower than the MTD and miss small QTc interval changes. The novel phase 0 concept of drug dosing, for no greater than seven days in less than 20 patients for tissue target validation only, may eventually result in fewer patients on oncology clinical trials, with an uncertain effect on finding cardiac toxicity before approval (29). Nonetheless, Rock et al. have described recommendations for altering the TQTS for oncology, with modifications that maintain the accuracy of the QTc interval data obtained; modifications that are feasible, ethical, and maintains patient safety in the cancer setting (26).
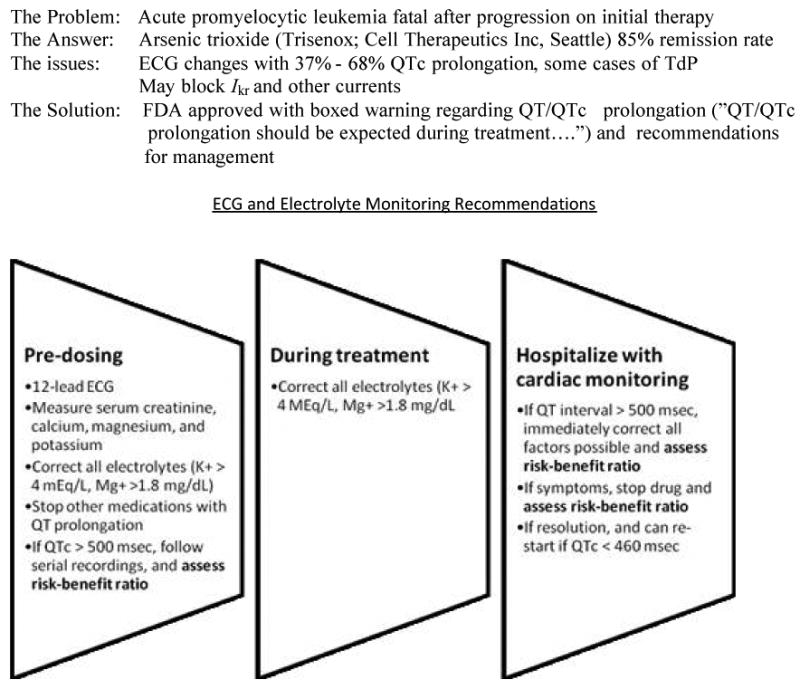
Table 1 details the complexities of QTc interval testing in cancer patients and provides suggestions for risk minimization. Risk minimization is the approach for strategically decreasing the risk of TdP in an individual patient. The usual clinical practice of dose reduction or drug discontinuation to minimize risk of TdP after approval, or even as an experimental agent in phase I studies, has the potential to reduce control of the cancer and may interfere with optimum target inhibition. Patients with unmanaged risk factors (such as low serum electrolytes, concomitant medications increasing risk of TdP, change in baseline creatinine clearance, among other factors) who develop pQTc can jeopardize further chemotherapeutic development, or even lead to drug withdrawal after approval, resulting in risk-reduced cancer patients from ever receiving a potentially beneficial agent. Since most TdP occurs in hosts with some controllable risk factors, risk minimization should be a priority at any stage in drug development and testing (20, 26). For example, risk minimization has allowed the approval of arsenic trioxide, with a high rate of pQT interval, for an uncommon adult leukemia [Figure 3].
TABLE 1. Modifications to Standard Drug Development Issues for QTc Interval Monitoring9,26-27.
| ICH E14/standard concepts | Modifications in oncology |
|---|---|
| Healthy volunteers | Oncology patients, usually heavily pre-treated with other therapies; accept other risks of anti-cancer therapy; older population; total number of exposed patients < 1,000 |
| Use of placebos, washout periods | Not appropriate |
| Use of positive controls | Only use drugs with benefit to cancer patients, such as anti-emetics |
| Supratherapeutic doses | No dosing higher than maximum tolerated or beneficial dose |
| Screen for cardiac disease, other medications | Many patients at high risk for cardiac disease; have other medical conditions |
| Normal QT interval values | Accept longer QTc interval at baseline and after dosing |
| Inpatient cardiac monitoring | Focus on drug concentration-QT interval association; average several ECG readings |
| Stop drug if QT interval prolonged | Dose reductions or drug holidays; re-consent with emphasis on greater TdP risk; risk minimization |
RISK MINIMIZATION
Make very high risk patients ineligible
Change all other medications with potential cardiac toxicity
Exhaustive review of risk factors (age, gender, CHF, sudden death family)
Assess renal and hepatic function
Increase ECG, electrolyte monitoring with aggressive supplementation of electrolytes
Extensive, personal informed consent
Repeat informed consent with ECG changes
Set individual parameters for agent discontinuation
Patient not to accept other medications from physicians unaware of arrhythmic potential
Figure 3. Risk minimization for arsenic trioxide 19, 30.
