Abstract
Conspicuous pathologic features of chronic liver allograft rejection include bile duct loss and chronic obliterative arteriopathy. A quantitative histometric analysis was performed to document the extent of bile duct loss, the size of the “vanished” ducts and the extent of chronic obliterative arteriopathy and to determine whether there was any relationship between chronic obliterative arteriopathy and bile duct loss. All failed liver allograft specimens with chronic rejection were reviewed and categorized according to the degree of chronic obliterative arteriopathy, assessed by the degree of luminal narrowing of hilar hepatic artery branches. Histometric analysis of the grafts revealed: (i) there was a loss of small portal arterioles (<35 μm); (ii) bile ducts which should accompany arteries <35, 35 to 54 or 55 to 74 μm in diameter were missing, with the greatest decrease occurring among the smallest ducts; (iii) bile duct loss was seen in the absence of significant large vessel chronic obliterative arteriopathy, and (iv) the severity of arteriole and bile duct loss, as well as the size of the vanished ducts, was directly proportional to the degree of chronic obliterative arteriopathy. Furthermore, the size of the “vanished” bile ducts in liver allografts appeared to differ from the size of ducts destroyed in primary biliary cirrhosis. These studies offer indirect, but suggestive proof that two mechanisms are operative in the bile duct loss seen in chronic rejection: direct lymphocytotoxicity and ischemic damage.
Chronic liver allograft rejection is responsible for at least 20% of the graft failures at the University of Pittsburgh. If early graft failures, which are often secondary to operative technical difficulties, are excluded, the figure approaches 50%. Generally accepted pathologic features of chronic liver allograft rejection include chronic obliterative arteriopathy (COA) and bile duct loss (1–5). The mechanisms involved in the development of COA, the major long-term complication of all solid organ allografts, are presently unknown. It seems likely that “immune injury” plays at least a partial role. Bile duct loss is thought to be due to direct lymphocytotoxic attack, which seems reasonable considering the histologic appearance (1–7). However, ischemia has been largely overlooked as a cofactor in the pathogenesis of bile duct injury.
The sole blood supply of the biliary tract in man is derived from the hepatic artery (8, 9). Therefore, COA may contribute to bile duct damage and loss. We undertook a histometric analysis of liver allografts which failed because of chronic rejection to determine whether there is any relationship between COA and bile duct loss.
MATERIALS AND METHODS
Source Material
Liver allografts which failed because of chronic rejection between 1981 and 1987 were reviewed (n = 28). The diagnosis of chronic rejection was based on a characteristic clinical course and confirmed by histologic analysis of the removed failed allograft. Allografts with complications other than rejection (e.g. hepatitis) were excluded from the analysis. Normal control livers were selected from organs obtained at autopsy from patients with no clinical or pathologic evidence of liver disease. Tissue sampling for histologic sections from failed allografts and from control livers was performed according to a predefined protocol (10). Three sections were taken from the hilum, two sections from the left lobe and three sections from the right lobe. All sections were cut at 3 μm and routinely stained with hematoxylin and eosin. No special staining techniques were routinely employed.
Analysis of the Degree of COA
The three sections taken from the hilum of each liver were examined blindly and independently by two of the authors (S. O., A. J. D.) for the degree of narrowing of hepatic arterial branches and the percentage of vessels affected. Thereafter, each case was subjectively scored on the basis of an overall assessment of the severity of the COA present. Specifically, deep hilar sections (10) from each case were examined. These sections contained first, second and third order branching arteries. If more than 70% of the arteries were narrowed by more than 75%, the case was classified as “severe COA.” Conversely, in “mild COA,” more than 70% of the arteries showed less than 35 % narrowing. Because this methodology was somewhat subjective, cases in which the determination of COA was not completely obvious were excluded. Also, omission of intermediate cases would accentuate any possible differences between these groups, which might result in recognition of the role of ischemia in bile duct loss. An example of severe COA is shown in Figure 1.
Fig. 1.
