Abstract
Connexin43 (Cx43), the predominant ventricular gap junction protein, is critical for maintaining normal cardiac electrical conduction, and its absence in the mouse heart results in sudden arrhythmic death. The mechanisms linking reduced Cx43 abundance in the heart and inducibility of malignant ventricular arrhythmias have yet to be established. In this report, we investigate arrhythmic susceptibility in a murine model genetically engineered to express progressively decreasing levels of Cx43. Progressively older cardiac-restricted Cx43 conditional knockout (CKO) mice were selectively bred to produce a heart-specific Cx43-deficient subline (“O-CKO” mice) in which the loss of Cx43 in the heart occurs more gradually. O-CKO mice lived significantly longer than the initial series of CKO mice but still died suddenly and prematurely. At 25 days of age, cardiac Cx43 protein levels decreased to 59% of control values (P<0.01), but conduction velocity was not significantly decreased and no O-CKO mice were inducible into sustained ventricular tachyarrhythmias. By 45 days of age, cardiac Cx43 abundance had decreased in a heterogeneous fashion to 18% of control levels, conduction velocity had slowed to half of that observed in control hearts, and 80% of O-CKO mice were inducible into lethal tachyarrhythmias. Enhanced susceptibility to induced arrhythmias was not associated with altered invasive hemodynamic measurements or changes in ventricular effective refractory period. Thus, moderately severe reductions in Cx43 abundance are associated with slowing of impulse propagation and a dramatic increase in the susceptibility to inducible ventricular arrhythmias.
Keywords: connexin43, arrhythmia, electrophysiology, heart, mice
Ventricular tachyarrhythmias are a frequent cause of sudden cardiac death in ischemic and nonischemic heart disease. Despite intense investigation, molecular mechanisms underlying the propensity of diseased myocardium to initiate and propagate lethal arrhythmias are incompletely understood. In recent years, a number of genetically engineered murine models have been developed to explore the pathophysiology of arrhythmogenesis. Although many such mice display increases in the frequency of spontaneous or inducible ventricular ectopy, in almost all cases this activity is self-limited and has not been shown to be the proximate cause of death.1–4 Several other mutant mouse models are able to support sustained ventricular arrhythmias, but these require provocative stimuli, such as anesthesia or exercise with administration of adrenergic agents.5–8 To date, only the heart-specific connexin43 (Cx43) conditional knockout (CKO) mouse has been shown to die prematurely from spontaneous sustained ventricular tachyarrhythmias.9 Because of the arrhythmic propensity of the Cx43 CKO mouse and the ease of inducible sustained arrhythmias,10 it has served as an ideal model for the study of basic mechanisms of arrhythmia.
In myopathic hearts, abnormal expression of Cx43 (gap junction remodeling) may contribute directly to the arrhythmic substrate.11–17 Perhaps the most striking example of this relationship is in the infarct border zone, where gap junction remodeling is associated with slowed conduction and formation of a substrate for reentrant arrhythmias.18–21 However, the severity of gap junction remodeling, in terms of the extent of connexin mislocalization away from the intercalated disc as well as the overall diminution of expression, can be highly variable. Thus, it remains uncertain how Cx43 expression in the heart relates to the likelihood of arrhythmogenesis. Accordingly, to explore this question, we studied impulse propagation and arrhythmia inducibility in a genetically engineered line of mice (“O-CKO” mice) in which cardiac Cx43 expression decreases progressively throughout the ventricular myocardium during postnatal stages.
Materials and Methods
Generation of the O-CKO Line
Progressively older Cx43 CKO mice were intercrossed for ten generations, resulting in a subline of cardiac-specific Cx43 CKO mice with reproducibly prolonged survival. This resulting substrain (“O-CKO” mice) was then used for all experiments reported in this study. All experiments were performed in accordance with the regulations of the Institutional Animal Care and Use Committees of the New York University School of Medicine and the Veterans Administration New York Harbor Healthcare Medical Center (New York, NY). A detailed description of the experimental methods is available in the online data supplement at http://circres.ahajournals.org.
Results
Delayed Sudden Death in Cx43 O-CKO Mice
To determine whether ventricular expression of Cx43 was related to inducibility of ventricular tachyarrhythmias in the Cx43 CKO mice, animals with cardiac restricted inactivation of the Cx43 gene were selectively bred to generate a line of CKO mice with a longer lifespan (O-CKO mice) than the previously described F1 generation of Cx43 CKO mice.9 We predicted that by selectively breeding progressively older CKO mice for several generations, we could produce a line with a more gradual loss of Cx43 in the heart that would allow us to more quantitatively study the relationship between loss of Cx43 and inducibility into ventricular arrhythmias.
After ten generations of selective inbreeding, we generated the O-CKO line, which survived for nearly twice as long as our initial series of CKO mice. Although mice in the initial F1 CKO series had an abrupt drop-off in survival with a mean lifespan of 43.9±2.4 days (n=15), the mean lifespan of O-CKO mice was increased to 81.4±3.3 days (n=190, P<0.0001; Figure 1A). Although longevity was prolonged in the O-CKO mice compared with the original F1 generation of Cx43 CKO mice, they nonetheless died prematurely in comparison to control (Cre−:Cx43flox/flox) littermates (P<0.0001; Figure 1B). In contrast to the Cx43-deficient mice, unexplained death was rare in the control littermates. Of 326 control mice monitored during the course of the F1 CKO and O-CKO studies, as well as intervening generations, only 14 (4.3%) died unexpectedly over the first year of life.
Figure 1.