Proceeding with a Prolonged QTc Interval
QTc prolongation approximately > 500 msec or a change from baseline of > 60 msecs is of clinical concern (2), and are common criteria to indicate potential cardiotoxicity and impede further development. If the QTc interval is discovered to be prolonged from baseline to just below those values, further drug development does not have to be necessarily discontinued, with the caveat that these numbers are biomarkers that do not always correspond with clinical outcomes. More credence can be placed on pQTc if there are other risk factors associated with TdP seen on cardiac monitoring, such as QT interval dispersion, bradycardia, and complex PVCs in couplets or ventricular runs, even in the absence of symptoms (21).
The overall benefits of the drug should be considered. Anti-cancer drug evaluation must consider the efficacy of the agent, other potential uses/indications for the drug, the number of patients who will be prescribed the drug, and current therapeutic options including the toxicity profile of the alternatives. The ability to diminish the risk and ensure patient safety is paramount if the agent appears to be beneficial.
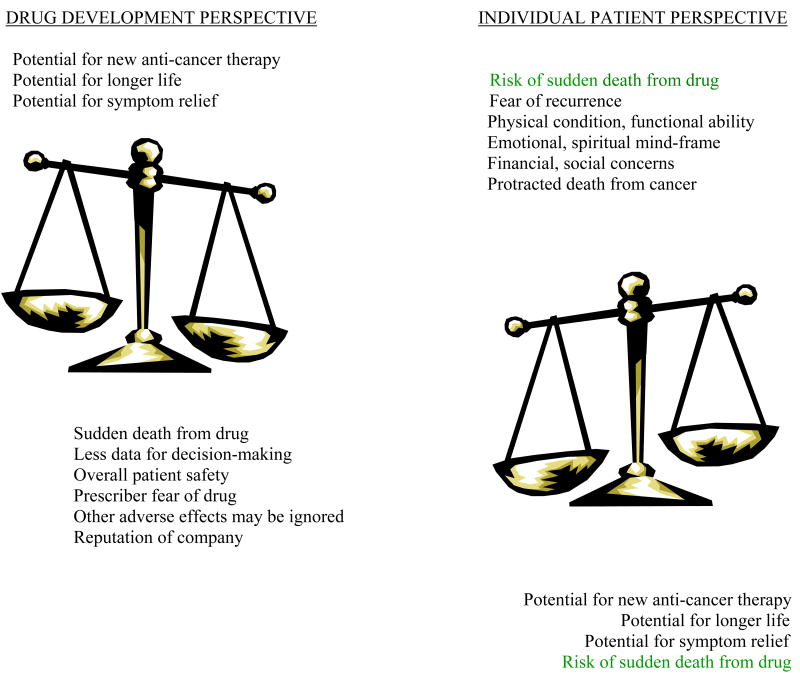
Sudden death associated with drug-induced TdP is of concern to all, not only to regulatory agencies. This trepidation is driven by the sporadic and unpredictable nature of this problem and the severity of the potential outcome. The concomitant use of ancillary medications, for non- life threatening indications, that result in dangerous drug-drug interactions requires some risk-taking for utilizing more than one medication at a time. In the oncology setting, as opposed to more elective clinical situations, the assessment of the risk-to-benefit ratio involves multiple factors. The obvious benefit in cancer therapeutic development is the detection of promising new agents, especially for malignancies with few treatment options, such as metastatic melanoma and pancreas carcinoma. Patients enrolled in early phase trials typically have incurable disease and are searching for options; some patients are on waiting lists for the opportunity to enroll and need only to have an expected lifespan measured in months to be eligible Sometimes eligibility requirements stipulate discontinuation of other medications with cardiac implications, so patients also become aware of the proarrhythmic potential of their current medications, lessening the perception of risk with the study drug; on the other hand, patients may opt out of a trial rather than change their other mediations. The methods by which cancer patients make decisions about their care have been studied and comprise a small body of literature. Agrawal, et al found that only 12% of patients thought potential toxicity was useful or important information when making decisions to participate in Phase I clinical trials. When queried about the potential risk of death from drug-induced toxicity, 91% of patients said even a 10% risk of death from toxicity would not deter them from phase I participation (31). However, this risk tolerance is more than the estimated risk of death from drug-induced TdP. Thus, the acceptance of toxicity is quite high in patients participating in early phase trials. It is important to remember that the ethical criteria for risk for oncology patients is different than for most other patient groups. Given the heterogeneity among cancer patients, therapeutic decisions are made at the individual patient-physician level, encompassing variables (such as family, spiritual, financial issues, altruism) in addition to toxicity [figure 2]. Fear of cancer progression or recurrence is a strong motivator to accept risk.