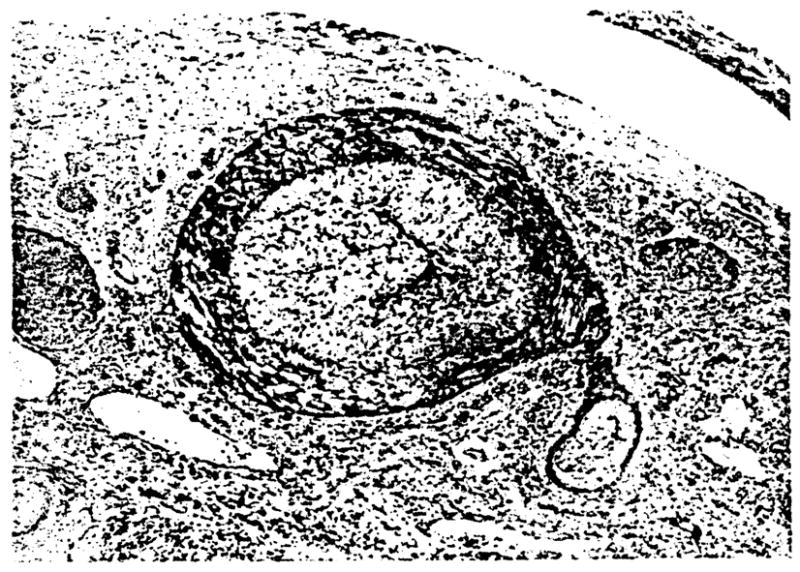
Cross-section of larger hilar artery with severe COA. Note the almost complete luminal obliteration by the subintimal foam cells. H & E, 150X.
Selection of Cases
Three subgroups of patients were then identified based on the degree of COA: none (normal controls, n = 14), mild (n = 10) and severe (n = 8) COA. General patient data are shown in Table 1. The average age of allograft patients with mild COA was 31.9 ± 11 years compared to 12.6 ± 10 years for the patients with severe COA (p > 0.05). There was a greater number of adult female patients in the mild COA group and more male children in the severe COA group. Among the control patients, there were nine females and five males (average age: 62 years). None had any clinical or pathologic evidence of hepatic disease or malfunction. The average graft survival was shorter in those with severe COA (7.7 ± 5.6 months) compared to those with mild COA (33.7 ± 43.9 months) (p < 0.05). Complete major histocompatibility typing of both donor and recipient was available in only eight of the 18 cases analyzed. No particular trend was noted in this small sample. The results were not further included because of the limited amount of data. All patients were maintained on baseline immunosuppression of cyclosporine and steroids. Episodes of acute cellular rejection were treated with a steroid bolus or recycling doses. If steroids were unsuccessful, OKT3 (Ortho Pharmaceuticals, Raritan, NJ) was used.
TABLE 1.
General data of patients experiencing chronic liver allograft rejection
| OLTa no. | Age (years) | Sex | Original diseaseb | Graft survival (mo) | Degree of COA |
|---|---|---|---|---|---|
| 235 | 44 | F | PBC | 3.0 | Mild |
| 303 | 23 | F | CAH | 1.3 | Mild |
| 056 | 20 | M | CHF | 140.5 | Mild |
| 722 | 34 | F | PBC | 4.0 | Mild |
| 466 | 47 | F | HCC | 6.3 | Mild |
| 578 | 45 | F | PBC | 6.2 | Mild |
| 137 | 14 | F | CAH | 71.5 | Mild |
| 328 | 33 | F | PBC | 39.5 | Mild |
| 339 | 33 | M | PSC | 24.7 | Mild |
| 412 | 26 | F | CAH | 40.7 | Mild |
| 346 | 16 | M | Wilson’s | 2.0 | Severe |
| 377 | 32 | M | PSC | 14.8 | Severe |
| 300 | 25 | M | HCC | 3.7 | Severe |
| 266 | 5 | F | A1ATD | 2.0 | Severe |
| 309 | 3 | F | 2°BC | 7.0 | Severe |
| 373 | 10 | M | SMN | 7.7 | Severe |
| 294 | 2 | M | A1ATD | 17.0 | Severe |
| 401 | 8 | M | SMN | 7.7 | Severe |
OLT = orthotopic liver transplantation.
PBC = primary biliary cirrhosis; CAH = chronic active hepatitis (non-A, non-B); CHF = congenital hepatic fibrosis; HCC = hepatocellular carcinoma; PSC = primary sclerosing cholangitis; A1ATD = α1-antitrypsin deficiency; 2°BC = secondary biliary cirrhosis; SMN = submassive necrosis.