Kaplan–Meier survival analysis comparing F1 CKO, O-CKO, and control mice. A, Median survival of O-CKO mice (solid line) was significantly longer than in the F1 CKO mice (dashed line; P<0.0001). B, Despite delayed sudden death in the O-CKO mice (solid line), their lifespan was significantly shorter than that of control littermates (dashed line; P<0.0001).
Despite their uniform incidence of sudden death, the O-CKO mice were indistinguishable from littermate controls. There were no signs of distress or obvious behavioral difference in the O-CKO mice compared with the controls. No significant differences in body weight, heart weight, or heart weight/body weight ratio were detected in O-CKO mice at 25, 35, or 45 days when compared with age-matched controls.
Loss of Cx43 Is Delayed in the O-CKO Mice
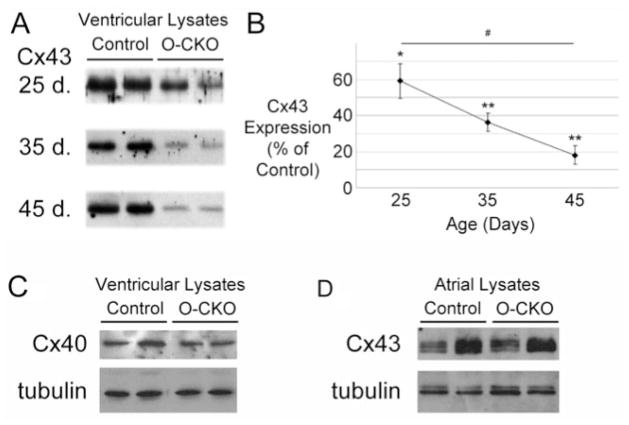
In the O-CKO mice, we surmised that Cre-dependent inactivation of the Cx43 gene was delayed compared with the initial series of CKO mice.9 To investigate this possibility, we collected total ventricular lysates from O-CKO and control mice at 25, 35, and 45 days after birth for immunoblotting. Western blot analysis of O-CKO samples from each time point showed a progressive decline in Cx43 expression relative to controls (59.1±9.6%, 36.2±5.1%, and 18.1±5.2%, at 25, 35, and 45 days, respectively; P<0.01 at 25 days and P<0.0001 at 35 days and 45 days compared with matched controls; Figure 2A and 2B). From 25 to 45 days of age, O-CKO Cx43 abundance was significantly reduced (P<0.01). In contrast to the O-CKO line, Cx43 protein in the F1 CKO mice was reduced to a much lower level at an earlier time point. Cx43 was reduced to 5% of control values by 28 days of age in the F1 generation of Cx43 CKO mice.9 These data suggest that the conditional knockout of Cx43 in the O-CKO mouse heart is temporally delayed compared with the F1 CKO mice. In keeping with previous findings from the F1 generation of Cx43 CKO mice, Cx40 expression was unchanged in the O-CKO ventricular lysates at 45 days of age compared with controls (Figure 2C). Cx45 protein was not detected by immunoblotting in either the control or the O-CKO ventricular lysates (data not shown). Atrial expression of Cx43 in the O-CKO hearts was reduced to 68.8±21.4% of control values by immunoblotting at 45 days, although this change did not reach statistical significance (n=4 per group; Figure 2D). Immunofluorescent staining indicated only limited areas of loss of Cx43 at day 45 and abundant staining for Cx40 in O-CKO atria (Figure S1 in the online data supplement).
Figure 2.
Connexin expression in O-CKO ventricular lysates. A, Representative lanes comparing Cx43 expression in O-CKO and control ventricular lysates at 25, 35, and 45 days of age demonstrate a progressive decline in Cx43 abundance in the O-CKO hearts. B, Expressed as a percentage of matched controls, Cx43 concentration is significantly lower at all time points in the O-CKO ventricles and decreases with time. *P<0.01 compared with matched controls; **P<0.001 compared with matched controls; #P<0.01. C, Representative immunoblots indicating no significant change in Cx40 expression in O-CKO ventricular lysates compared with controls at 45 days. D, Representative immunoblots of atrial lysates demonstrating no significant difference in Cx43 abundance between control and O-CKO samples at 45 days. Blotting for tubulin was performed to indicate relative loading.
To visualize the extent of Cx43 knockout in the O-CKO ventricles, we immunostained mid-left ventricular sections from the O-CKO mice at 25, 35, and 45 days of age. Similar to our immunoblotting experiments with ventricular lysates, a progressive decline in Cx43 expression was observed with immunofluorescence labeling (Figure 3). Net mean fluorescence levels (total fluorescence minus background) of Cx43 signal in O-CKO hearts progressively declined from 25 to 35 and 45 days of age, with Cx43 levels relative to controls very similar to those obtained by immunoblotting. In 25-day-old O-CKO ventricles, Cx43 fluorescence signal was reduced compared with age-matched control littermates but did not reach statistical significance (64.1±7.9% of control fluorescence; P=0.08; n=3 O-CKO and 4 control hearts). By 35 and 45 days of age, Cx43 fluorescence signal is progressively and significantly reduced in the O-CKO ventricles compared with littermate controls (34.1±11.3%, P<0.01, and 22.4±4.8%, P<0.001, of control fluorescence levels, respectively; n=4 O-CKO and controls hearts at each time point). From 25 to 45 days of age, O-CKO Cx43 fluorescence was significantly reduced (P<0.05). There was no significant difference in net mean fluorescence levels in O-CKO left ventricular sections compared with those from the right ventricle at 45 days of age (net mean fluorescence differed by <6% between left ventricle and right ventricle of 4 O-CKO hearts, P=0.8).