FIGURE 2. Issues in Decision Making for Anti-Cancer Drug Development with Prolonged QTc Interval.
As the complexity of anti-cancer treatments continue to expand, the possibility of life-threatening treatment-induced toxicity is a significant factor. When therapies are applied to a less ill population and used in an adjuvant setting, toxicity is less acceptable. These include curable metastatic cancers such as some lymphomas and testicular cancer; adjuvant use in multiple diseases (such as early stage breast and colon cancer) where the majority survive; and possibly for cancer prevention in high-risk individuals. In these situations, patients are taking treatment for a specified period of time only, as opposed to taking treatment until it is no longer effective. This expansion of therapeutic indications is rarely developed until years after the first few clinical trials and may be established long after initial drug approval. There may be increased reports of TdP as more patients have been exposed to these agents, but typical monitoring outside of clinical trials in haphazard at best and is reported very infrequently. The FDA labeling changes to cisapride are based on the discovery of such drug interactions. It is for this reason that comprehensive post-marketing surveillance for TdP-associated therapies is a critical part of the future of clinical practice, for cardiologists and oncologists. The application of efficacious oncology agents with a proarrhythmic predilection in a healthier population will require discussion at both the regulatory level and the physician-patient level. For cancer patients, especially those in the initial clinical trials, the risk of premature loss of a potentially efficacious agent may sometimes be greater than the risk of sudden death due to TdP.
Summary
There are several key points regarding the investigation of the QTc interval in anti-cancer drug development:
QTc interval is imprecisely measured because of wide diurnal variations; the incidence of drug-induced pQTc leading to TdP is estimated as small, but would be unacceptable for a healthy population
Oncology patients have multiple risk factors for pQTc at presentation, so the incidence of TdP is theoretically higher, but may not be attributable to drug alone, confounding assessment new agents,
Advanced cancer patients are heterogeneous and individually unique regarding their disease burden, the influence of other circumstances on therapy decisions, and risk perception. The risk of sudden death from treatment is only one factor controlling drug development for these patients.
QT assessment can be modified in advanced cancer patients, but pro-arrhythmic agents must be identified so that the population that will ultimately receive the agent can benefit from risk modification plans.
Current State
The FDA has convened a “QT interdisciplinary Review Team” with the expertise to assess QT interval studies and discuss plans for modified TQT studies (27). Published lists of medications associated with prolonged QTc interval steer healthcare providers from possible additive risks of pQTc interval, especially after drug approval (32). In this way, much-needed cancer therapeutics such as romidepsin (ISTODAX, Gloucester Pharmaceuticals Inc.) (33) for cutaneous T cell lymphoma are being approved (November 2009), with the issues of QTc prolongation and sudden death addressed (34, 35) and warning regarding potential ECG changes (33).
Future Directions
The development of new powerful cancer drugs that do not cause TdP will proceed on many fronts:
Expanding knowledge of multiple ion channels and pathways involved with repolarization, aiming for improved preclinical models, such that only a small fraction of QTc prolonging drugs progress to human studies
Discover ing genetic changes such as polymorphisms involved in drug-induced QT prolongation, such that future testing, can be tailored to a patient's unique susceptibility (36), avoiding drug exposure in patients at high risk for TdP
Designing new oncology and supportive care agents to circumvent hERG and other channel functioning to decrease arrhythmic toxicity
Assessment and input from cardiologists regarding patients' risk and reduction of TdP as well as devising more accurate methods to assess QT interval length, such as standardized corrections for abnormal ECGs
Continuation of tight post-market surveillance, especially since smaller numbers of patients are required in FDA approval trials than for non-oncologic drugs.
Linking and sharing clinical data (ECG data correlated with patient characteristics, heart rate, concomitant medications, DNA samples, etc) for risk assessment, drug development decisions, and risk minimization; educating prescribers about risk minimization with potentially proarrhythmic drugs to improve skill in managing these specific medications
Cardiologists and oncologists have complementary roles regarding measurement of TdP risk and anti-cancer drug development. The development of cardiotoxic molecularly targeted treatments mandates that cardiologists have an established role in risk assessment and decision-making in the multidisciplinary care of cancer. With shared input on risk management strategies and preclinical “no-go” assessments, the clinical incidence of new drug induced TdP should decrease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vertex Pharmaceuticals press releases. 2008 available at http://investors.vrtx.com/releaseDetail.cfm?releaseid=325650. http://investors.vrtx.com/releasedetail.cfm?ReleaseID=276543 2007.