Histometric Analysis of Portal Arterial Size and Distribution
The presence or absence of any artery within the confines of the portal tract and the size of the largest (or only) artery in that tract was recorded. This assessment was repeated for all portal tracts in every section of the selected cases (average: 200 to 300 portal tracts per case). The size of the artery was determined by measuring the diameter of the vessel to the outer extent of the media using an ocular micrometer. Tangentially cut arteries were handled as follows. Most arteries were, to some extent, cut tangentially. If the artery of interest was located in a portal tract, which was defined on all sides by a limiting plate, the shortest diameter was measured. This measurement is closest to the true vessel diameter. If, however, that portal tract was not defined on all sides by a limiting plate and ran longitudinally through the section, the artery was ignored. The percentage of portal tracts with arteries and the Size distribution of the largest (or only) artery was then calculated and tabulated. Recognition of portal tracts in the failed allografts was sometimes difficult because of the lack of easily identifiable architectural features (i.e. bile ducts and arteries). In these livers, the appearance of the connective tissue, particularly surrounding the vein, and the location of cholestasis (invariably present in failed allografts; centrilobular) were used as landmarks. An example of a portal tract with no bile duct and no artery is shown in Figure 2.
Fig. 2.
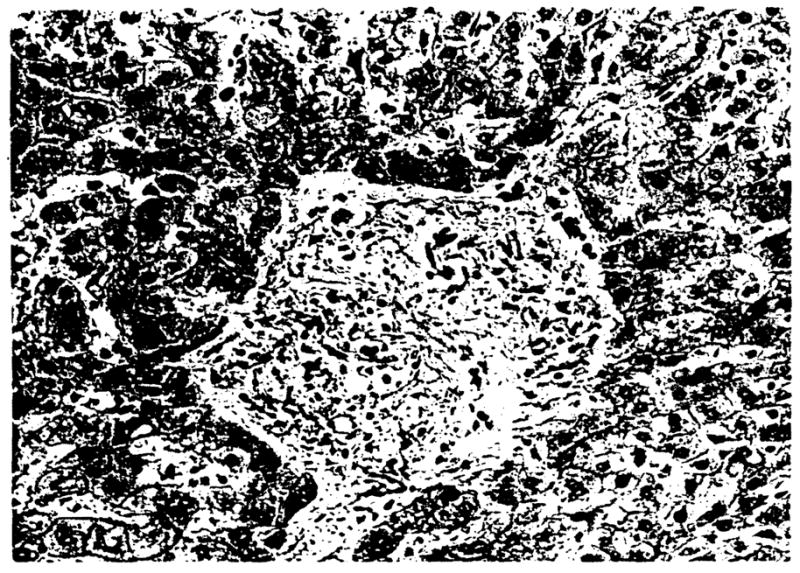
Portal tract without an identifiable bile duct or artery. H & E, 310x.
Histometric Analysis of Bile Duct Loss
We performed an analysis similar to that done by Nakanuma and Ohta (11) to determine the extent of duct loss and the relative size of vanished bile ducts in the selected cases. In brief, it is known that arteries and bile ducts of a similar diameter occur in parallel in the portal tracts of normal livers. Precise measurement of the greatest diameter (outer extent of media) of the largest (or only) artery in each portal tract was recorded. Tracing an imaginary circle 3 times the recorded arterial diameter from the external media of that vessel circumscribed the area in which the accompanying bile duct should be detected. An example of a portal tract which contains an artery, but no bile duct, is shown in Figure 3. The presence or absence of a bile duct within this area was then recorded for each portal tract of every section. The results were tabulated according to a predefined range of arterial diameter (<35, 35 to 54, 55 to 74 and ≥ 75 μm) for each liver and expressed as the rate of bile duct-artery (BD-A) parallelism. Arteries >75 μm were not separated into distinct groups. Too few were present for a meaningful statistical analysis if subcategories were made.
Fig. 3.