Figure 3.

Immunofluorescent staining for connexin43 in control and O-CKO mid-ventricular sections. Abundant Cx43 staining (bright green signal) is seen in sections from 25-, 35-, and 45-day-old control ventricles (A to C). In contrast, gradual loss of Cx43 expression is evident in O-CKO ventricular sections from the same time points (D to F). Variable expression of Cx43 is observed in the ventricular myocardium of a 45-day-old O-CKO heart (G to I). Scale bar=200 μm in panels A to F, 100 μm in panels G to I.
Both the number of immunofluorescent clusters and the percent area they occupy declined with time in the control mice, likely reflecting consolidation of Cx43 expression at the intercalated discs over the time points assessed.22,23 In the O-CKO hearts, Cx43 immunofluorescence-positive clusters (Figure 4A) and their percent area (Figure 4C) were both significantly decreased compared with controls at all time points. Moreover, these parameters declined at a significantly more accelerated rate in the O-CKO hearts when compared with controls. From 25 to 45 days, immunofluorescent clusters in the O-CKO hearts decreased from 67% of control to 34% (Figure 4B) and percent area from 59% to 30% (Figure 4D; P<0.01 for the change in both parameters). There was no significant change in the average area or mean fluorescence of the immunofluorescent clusters of O-CKO hearts compared with controls. These data indicate that the size and Cx43 content of individual gap junction plaques in the O-CKO hearts were no different than in controls—rather, there simply were progressively fewer plaques as the mice aged.
Figure 4.
Quantification of connexin43 immunofluorescent signal in O-CKO and control heart sections. A, Graph of immunofluorescent clusters (IFC) per 20× field in control (solid bars) and O-CKO hearts (shaded bars) shows a significant decline in both groups with time, whereas O-CKO values are significantly less than those of controls at each time point. B, IFC per 20× field in O-CKO hearts as a percentage of age-matched controls decreases significantly over time. C, Percent area occupied by Cx43 immunofluorescent signal in control (solid bars) and O-CKO hearts (shaded bars) shows a similar relationship to that seen for IFC in (A). D, Percent area of Cx43 signal expressed relative to age-matched controls decreases significantly over time. *P<0.05; **P<0.01.
For the assessment of heterogeneity of Cx43 expression, coefficients of variance of Cx43-positive percent area were calculated from 700-μm×500-μm images taken from O-CKO and control hearts at each time point. There was no significant difference in mean coefficient of variance between the two groups at 25 days of age (26.2±5.7% for O-CKOs versus 12.6±2.7% for controls, P=0.11). However, by 35 days of age (43.3±5.2% versus 13.0±2.8%, P<0.01) and at 45 days (55.8±12.7% versus 12.2±2.1%, P<0.05), expression of Cx43 was significantly more heterogeneous in the O-CKO hearts than in controls. This phenomenon is evident in higher power views of 45-day-old O-CKO hearts, where regions with only rare gap junction plaques, as well as those with clusters of positively staining cells, are visible within the same heart (Figure 3G, 3H, and 3I).
Decreased Cx43 Content Is Associated With Increased Inducibility Into Sustained Arrhythmias
Baseline electrocardiographic parameters were obtained and programmed electrical stimulation (PES) was performed to determine the effects of progressive loss of Cx43 expression on in vivo electrophysiology and arrhythmic susceptibility. Baseline heart rates of anesthetized O-CKO and control animals were no different at any of the time points tested. There also were no significant differences in the P wave amplitude, P wave duration, or PR interval between O-CKO and matched control mice at any of the time points (Table). A modest increase in QRS duration was detected only at 25 days in the O-CKO mice (P=0.03). At 35 and 45 days of age, however, there was no significant difference in the QRS duration between the O-CKO and littermate controls. Interestingly, the QRS amplitude showed an age-dependent decrease in the O-CKO mice, which closely paralleled the loss of Cx43 expression (Figure 5). This finding was not associated with pericardial effusion, anasarca, or gross ventricular dilatation.
Table.
Comparison of Electrocardiographic Data in Control and O-CKO Mice
| 25 Days Old |
35 Days Old |
45 Days Old |
||||
|---|---|---|---|---|---|---|
| Control | O-CKO | Control | O-CKO | Control | O-CKO | |
| P wave amplitude | 14.9±1.3 | 12.8±1.0 | 12.6±1.7 | 12.0±1.5 | 11.8±0.8 | 13.7±1.3 |
| P wave duration | 17.1±0.7 | 18.2±0.8 | 16.8±0.4 | 17.3±0.7 | 18.0±0.9 | 18.8±0.4 |
| PR interval | 37.5±1.2 | 39.4±1.1 | 37.2±1.2 | 36.5±0.9 | 36.7±1.4 | 38.6±1.1 |
| QRS duration | 7.4±0.3 | 8.8±0.5* | 7.3±0.4 | 7.9±0.3 | 9.4±0.3 | 10.4±0.5 |
| RR interval | 156±9 | 162±8 | 158±7 | 140±4 | 145±6 | 140±5 |
| QRS amplitude | 148±10 | 61±12† | 146±10 | 47±10† | 122±16 | 23±4† |
Amplitudes are presented in μV; durations and intervals are presented in milliseconds as group means±SEM; n=10 for all groups.
P<0.05;
P<0.0001.
Figure 5.
QRS amplitude progressively decreases in O-CKO mice. Representative electrocardiograms from control and 25-, 35-, and 45-day-old O-CKO mice demonstrate a gradual loss of QRS amplitude in the O-CKO mice. Note that P wave amplitude remains unchanged.