- 2.The Clinical Evaluation of QT/QTc Prolongation and Pro-arrhythmic Potential for Non-Antiarrhythmic Drugs: E14. Geneva, Switzerland: International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use; 2005. Available at http://www.ich.org/LOB/media/MEDIA1476.pdf. [Google Scholar]
- 3.Darpo B. Spectrum of drugs prolonging QT interval and the incidence of torsades de pointes. European Heart Journal Supplements. 2003;3K:K70–K80. [Google Scholar]
- 4.Whellan DJ, Green CL, Piccini JP, Krucoff MW. QT as a safety biomarker in drug development. Clin Pharmacol & Therapeutics. 2009;86:101–104. doi: 10.1038/clpt.2009.70. [DOI] [PubMed] [Google Scholar]
- 5.HaverKamp W, Breithardt G, Camm JA, et al. Potential for QT prolongation and proarrhythmia by non-anti-arrhythmic drugs: clinical and regulatory implications report on a Policy Conference of the European Society of Cardiology. Cardiovascular Res. 2000;47:219–223. doi: 10.1016/s0008-6363(00)00119-x. [DOI] [PubMed] [Google Scholar]
- 6.Moss AJ. QT interval and torsades de pointe. Drug safety. 1999;21(Suppl):5–10. doi: 10.2165/00002018-199921001-00002. [DOI] [PubMed] [Google Scholar]
- 7.Vitola J, Vukanovic J, Roden DM. Cisapride-induced torsades de pointes. J of CardiovascularElectrophysiology. 1998;9:1109–1113. doi: 10.1111/j.1540-8167.1998.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 8.Strevel EL, Ing DJ, Su LL. Molecularly targeted oncology therapeutics and prolongation of the QT interval. J Clin Oncol. 2007;25:3262–3371. doi: 10.1200/JCO.2006.09.6925. [DOI] [PubMed] [Google Scholar]
- 9.Fingert H, Varterasain M. Safety biomarkers and the clinical development of oncology therapeutics: considerations for cardiovascular risk and safety management. The AAPS Journal. 2006;8(1):E89–E94. doi: 10.1208/aapsj080110. article 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bednar MM, Harrigan EP, Anziano RJ, Camm AJ, Ruskin JN. The QT interval. Progress in Cardiovascular Disease. 2001;43(No 5 suppl):1–45. doi: 10.1053/pcad.2001.21469. [DOI] [PubMed] [Google Scholar]
- 11.Zehender M, Hohnloser S, Just H. QT-interval prolonging drugs: mechanisms and clinical relevance of their arrhythmic hazards. Cardiovascular Drugs and Therapy. 1991;5:515–530. doi: 10.1007/BF03029779. [DOI] [PubMed] [Google Scholar]
- 12.Molnar J, Zhang F, Weiss J, Ehlert F, Rosenthal JE. Diurnal pattern of QTc interval: how long is prolonged? Possible Relation to circadian triggers of cardiovascular events. J Am College of Cardiol. 1996;27:76–83. doi: 10.1016/0735-1097(95)00426-2. [DOI] [PubMed] [Google Scholar]
- 13.Agin MA, Kazierad DJ, Abel R, et al. Assessing QT variability in normal volunteers. Proc American College of Clinical Pathology. 2003 [Google Scholar]
- 14.Priori SG, Schwartz PJ, Napolitano C, et al. Risk Stratification in the long-QT syndrome. New Engl J Med. 2003;348:1866–1874. doi: 10.1056/NEJMoa022147. [DOI] [PubMed] [Google Scholar]
- 15.Andersom ME, Al-Khatib SM, Roden DM, Califf RM. Cardiac repolarization: current knowledge, critical gaps, and new approaches to drug development and patient management. Am Heart Journal. 2002;144:769–781. doi: 10.1067/mhj.2002.125804. [DOI] [PubMed] [Google Scholar]
- 16.Redfern WS, Carlsson, Davis AS, et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsades de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovascular Research. 2003;58:32–45. doi: 10.1016/s0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- 17.Roden DM. Drug-induced prolongation of the QT interval. N ENgl J Med. 2004;350:1013–1022. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 18.Yang T, Snyders D, Roden DM. Drug block of IKr: model systems and relevance to human arrhythmias. J Cardiovascular Pharmacol. 