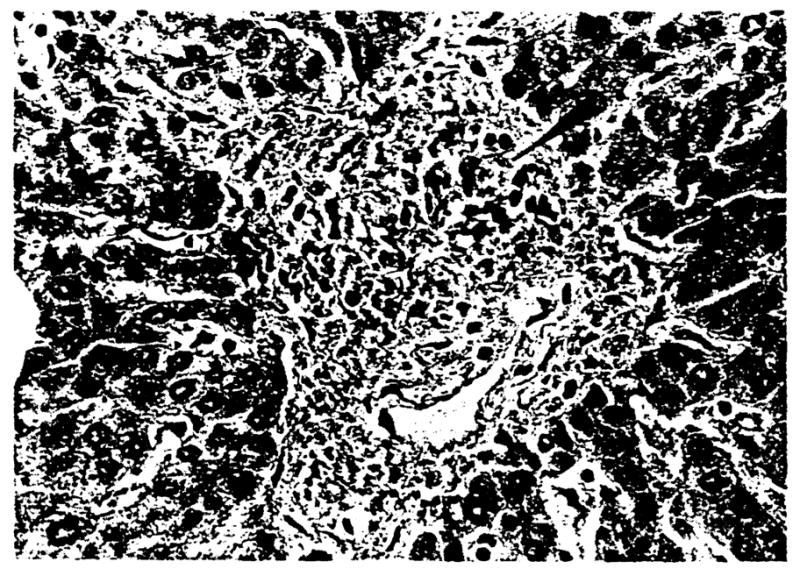
Portal tract containing an artery (arrowhead), but no bile duct. H & E, 500X.
It must be noted that the rate of parallelism was determined only for those portal tracts which contained arteries. Portal tracts without arteries and bile ducts were excluded from the determinations of BD-A parallelism.
RESULTS
Pathologic Findings in Failed Liver Allografts
Grossly, the failed allografts with chronic rejection were slightly enlarged and bile stained. No graft was cirrhotic with grossly evident regenerative nodularity. Hilar arteries had thickened walls. Microscopically, the lobular architecture was distorted secondary to portal and perivenular fibrosis. A true cirrhosis was not seen in any of the livers. The fibrotic portal tracts were conspicuous for an absence of small bile ducts and arteries. A mild predominantly lymphohistiocytic and plasmacytic portal inflammatory infiltrate was scattered throughout the sections. Many portal tracts were free of inflammation. Often, the tracts without ducts were also devoid of inflammation. Centrilobular hepatocellular swelling was a conspicuous feature, and invariable prominent centrilobular hepatocanalicular cholestasis was present. First, second and third order branching vessels of the hepatic artery in the hilum demonstrated luminal narrowing secondary to subintimal foam cells and fibrointimal hyperplasia (COA). Intramural and periadventitial inflammation was also present in some cases. The grafts were categorized according to the degree of COA, which is shown in Table 1.
Distribution and Size of Portal Tract Arteries
The percentage of portal tracts containing arteries or arterioles was computed for each of the 14 subjects with no COA (controls), the 10 subjects with mild COA and the eight subjects with severe COA (Fig. 4). Overall, 90.9% of the portal tracts in controls, 61.3% of the portal tracts in cases with mild COA and 25.3% of the portal tracts in cases with severe COA had at least one artery or arteriole. The logistic regression model (12) was used to see whether there was an association between severity of COA and presence of arteries in the portal tract. A statistically significant (p < 0.001) association was found, with the differences between each pair of groups found to be significantly (p < 0.001) greater than zero.
Fig. 4.
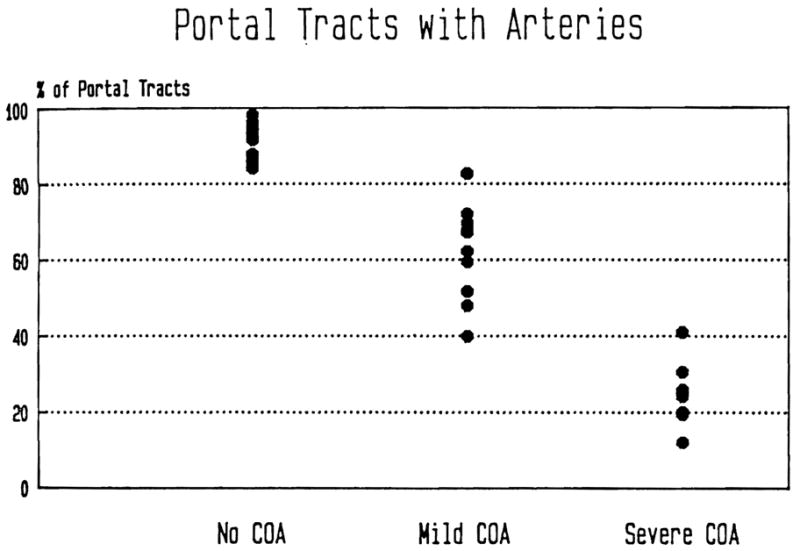
The percentage of portal tracts from normal control and failed allograft livers containing at least one identifiable artery is represented. Note the absolute loss of identifiable portal arteries in failed allografts.