For PES studies, hearts were paced in situ using a novel subdiaphragmatic approach that we have recently developed.10 Unlike other experimental approaches to invasive electrophysiological studies, this procedure does not result in high mortality. In this study, aside from those mice that were induced into lethal arrhythmias, none of the animals died during the procedure.
After a peritoneal incision was made, the stimulating electrode was inserted until contact was made with the epicardial surface of the apex of the heart. A train of 8 stimuli was delivered at pacing cycle lengths of 120, 100, and 80 ms with single extrastimuli added at progressively shorter intervals to determine the ventricular effective refractory period (VERP). There were no significant differences in VERP between O-CKO and control mice at 25, 35, or 45 days of age (Table S1 in the online data supplement).
After the VERP was determined in each mouse, we delivered double extrastimuli at each cycle length to assess the inducibility of ventricular tachyarrhythmias. Mice were categorized as having no ventricular tachycardia (VT), nonsustained VT (NSVT), or sustained VT (Figure 6). Overall, in agreement with results of in vivo PES from other investigators,24 NSVT was induced in one-third of the control mice (10 of 30). Sustained VT could not be induced in any of the controls (Table S2 in the online data supplement). In O-CKO mice at 25 days of age, NSVT was induced in 7 of 10 animals, although there were no episodes of sustained VT. At 35 days of age, 6 of 10 O-CKO mice developed NSVT and one mouse was induced into sustained VT, which degenerated into polymorphic VT and then ventricular fibrillation (VF). By 45 days of age, however, 8 of 10 O-CKO mice developed intractable polymorphic VT/ventricular fibrillation during PES (P<0.001 for 45-day-old O-CKO mice versus age-matched controls and O-CKO mice at 25 or 35 days of age for sustained VT). All mice that developed sustained VT ultimately degenerated into lethal polymorphic VT/ventricular fibrillation and none could be successfully terminated by overdrive pacing.
Figure 6.

Programmed electrical stimulation in an O-CKO mouse. A, Several beats of nonsustained ventricular tachycardia are induced by a single extrastimulus. S1-S1, 120 ms; S1–S2, 35 ms. B, Sustained ventricular tachycardia with a cycle length of ≈40 ms is induced by double extrastimuli. S1-S1, 120 ms; S1–S2, 35 ms; S2–S3, 35 ms. C, Rapid incessant ventricular tachycardia persists despite attempts at overdrive pacing. D, Within 2 minutes of induction of ventricular tachycardia, the arrhythmia degenerates into coarse ventricular fibrillation.
Further analysis revealed that no O-CKO mice with Cx43 abundance in the heart ≥40% of matched control values were inducible into sustained lethal ventricular arrhythmias. Those O-CKO mice with Cx43 levels of <40% of controls were significantly more likely to be induced into sustained arrhythmias than O-CKO mice with levels ≥40% of control (P=0.002). Not surprisingly, Cx43 levels in all O-CKO mice that were inducible into sustained arrhythmias (14.2±4.3% of matched control values) were significantly lower than that of O-CKO mice that were not induced into sustained VT (47.2±5.4%; P=0.001; Figure 7). None of the control mice had Cx43 levels of ≤50% of the mean control values at any age.
Figure 7.

Connexin43 abundance in inducible and noninducible O-CKO mice. Cx43 abundance as a percentage of control levels in individual O-CKO mice with and without inducible sustained VT is shown. Mean±SEM for each population is indicated. *P=0.001.
Loss of Cx43 Is Associated With Conduction Velocity Slowing
To assess the effect of loss of Cx43 expression on ventricular impulse propagation, we optically mapped cardiac activation patterns and calculated conduction velocity in isolated perfused O-CKO and control hearts at 25 and 45 days of age. As connexin levels decreased in the O-CKO population from 25 to 45 days, conduction velocity slowed significantly. Minimum conduction velocity (CVmin) at 25 days was 42.4±2.8 cm/s in controls and 34.0±3.7 cm/s in O-CKO hearts (P=0.11; n=5 in each group). By 45 days, CVmin in the O-CKO hearts had slowed significantly to 21.8±2.9 cm/s compared with 43.2±2.1 cm/s in controls (P<0.001; n=5 in each group). Conduction velocity at 45 days in the O-CKO hearts was significantly slowed not only in comparison to age-matched controls but also when compared with 25 day old O-CKO hearts (P<0.01). The patterns of excitation in the O-CKO hearts showed smooth anisotropic activation and were similar to those previously published for the F1 CKO mice.9
Ventricular Contractility Is Preserved Despite Loss of Cardiac Cx43 Expression
To determine whether the progressive loss of Cx43 expression in the O-CKO hearts affected the contractile properties of the ventricle, invasive hemodynamic monitoring was performed at 30 and 45 days of age. There were no differences in overall contractility as measured by dP/dTmax (Table S3 in the online data supplement) at either time point in O-CKO mice compared with age-matched controls. Other invasive hemodynamic parameters were unchanged in the O-CKO mice in comparison to controls, as well. These data are in agreement with the finding of normal echocardiographic parameters in the F1 generation of Cx43 CKO mice.9 Thus, despite the loss of Cx43 expression in the O-CKO ventricle and increasing inducibility into lethal arrhythmias, ventricular contractility in the O-CKO mice remained no different than in controls.