2001;38:737–744. doi: 10.1097/00005344-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Barbey JT, Pezzullo JC, Saignet SL. Effect of arsenic trioxide on QT interval in patients with advanced malignancies. J Clin Oncol. 2003;21:3609–3615. doi: 10.1200/JCO.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Varterasian M, Meyer M, Fingert, et al. Baseline heart rate-corrected QT and eligibility for clinical trials in oncology. J Clin Oncol. 2003;21:3378–3379. doi: 10.1200/JCO.2003.99.104. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad K, Dorian P. Drug-induced QT prolongation and proarrhythmia: an inevitable link? Europace-European Society for Cardiology. 2007:iv16–iv22. doi: 10.1093/europace/eum167. [DOI] [PubMed] [Google Scholar]
- 22.Floyd JD, Nguyen DT, Lobins RL, Bushir Q, Doll DC, Perry MC. Cardiotoxicty of cancer therapy. J Clin Oncol. 2005;23:7685–7696. doi: 10.1200/JCO.2005.08.789. [DOI] [PubMed] [Google Scholar]
- 23.DePonti F, Poluzzi E, Montanaro N. QT-interval prolongation by non-cardiac drugs: lessons to be learned from recent experience. Eur J Clin Pharmacol. 2000;56:1–18. doi: 10.1007/s002280050714. [DOI] [PubMed] [Google Scholar]
- 24.Sarapa N, Britto MR. Challlenges of characterizing proarrythmic risk due to QTc prolongation induced by nonadjuvant anticancer agents. Expert Opin Drug Saf. 2008;7:305–318. doi: 10.1517/14740338.7.3.305. [DOI] [PubMed] [Google Scholar]
- 25.The Non-Clinical Evaluation of the Potential for Delayed Ventricular Repolarization (Qt interval prolongation) by Human Pharmaceuticals: S7B. Geneva, Switzerland: International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use; 2005. Available at http://www.ich.org/LOB/media/MEDIA2192.pdf. [Google Scholar]
- 26.Rock EP, Finkle J, Fingert HJ, et al. Assessing proarrythmic potential of drugs when optimal studies are infeasible. Am Heart J. 2009;157:827–836. doi: 10.1016/j.ahj.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 27.Shah RR. If a drug deemed “safe” in nonclinical tests subsequently prolongs QT in phase 1 studies, how can its sponsor convince regulators to allow development to proceed? Pharmacology & Therapeutics. 2008;119:215–221. doi: 10.1016/j.pharmthera.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Garnett CE, Beasley N, Bhattaram VA, et al. Concentration-QT relationships play a key role in evaluation of proarrhythmic risk during regulatory review. J Clin Pharmacol. 2008;48:13–18. doi: 10.1177/0091270007307881. [DOI] [PubMed] [Google Scholar]
- 29.Kummar S, Kinders R, Rubinstein L, et al. Compressing drug development timelines in oncology using phase “0” trials. Nature Reviews Cancer. 2007;7:131–139. doi: 10.1038/nrc2066. [DOI] [PubMed] [Google Scholar]
- 30.Arsenic trioxide prescribing information. available at http://www.trisenox.com/490-Trisenox-Prescibing-Info.aspx.
- 31.Agrawal M, Grady C, Fairclough DL, Meropol NJ, Maynard K, Emanuel EJ. Patients' decision-making process regarding participation in phase I oncology research. J Clin Oncol. 2006;24:4479–4484. doi: 10.1200/JCO.2006.06.0269. [DOI] [PubMed] [Google Scholar]
- 32.QTc prolonging agents. available at http://www.qtdrugs.org.
- 33.Romidepsin prescribing information. available at www.accessdata.fda.gov/drugsatfda_docs/label/2009/022393lbl.pdf.
- 34.Bates SE, Rosing DR, Fofo T, Piekarz RL. Challenges of evaluating cardiac effects of anticancer agents. Clin Cancer Res. 2006;12:3871–3873. doi: 10.1158/1078-0432.CCR-06-1017. [DOI] [PubMed] [Google Scholar]
- 35.Piekarz RL, Frye AR, Wright JJ, et al. Cardiac studies in patients treated with depsipeptide, FK228, in a phase II trial for T-cell lymphoma. Clin Cancer Res. 2006;12:3762–3773. doi: 10.1158/1078-0432.CCR-05-2095. [DOI] [PubMed] [Google Scholar]
- 36.Fitzgerald PT, Ackerman MJ. Drug-induced torsades de pointes: evolving role of pharmacogenomics. Heart Rhythm. 2005;2:S30–S37. doi: 10.1016/j.hrthm.2005.08.007. [DOI] [PubMed] [Google Scholar]