Figure 5 shows the percentage of portal tracts in which the largest or only artery is <35, 35 to 54, 55 to 74 or ≥75 μm in diameter for each subject, classified by degree of COA. Overall, the distribution of the size of the largest (or only) artery in those portal tracts with at least one artery is significantly (p < 0.001) different among groups, using the χ2 test of association for independent samples. Pairwise comparison of the three groups (control, mild COA, severe COA) using the same statistical technique demonstrated that the distributions of the size of largest (or only) artery differ significantly (p < 0.001) between each pair of groups. Among 81 % of the portal tracts of controls, the largest or only artery was less than 35 μm, compared to about 58% of the portal tracts in cases with mild COA and less than 41 % of the portal tracts in cases with severe COA. These differences are statistically significant (p < 0.001). At the other extreme, only about 4 % of the portal tracts in controls contained a single or largest artery 75 μm or greater in diameter, ranging from 1.9 to 7.9% among the 14 subjects. This compares to 6% of portal tracts in cases with mild COA (range: 3.1 to 12.6%) and over 14 % of portal tracts in cases with severe COA (range: 3.1 to 30.8%). These differences are also significant (p < 0.001).
Fig. 5.
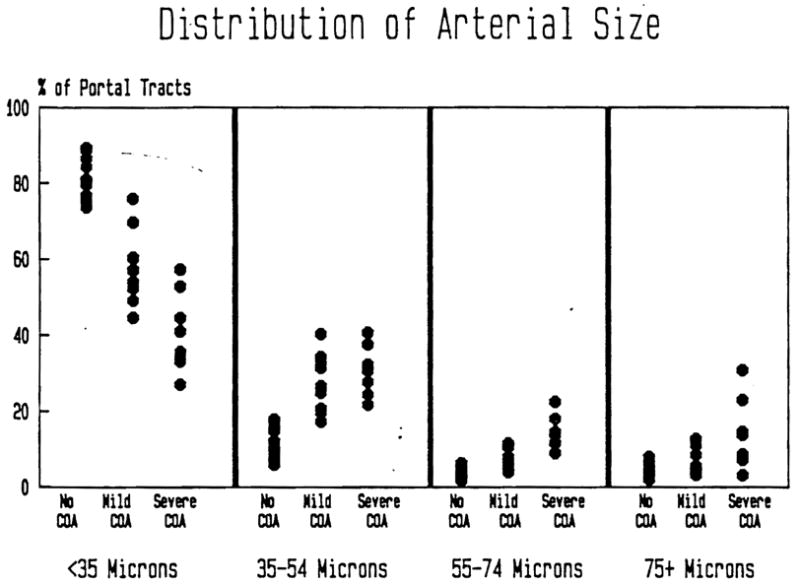
The percentage of portal tracts according to the size of the largest, or only, portal artery is represented. Note the relative decrease in small portal arterioles and relative increase in larger arteries.
Bile Duct-Arterial Parallelism
The mean rate of BD-A parallelism vs. arterial size, classified by degree of COA, is shown in Figure 6. Using a test for linear trend (13), there is a statistically significant (p < 0.001) trend toward decreasing parallelism with decreasing artery size in both the mild and severe COA groups. Using the χ2 test for independent samples, statistically significant differences (p < 0.01) for BD-A parallelism between groups (controls vs. mild COA, controls vs. severe COA and mild COA vs. severe COA) were found in all size categories, except between control and mild COA (p > 0.05) for arteries greater than or equal to 75 μm in diameter.
Fig. 6.