Discussion
In this study, we explored the relationship between cardiac Cx43 protein expression and arrhythmic susceptibility. Previous studies from our laboratory and from others have shown that widespread loss of Cx43 in the heart produces a highly arrhythmogenic substrate, with spontaneous sudden cardiac death.9,10,25 Here, we used a novel model, the O-CKO mouse, in which the extent of the knockout progresses over time. By 45 days of age, Cx43 protein content in O-CKO hearts was decreased to 18% of control levels, ventricular impulse propagation was slowed to half of that observed in control hearts, and 80% of O-CKO mice were inducible into lethal tachyarrhythmias. No O-CKO mice with Cx43 levels ≥40% of matched controls were inducible into sustained arrhythmias.
What accounts for the increasing susceptibility of the O-CKO mice to the induction of sustained arrhythmias as a function of time? Reentry requires the formation of wave breaks in a propagating wave front and sufficient room for the free end (wave tip) to curl and form a rotor.26 The progressive and heterogeneous loss of Cx43 in the heart of O-CKO mice may influence both of these factors.
Previous studies have demonstrated that pharmacological uncoupling may enhance intrinsic nonuniformities in conduction. In response to external (eg, S2) or intrinsic (premature beats) stimuli, these nonuniformities may progress to the development of zones of functional block and wave breaks.27 Moreover, the heterogeneous nature of loss of Cx43 in the O-CKO heart may exacerbate these nonuniformities. Loss of Cx43 in our conditional knockout model occurs in a binary fashion in individual cells.28 As a result, randomly interspersed among Cx43-deficient myocytes are single cells and more complex higher-order clusters of cells that express normal levels of Cx43. Interestingly, unlike the Cx43-null chimeric mice, in which isolated macroscopic Cx43-deficient foci in an otherwise well-coupled myocardium are associated with discrete areas of conduction delay,29 the activation wave front is not deformed in the O-CKO hearts. Nonetheless, the microscopic cellular heterogeneity in the O-CKO hearts may result in spatial dispersion of action potential duration and conduction velocity restitution properties, potentially contributing to the arrhythmic phenotype.30 Local heterogeneities of cellular coupling may contribute to the formation of unidirectional conduction block,31 as Rohr et al have elegantly demonstrated in cultured partially uncoupled myocytes.32
Inasmuch as VERP values were not significantly different in control and O-CKO mice, our data imply a progressive reduction in calculated wavelength (CV × VERP) in O-CKO hearts compared with controls (6.6 mm versus 13.2 mm, respectively, at 45 days of age). Although CV values are likely to differ during ventricular tachycardia compared with ventricular pacing, the slower impulse propagation in the O-CKO hearts should allow more “room” for rotor formation, presumably contributing to their increased susceptibility to inducible ventricular tachycardia.33 Rotor formation may be favored in heterogeneously uncoupled cardiac tissue in particular. Inexcitable obstacles with sharp edges, such as small groups of Cx43-deficient cells, may destabilize the propagation of the activation wave front, causing the formation of self-sustained vortices and turbulent cardiac electrical activity, a phenomenon known as “vortex shedding.”34
It is conceivable that compensatory changes in other connexins or secondary electrical remodeling may also play a role in the arrhythmic phenotype.35 We found no alterations in the expression of Cx40 or Cx45 in the heart and no statistically significant changes in VERP. Additional studies of cellular electrophysiology will be necessary to address the possibility of remodeling of other sarcolemmal ion channels.
The relationship between loss of Cx43 expression in the heart and the resulting slowing of impulse propagation and inducibility is intriguing in view of results obtained in studies of Cx43 heterozygous-null mice, which express ≈50% of wild-type levels.14 In the absence of secondary stimuli, Cx43+/− mice do not develop spontaneous ventricular arrhythmias, consistent with our findings here. However, in the presence of ischemia, Cx43+/− mice have an increased incidence and duration of ventricular tachyarrhythmias compared with controls. Together, these data suggest that whereas profound reductions in Cx43 expression may be sufficient to create a highly arrhythmogenic substrate, more moderate diminutions of Cx43 expression, as might be seen clinically with gap junction remodeling, may require “second hits” for arrhythmogenesis, such as ischemia, structural remodeling, changes in autonomic tone, or electrical remodeling of active membrane currents.
Conduction slowing in hearts lacking Cx43 is not surprising given the profound decreases consistently reported in gap junctional conductance between Cx43-deficient myocytes.28,36,37 We previously found that junctional coupling between end-to-end coupled cell pairs from adult Cx43 CKO mice was reduced to 11 nS or 2% of control values.28 What is unexpected, however, is the relative preservation of propagation velocity in O-CKO hearts, which is slowed by only ≈50%. Interestingly, Kleber et al recently reported that propagation velocity in neonatal myocyte strands from germline Cx43-null neonatal myocytes was much more severely affected, ≈2 cm/s, leading them to propose that the presence of a residual population of Cx43-positive cells distributed throughout an otherwise weakly coupled syncytium might account for the relative preservation of conduction velocity in the CKO heart.37 Clearly, it will be of interest to examine propagation characteristics in mixed cultures of Cx43-expressing and Cx43-deficient neonatal myocytes.
The pattern of loss of Cx43 may play an important role in the resulting effect on contractility by influencing the spatial profile of ventricular excitation and contraction. In O-CKO mice, as in other models in which reduction of Cx43 expression is widespread and diffuse, systolic function appears to be preserved. For example, transgenic mice expressing mitogen-activated protein kinase 7D (MKK-7D), an upstream activator of c-Jun N-terminal kinase (JNK), have Cx43 levels of only 10% of control littermates38 and significantly slowed propagation velocity.39 Systolic function remains normal in these mice, although diastolic relaxation is impaired, likely the result of increased fibronectin expression in the heart.39 Heterozygous germline deficiency of Cx43 is associated with a decreased fractional shortening of <10%, which was only of marginal statistical significance.40 In our previous F1 series of cardiac-specific Cx43 knockout mice, there was no significant decline in systolic function in the mutant mice at 1 month of age.9
Curiously, the mean QRS duration of 45-day-old O-CKO mice is not widened despite significant CV slowing. QRS widening with uncoupling has been controversial in the literature. Morley et al41 found no change in either CVmin or QRS duration in heterozygous Cx43-null mice, whereas Guerrero et al42 observed changes in both conduction velocity and QRS duration in the Cx43+/− mice. Most recently, however, Eckardt et al43 and van Rijen et al25 presented data on QRS duration in their Cre-ER(T)/fl (tamoxifen-inducible Cx43 knockout) model that are very similar to our O-CKO data. When CVmin is reduced by ≈50% from wild-type levels in either the 45-day-old O-CKO or the Cre-ER(T)/fl mice, there was no significant increase in QRS duration. Only when Cre-mediated loss of Cx43 is induced in the Cx43 Cre-ER(T)/fl mice by administration of 4-hydroxytamoxifen does CV slow even more dramatically, with resulting QRS widening.25,43 Thus, it appears that greater degrees of CV slowing than those observed in the 45-day-old O-CKO hearts are required to result in QRS widening.
In conclusion, our studies strongly support the growing recognition that gap junction remodeling is a major contributor to the arrhythmogenic substrate in the diseased heart and suggest that uncoupling as a result of diminished Cx43 expression plays a mechanistic role in the formation of a highly arrhythmogenic substrate. These data further support exploring gap junction channels as potential anti-arrhythmic therapeutic targets.
Supplementary Material
Acknowledgments
Supported by a Grant-in-Aid from the American Heart Association; HL04222 (D.E.G.), HL30557, and HL64757 from the National Institutes of Health; and a Burroughs-Wellcome Fund Clinical Scientist Award in Translational Research (G.I.F.).
References
- 1.London B, Jeron A, Zhou J, Buckett P, Han X, Mitchell GF, Koren G. Long QT and ventricular arrhythmias in transgenic mice expressing the N terminus and first transmembrane segment of a voltage-gated potassium channel. Proc Natl Acad Sci U S A. 1998;95:2926–2931. doi: 10.1073/pnas.95.6.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeron A, Mitchell GF, Zhou J, Murata M, London B, Buckett P, Wiviott SD, Koren G. Inducible polymorphic ventricular tachyarrhythmias in a transgenic mouse model with a long Q-T phenotype. Am J Physiol Heart Circ Physiol. 2000;278:H1891–H1898. doi: 10.1152/ajpheart.2000.278.6.H1891. [DOI] [PubMed] [Google Scholar]
- 3.Guo W, Li H, London B, Nerbonne JM. Functional consequences of elimination of i(to,f) and i(to,s): early afterdepolarizations, atrioventricular block, and ventricular arrhythmias in mice lacking Kv1.4 and expressing a dominant-negative Kv4 alpha subunit. Circ Res. 2000;87:73–79. doi: 10.1161/01.res.87.1.73. [DOI] [PubMed] [Google Scholar]
- 4.Brunner M, Guo W, Mitchell GF, Buckett PD, Nerbonne JM, Koren G. Characterization of mice with a combined suppression of I(to) and I(K,slow) Am J Physiol Heart Circ Physiol. 2001;281:H1201–H1209. doi: 10.1152/ajpheart.2001.281.3.H1201. [DOI] [PubMed] [Google Scholar]
- 5.Baker LC, London B, Choi BR, Koren G, Salama G. Enhanced dispersion of repolarization and refractoriness in transgenic mouse hearts promotes reentrant ventricular tachycardia. Circ Res. 2000;86:396–407. doi: 10.1161/01.res.86.4.396. [DOI] [PubMed] [Google Scholar]
- 6.Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, Song LS, Haurogne K, Kyndt F, Ali ME, Rogers TB, Lederer WJ, Escande D, Le Marec H, Bennett V. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003;421:634–639. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- 7.Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, Sun J, Guatimosim S, Song LS, Rosemblit N, D’Armiento JM, Napolitano C, Memmi M, Priori SG, Lederer WJ, Marks AR. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 8.Nuyens D, Stengl M, Dugarmaa S, Rossenbacker T, Compernolle V, Rudy Y, Smits JF, Flameng W, Clancy CE, Moons L, Vos MA, Dewerchin M, Benndorf K, Collen D, Carmeliet E, Carmeliet P. Abrupt rate accelerations or premature beats cause life-threatening arrhythmias in mice with long-QT3 syndrome. Nat Med. 2001;7:1021–1027. doi: 10.1038/nm0901-1021. [DOI] [PubMed] [Google Scholar]