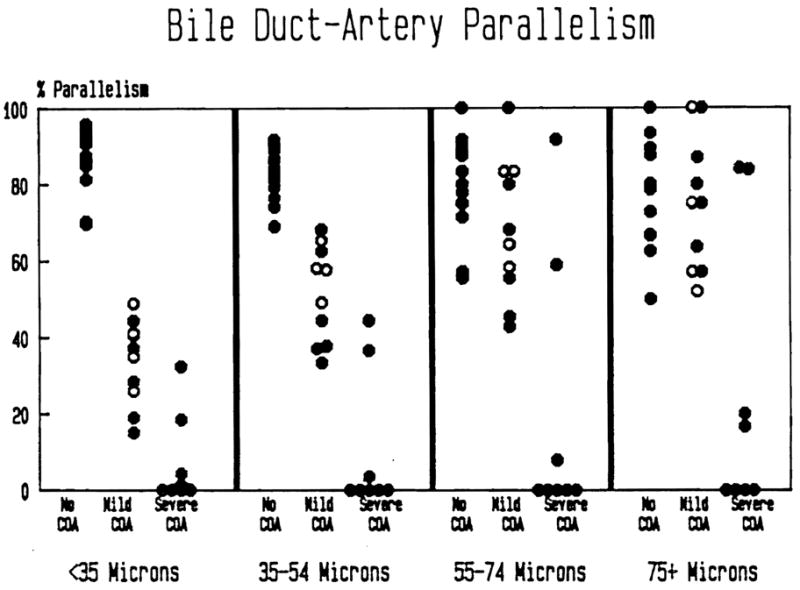
The percentage of portal tracts in which a bile duct is identified along with the corresponding artery is represented. Note the severe loss of small bile ducts in the failed allografts. Duct loss corresponding to larger arteries is seen only in allografts with severe COA. Patients with PBC prior to transplantation are represented by the open circles.
It must be remembered that the rate of BD-A parallelism was determined using only those portal tracts which contained arteries. Bile duct loss with associated artery loss was not specifically tabulated. Therefore, absolute bile duct loss, particularly involving the smaller ducts (<35 μm)is even greater than that expressed as a decrease in the rate of parallelism.
DISCUSSION
Analysis of the data reveals that quantifiable bile duct and small arterial loss are features of chronic liver allograft rejection. The degree of damage to both arteries and bile ducts increases as the size of each decreases. Additionally, the severity and size of arterial loss and vanished ducts is directly proportional to the degree of COA.
Bile duct loss was recognizable under two circumstances. One occurred when an artery without a corresponding bile duct was found (decreased rate of BD-A parallelism). The other was recognized when there was loss of both the artery and the bile duct (absolute arterial loss). Therefore, the results of this study offer indirect but supportive evidence that two mechanisms may be responsible for bile duct loss: direct immunologic attack and ischemic injury.
The absence of a bile duct when an artery is found is supporting evidence for a direct (probably lymphocytotoxic) immunologic attack on the biliary epithelium. Other lines of evidence also point to a primary immunologic insult upon the biliary epithelium. The histologic appearance of acute cellular rejection is characterized by lymphocytic invasion and evidence of degenerative changes in the biliary epithelium (1–7). Biliary inflammation is less severe in chronic rejection (5, 10). Nevertheless, careful light microscopic examination of livers undergoing chronic rejection often reveals lymphocytic cells within the biliary epithelium. Ultrastructural examination of chronically rejected liver allografts by Fennel and Vierling (14) revealed lymphocytes in close contact with degenerating bile duct cells, similar to that necessary for cytotoxic T-lymphocyte reactions. In vitro functional analysis of graft-infiltrating lymphocytes also offers supportive evidence. Lymphocyte cultures generated from liver allograft tissue undergoing rejection have demonstrated cytotoxic activity directed at donor MHC antigens (15). These MHC antigens are expressed strongly on bile ducts (16, 17), particularly during a rejection reaction.
The absence of a bile duct in conjunction with arterial loss is indirect but strongly suggestive evidence that ischemic injury contributes to duct loss. We also found that the severity of duct loss and the size of the “vanished” ducts is directly proportional to the degree of COA. Since the biliary tree in man receives its blood supply exclusively from the hepatic arterial system (8, 9), COA and peripheral arterial loss compromise blood flow to the dependent bile ducts. Furthermore, the damage to larger ducts seen in the allografts with severe COA probably accounts for the biliary strictures detected by cholangiography in some patients with chronic rejection (10). Severe COA and small arterial loss also help explain the appearance of hepatic arterial angiograms done in patients experiencing chronic liver rejection (18), with markedly narrowed main vessels, branch vessel occlusions and no peripheral filling.