- 9.Gutstein DE, Morley GE, Tamaddon H, Vaidya D, Schneider MD, Chen J, Chien KR, Stuhlmann H, Fishman GI. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ Res. 2001;88:333–339. doi: 10.1161/01.res.88.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutstein DE, Danik SB, Sereysky JB, Morley GE, Fishman GI. Subdiaphragmatic murine electrophysiological studies: sequential determination of ventricular refractoriness and arrhythmia induction. Am J Physiol Heart Circ Physiol. 2003;285:H1091–H1096. doi: 10.1152/ajpheart.00100.2003. [DOI] [PubMed] [Google Scholar]
- 11.Peters NS, Green CR, Poole-Wilson PA, Severs NJ. Reduced content of connexin43 gap junctions in ventricular myocardium from hypertrophied and ischemic human hearts. Circulation. 1993;88:864–875. doi: 10.1161/01.cir.88.3.864. [DOI] [PubMed] [Google Scholar]
- 12.Peters NS, Wit AL. Myocardial architecture and ventricular arrhythmogenesis. Circulation. 1998;97:1746–1754. doi: 10.1161/01.cir.97.17.1746. [DOI] [PubMed] [Google Scholar]
- 13.Kaprielian RR, Gunning M, Dupont E, Sheppard MN, Rothery SM, Underwood R, Pennell DJ, Fox K, Pepper J, Poole-Wilson PA, Severs NJ. Downregulation of immunodetectable connexin43 and decreased gap junction size in the pathogenesis of chronic hibernation in the human left ventricle. Circulation. 1998;97:651–660. doi: 10.1161/01.cir.97.7.651. [DOI] [PubMed] [Google Scholar]
- 14.Lerner DL, Yamada KA, Schuessler RB, Saffitz JE. Accelerated onset and increased incidence of ventricular arrhythmias induced by ischemia in Cx43-deficient mice. Circulation. 2000;101:547–552. doi: 10.1161/01.cir.101.5.547. [DOI] [PubMed] [Google Scholar]
- 15.Wit AL. Remodeling of cardiac gap junctions: the relationship to the genesis of ventricular tachycardia. J Electrocardiol. 2001;34 (Suppl):77–83. doi: 10.1054/jelc.2001.28834. [DOI] [PubMed] [Google Scholar]
- 16.Spragg DD, Leclercq C, Loghmani M, Faris OP, Tunin RS, DiSilvestre D, McVeigh ER, Tomaselli GF, Kass DA. Regional alterations in protein expression in the dyssynchronous failing heart. Circulation. 2003;108:929–932. doi: 10.1161/01.CIR.0000088782.99568.CA. [DOI] [PubMed] [Google Scholar]
- 17.Kitamura H, Ohnishi Y, Yoshida A, Okajima K, Azumi H, Ishida A, Galeano EJ, Kubo S, Hayashi Y, Itoh H, Yokoyama M. Heterogeneous loss of connexin43 protein in nonischemic dilated cardiomyopathy with ventricular tachycardia. J Cardiovasc Electrophysiol. 2002;13:865–870. doi: 10.1046/j.1540-8167.2002.00865.x. [DOI] [PubMed] [Google Scholar]
- 18.Yao JA, Hussain W, Patel P, Peters NS, Boyden PA, Wit AL. Remodeling of gap junctional channel function in epicardial border zone of healing canine infarcts. Circ Res. 2003;92:437–443. doi: 10.1161/01.RES.0000059301.81035.06. [DOI] [PubMed] [Google Scholar]
- 19.Peters NS, Coromilas J, Severs NJ, Wit AL. Disturbed connexin43 gap junction distribution correlates with the location of reentrant circuits in the epicardial border zone of healing canine infarcts that cause ventricular tachycardia. Circulation. 1997;95:988–996. doi: 10.1161/01.cir.95.4.988. [DOI] [PubMed] [Google Scholar]
- 20.Matsushita T, Oyamada M, Fujimoto K, Yasuda Y, Masuda S, Wada Y, Oka T, Takamatsu T. Remodeling of cell-cell and cell-extracellular matrix interactions at the border zone of rat myocardial infarcts. Circ Res. 1999;85:1046–1055. doi: 10.1161/01.res.85.11.1046. [DOI] [PubMed] [Google Scholar]
- 21.Ohara T, Ohara K, Cao JM, Lee MH, Fishbein MC, Mandel WJ, Chen PS, Karagueuzian HS. Increased wave break during ventricular fibrillation in the epicardial border zone of hearts with healed myocardial infarction. Circulation. 2001;103:1465–1472. doi: 10.1161/01.cir.103.10.1465. [DOI] [PubMed] [Google Scholar]
- 22.Peters NS, Severs NJ, Rothery SM, Lincoln C, Yacoub MH, Green CR. Spatiotemporal relation between gap junctions and fascia adherens junctions during postnatal development of human ventricular myocardium. Circulation. 1994;90:713–725. doi: 10.1161/01.cir.90.2.713. [DOI] [PubMed] [Google Scholar]
- 23.Litchenberg WH, Norman LW, Holwell AK, Martin KL, Hewett KW, Gourdie RG. The rate and anisotropy of impulse propagation in the postnatal terminal crest are correlated with remodeling of Cx43 gap junction pattern. Cardiovasc Res. 2000;45:379–387. doi: 10.1016/s0008-6363(99)00363-6. [DOI] [PubMed] [Google Scholar]
- 24.Maguire CT, Wakimoto H, Patel VV, Hammer PE, Gauvreau K, Berul CI. Implications of ventricular arrhythmia vulnerability during murine electrophysiology studies. Physiol Genomics. 2003;15:84–91. doi: 10.1152/physiolgenomics.00034.2003. [DOI] [PubMed] [Google Scholar]