The arterial damage seen in COA may also be directly or indirectly lymphocyte mediated. Alternatively, small portal arteries may be subject to ischemic necrosis because of more proximal vessel occlusion. Moreover, these small arteries may be damaged by cytokines (e.g. TNF, IL-1) released in the portal tract during recurrent acute cellular rejection episodes. Regardless of the mechanism, the result is the same: diminished blood flow to bile ducts.
One could argue that arterial and bile duct damage and loss occur in parallel, but not dependently. Although this line of reasoning is possible, we feel it is unlikely to be correct. First, as our evidence shows, severe duct loss was seen only in association with severe COA. In fact, even when it appeared that large vessels were relatively unaffected by COA, small peripheral artery loss was seen. Secondly, it is known that arterial ischemia in nonallograft livers may also produce bile duct loss (19). Thirdly, hilar bile duct strictures may occur in liver allografts with severe COA, presumably due to an ischemic insult. Resolution may require the development of an animal model. Care must be taken, however, to ensure that the hemodynamics of such a model are analogous to the human liver allograft.
It is noteworthy that the patients with most severe COA and bile duct loss had the shorter graft survival (Table 1, Fig. 6). This suggests that immunologic factors probably playa major role in the development of COA.
The results of this study also offer some insight into the puzzling question of whether primary biliary cirrhosis (PBC) recurs in liver allografts (20, 21). Nakanuma and Ohta (11) discovered that there was a decreased rate of BD-A parallelism for arteries 35 to 54, 55 to 74 and 75 to 94 μm in PBC livers, compared to controls. In contrast, we found that the loss of bile ducts in the liver allografts was more restricted. Only those ducts with corresponding arteries less than 75 μm in diameter were missing. A decreased rate of BD-A parallelism for larger arteries was seen only when there was coexistent severe COA. However, all of the patients transplanted for PBC in this study fell into the group with mild COA. Therefore, none of the patients with PBC prior to transplantation who were included in this study demonstrated loss of larger ducts as shown by Nakanuma and Ohta (11). Furthermore, large excretory bile duct damage, similar to that seen in sclerosing cholangitis, is observed in liver allografts with COA but not in PBC (21). Admittedly, we did not subdivide arteries >75 μm as Nakanuma and Ohta have done. The reason for the different methodology is outlined in the “Materials and Methods.”
Finally, the pathogenesis of graft COA, or as some have termed it, “graft atherosclerosis,” remains obscure. It is probable that many factors are involved, but we feel that immunologic injury should be strongly considered, as originally suggested by Porter et a1. (22). Since COA is the major obstacle to long-term survival of heart (23), kidney (22) and liver (24) allografts, the attention of more transplant biologists should be focused on elucidating the pathogenesis of this lesion (25, 26).
Acknowledgments
We would like to thank Dr. Ron Jaffe for his cooperation.
References
- 1.Porter K. Pathology of the orthotopic homograft and heterograft. In: Stanl TE, editor. Experience in hepatic transplantation. Philadelphia: Saunders; 1969. pp. 422–468. [Google Scholar]
- 2.Fennel RH. Ductular damage in liver transplant rejection: its similarity to that of primary biliary cirrhosis and graft-versus-host disease. Pathol Annu. 1981;16:289–294. [PubMed] [Google Scholar]
- 3.Vierling JM, Fennel RH. Histopathology of early and late human hepatic rejection: evidence of progressive destruction of interlobular bile ducts. Hepatology. 1985;5:1076–1082. doi: 10.1002/hep.1840050603. [DOI] [PubMed] [Google Scholar]
- 4.Demetris AJ, Lasky S, Van Thiel DH, et al. Pathology of hepatic transplantation: a review of 62 adult allograft recipients immunosuppressed with a cyclosporine/steroid regimen. Am J Pathol. 1985;118:151–161. [PMC free article] [PubMed] [Google Scholar]
- 5.Wight DGD, Portmann B. Pathology of liver transplantation. 2. Orlando, Florida: Grune & Stratton; 1987. pp. 385–435. [Google Scholar]
- 6.Hubscher SG, Clements D, Elias E, et al. Biopsy findings in cases of rejection of liver allografts. J Clin Pathol. 1985;38:1366–1373. doi: 10.1136/jcp.38.12.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snover DC, Sibley RK, Freese DK, et al. Orthotopic liver transplantation: a pathological study of 63 serial liver biopsies from 17 patients with special reference to the diagnostic features and natural history of rejection. Hepatology. 1984;4:1212–1222. doi: 10.1002/hep.1840040620. [DOI] [PubMed] [Google Scholar]
- 8.Male AJ. The minute structure of the liver: a review. Glasgow Med J. 1951;32:283–301. [PMC free article] [PubMed] [Google Scholar]
- 9.Mitra SK. The terminal distribution of the hepatic artery with special reference to arterio-portal anastomosis. J Anat. 1966;100:651–663. [PMC free article] [PubMed] [Google Scholar]
- 10.Demetris AJ, Jaffe R, Starzl TE. A review of adult and pediatric post-transplant liver pathology. Pathol Annu (Part II) 1987:347–386. [PubMed] [Google Scholar]
- 11.Nakanuma Y, Ohta G. Histometric and serial section observations of the intrahepatic bile ducts in primary biliary cirrhosis. Gastroenterology. 1979;76:1326–1332. [PubMed] [Google Scholar]
- 12.Cox DR. The analysis of binary data. London: Methuen; 1970. [Google Scholar]
- 13.Cochran WG. Some methods for strengthening the common χ2 tests. Biometrics. 1954;10:417–441. [Google Scholar]
- 14.Fennel RH, Vierling JM. Electron microscopy of rejected human liver allografts. Hepatology. 1985;5:1083–1087. doi: 10.1002/hep.1840050604. [DOI] [PubMed] [Google Scholar]
- 15.Fung JJ, Zeevi A, Stanl TE, et al. Functional characterization of inflitrating T lymphocytes in human hepatic allografts. Hum Immunol. 1986;16:182–199. doi: 10.1016/0198-8859(86)90047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daar AS, Fuggle SV, Fabre JW, et al. The detailed distribution of MHC class II antigens in normal human organs. Transplantation. 1984;38:293–298. doi: 10.1097/00007890-198409000-00019. [DOI] [PubMed] [Google Scholar]
- 17.Demetris AJ, Lasky S, Van Thiel DH, et al. Induction of DR/Ia antigens in human liver allografts. Transplantation. 1985;40:504–509. doi: 10.1097/00007890-198511000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White RM, Zajko AB, Demetris AJ, et al. Liver transplant rejection: angiographic findings in 35 patients. Am J Roentgenol. 1987;148:1095–1098. doi: 10.2214/ajr.148.6.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherlock S. The syndrome of disappearing intra-hepatic bile ducts. Lancet. 1987;2:493–496. doi: 10.1016/s0140-6736(87)91802-2. [DOI] [PubMed] [Google Scholar]
- 20.Neuberger J, Portmann B, MacDougall BRD, et al. Recurrence of primary biliary cirrhosis after liver transplantation. N Engl J Med. 1982;306:1–4. doi: 10.1056/NEJM198201073060101. [DOI] [PubMed] [Google Scholar]
- 21.Demetris AJ, Markus BH, Esquivel C, et al. Pathologic analysis of liver transplantation for primary biliary cirrhosis. Hepatology. 1988;8:939–947. doi: 10.1002/hep.1840080439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porter KA, Thomson WB, Owen K, et al. Obliterative vascular changes in four human kidney homotransplants. Br J Med. 1963;2:639–645. doi: 10.1136/bmj.2.5358.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao SZ, Schroeder JS, Alderman EL, et al. Clinical and laboratory correlates of accelerated coronary artery disease in the cardiac transplant patient. Circulation. 1987;76(Suppl V):V–56. [PubMed] [Google Scholar]
- 24.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Evolution of liver transplantation. Hepatology. 1982;2:614–636. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Billingham ME. Cardiac transplant atherosclerosis. Transpl Proc. 1987;19:19–25. [PubMed] [Google Scholar]
- 26.Oguma S, Okazaki H, Jimbo M, et al. Vascular rejection and arteriosclerosis. Transpl Proc. 1987;19:63–70. [PubMed] [Google Scholar]