- 25.van Rijen HV, Eckardt D, Degen J, Theis M, Ott T, Willecke K, Jongsma HJ, Opthof T, de Bakker JM. Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation. 2004;109:1048–1055. doi: 10.1161/01.CIR.0000117402.70689.75. [DOI] [PubMed] [Google Scholar]
- 26.Tung L, Bursac N, Aguel F. Rotors and spiral waves in two dimensions. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology: From Cell to Bedside. 4. Philadelphia: WB Saunders; 2004. pp. 336–344. [Google Scholar]
- 27.Ohara T, Qu Z, Lee MH, Ohara K, Omichi C, Mandel WJ, Chen PS, Karagueuzian HS. Increased vulnerability to inducible atrial fibrillation caused by partial cellular uncoupling with heptanol. Am J Physiol Heart Circ Physiol. 2002;283:H1116–H1122. doi: 10.1152/ajpheart.00927.2001. [DOI] [PubMed] [Google Scholar]
- 28.Yao JA, Gutstein DE, Liu F, Fishman GI, Wit AL. Cell coupling between ventricular myocyte pairs from connexin43-deficient murine hearts. Circ Res. 2003;93:736–743. doi: 10.1161/01.RES.0000095977.66660.86. [DOI] [PubMed] [Google Scholar]
- 29.Gutstein DE, Morley GE, Vaidya D, Liu F, Chen FL, Stuhlmann H, Fishman GI. Heterogeneous expression of Gap junction channels in the heart leads to conduction defects and ventricular dysfunction. Circulation. 2001;104:1194–1199. doi: 10.1161/hc3601.093990. [DOI] [PubMed] [Google Scholar]
- 30.Weiss JN, Chen PS, Qu Z, Karagueuzian HS, Garfinkel A. Ventricular fibrillation: how do we stop the waves from breaking? Circ Res. 2000;87:1103–1107. doi: 10.1161/01.res.87.12.1103. [DOI] [PubMed] [Google Scholar]
- 31.Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev. 2004;84:431–488. doi: 10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
- 32.Rohr S, Kucera JP, Fast VG, Kleber AG. Paradoxical improvement of impulse conduction in cardiac tissue by partial cellular uncoupling. Science. 1997;275:841–844. doi: 10.1126/science.275.5301.841. [DOI] [PubMed] [Google Scholar]
- 33.Vaidya D, Morley GE, Samie FH, Jalife J. Reentry and fibrillation in the mouse heart. A challenge to the critical mass hypothesis. Circ Res. 1999;85:174–181. doi: 10.1161/01.res.85.2.174. [DOI] [PubMed] [Google Scholar]
- 34.Cabo C, Pertsov AM, Davidenko JM, Baxter WT, Gray RA, Jalife J. Vortex shedding as a precursor of turbulent electrical activity in cardiac muscle. Biophys J. 1996;70:1105–1111. doi: 10.1016/S0006-3495(96)79691-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson CM, Kanter EM, Green KG, Laing JG, Betsuyaku T, Beyer EC, Steinberg TH, Saffitz JE, Yamada KA. Redistribution of connexin45 in gap junctions of connexin43-deficient hearts. Cardiovasc Res. 2002;53:921–935. doi: 10.1016/s0008-6363(01)00522-3. [DOI] [PubMed] [Google Scholar]
- 36.Vink MJ, Suadicani SO, Vieira DM, Urban-Maldonado M, Gao Y, Fishman GI, Spray DC. Alterations of intercellular communication in neonatal cardiac myocytes from connexin43 null mice. Cardiovasc Res. 2004;62:397–406. doi: 10.1016/j.cardiores.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 37.Beauchamp P, Choby C, Desplantez T, de Peyer K, Green K, Yamada KA, Weingart R, Saffitz JE, Kleber AG. Electrical propagation in synthetic ventricular myocyte strands from germline connexin43 knockout mice. Circ Res. 2004;95:170–178. doi: 10.1161/01.RES.0000134923.05174.2f. [DOI] [PubMed] [Google Scholar]
- 38.Petrich BG, Gong X, Lerner DL, Wang X, Brown JH, Saffitz JE, Wang Y. c-Jun N-terminal kinase activation mediates downregulation of connexin43 in cardiomyocytes. Circ Res. 2002;91:640–647. doi: 10.1161/01.res.0000035854.11082.01. [DOI] [PubMed] [Google Scholar]
- 39.Petrich BG, Eloff BC, Lerner DL, Kovacs A, Saffitz JE, Rosenbaum DS, Wang Y. Targeted activation of c-Jun N-terminal kinase in vivo induces restrictive cardiomyopathy and conduction defects. J Biol Chem. 2004;279:15330–15338. doi: 10.1074/jbc.M314142200. [DOI] [PubMed] [Google Scholar]
- 40.Betsuyaku T, Kovacs A, Saffitz JE, Yamada KA. Cardiac structure and function in young and senescent mice heterozygous for a connexin43 null mutation. J Mol Cell Cardiol. 2002;34:175–184. doi: 10.1006/jmcc.2001.1499. [DOI] [PubMed] [Google Scholar]
- 41.Morley GE, Vaidya D, Samie FH, Lo C, Delmar M, Jalife J. Characterization of conduction in the ventricles of normal and heterozygous Cx43 knockout mice using optical mapping. J Cardiovasc Electrophysiol. 1999;10:1361–1375. doi: 10.1111/j.1540-8167.1999.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 42.Guerrero PA, Schuessler RB, Davis LM, Beyer EC, Johnson CM, Yamada KA, Saffitz JE. Slow ventricular conduction in mice heterozygous for a connexin43 null mutation. J Clin Invest. 1997;99:1991–1998. doi: 10.1172/JCI119367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eckardt D, Theis M, Degen J, Ott T, van Rijen HV, Kirchhoff S, Kim JS, de Bakker JM, Willecke K. Functional role of connexin43 gap junction channels in adult mouse heart assessed by inducible gene deletion. J Mol Cell Cardiol. 2004;36:101–110. doi: 10.1016/j.yjmcc.2003.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.