Abstract
Hepatic retransplantation is controversial because the results are inferior to primary transplants and organs are so scarce. To determine the factors that are associated with poor outcome within the first year following retransplantation, we performed a multivariate analysis, using stepwise logistic regression, of 418 hepatic retransplantations performed at a single institution from November 1987 to December 1993. The minimum follow-up was 1 year. Seven variables were found to be independently associated with subsequent graft failure (defined as either patient death or retransplantation): donor age (odds ratio 2.2 for each 10-year increase over age 45, 95% CI 1.3 to 3.7), female donor sex (odds ratio 1.7,95% CI 1.05 to 2.7), recipient age (odds ratio 1.6 for each 10-year increase over age 45, 95% CI 1.2 to 2.3), need for preoperative mechanical ventilation (odds ratio 1.8, 95% CI 1.1 to 2.9), pretransplant serum creatinine (odds ratio 1.24 for each increase of 1 mg/dl, 95% CI 1.1 to 1.4), pretransplant total serum bilirubin (odds ratio 1.4 for each 10-mg/dl increase over 15 mg/dl, 95% CI 1.1 to 1.8), and the primary immunosuppressant, using tacrolimus as the reference category (odds ratio for cyclosporine-based immunosuppression 3.9, 95% CI 2.3 to 6.8). Although not part of the logistic regression model, the timing of retransplantation was also found to be important, with the overall probability of failure increasing from 0.58 on day 0 to a peak of 0.8 on day 38 and decreasing slowly after that. The implications of these results regarding the appropriateness of retransplantation are discussed.
Hepatic retransplantation is a technically demanding procedure, and the overall results are inferior to those of primary grafting (1–9). Although some reports have stressed that outcome has improved in recent years (5, 9), especially when considering so-called “elective” retransplantations (9, 10), others feel that the failure rate is so high that the practice of retransplantation should be curtailed (11).
An outright ban on hepatic retransplantation raises troubling ethical questions, especially for those of us still grappling with the apparent changing role of health professionals: from the patient's advocate to agents of society (12, 13). It has also been pointed out that foreclosing the option of retransplantation would have a chilling effect on donor acceptance, as patients and their surgeons refuse to use anything but the highest quality grafts (1). This could well have the paradoxical effect of further limiting access to primary grafting.
Practical and ethical considerations aside, our severe organ shortage dictates that we apply the lessons learned in the past, so that we can make more efficient use of our scarce resources. To that end, we conducted a retrospective analysis of hepatic retransplantations carried out since the University of Wisconsin (UW)* (14) solution was adopted for organ preservation, in order to identify those risk factors that play a role in outcome.
MATERIALS AND METHODS
Patient population
From November 1, 1987 to December 31, 1993 a total of 2019 adults underwent 2376 liver transplantations at Presbyterian University Hospital and the Veterans Administration Medical Center, Pittsburgh, Pennsylvania. Of these, 418 were retransplantations, and constitute the basis for this report. Cases were excluded if the liver was received as part of a multivisceral transplant that included intestine. Minimum follow-up time was at least one year (censoring date: January 18, 1995). All grafts were flushed with UW solution (14) during cold preservation.
Variables studied and endpoints
Recipient variables were age, sex, primary diagnosis, indication for retransplantation, time to retransplantation, UNOS status (United Network for Organ Sharing classification), need for preoperative mechanical ventilation, primary immunosuppressive agent (tacrolimus, cyclosporine, or tacrolimus rescue), whether the graft was an ABO mismatch, and the following preoperative laboratory measurements: total serum bilirubin, serum creatinine, and prothrombin time (as measured on the day of the retransplant or immediately before).
Donor variables were age, sex, and total ischemia time. The primary endpoint was retransplanted graft failure; the cause of failure was the secondary endpoint.
Definitions
Graft failure: Patient death or retransplantation.
Medical urgency: UNOS 1–stable patient, waiting at home; UNOS 2–waiting at home, but requiring medical support; UNOS 3–unstable, in need of continuous hospitalization; or UNOS 4–requiring life-support systems. We should note that this classification was changed on April 1, 1995, but we use here the classification that was in effect during the study period.
Total ischemia time: time elapsed from aortic cross-clamping in the donor to portal or arterial revascularization, or both simultaneously, in the recipient.
Primary diagnosis: original liver disease (Table 1).
Table 1.
Primary diagnoses
| Category | Diagnoses |
|---|---|
| Autoimmune | Autoimmune hepatitis |
| Cholestatic | PBC,a PSC, cystic fibrosis, secondary biliary cirrhosis, biliary atresia, etc. |
| Alcoholic | Ethanol-induced cirrhosis |
| Hepatitic | Hepatitis B, hepatitis C, etc. |
| Metabolic | a-1-antitrypsin deficiency, Wilson's disease, hemochromatosis, etc. |
| Cryptogenic | All other etiologies of cirrhosis excluded |
| FHF | Fulminant hepatic failure |
| HCC-Cholangio. | Hepatocellular carcinomab and cholangiocarcinoma |
| Other malignancy | Secondary hepatic malignancies |
| Other | Budd-Chiari syndrome, benign tumors, etc. |
PBC=primary biliary cirrhosis; PSC=primary sclerosing cholangitis.
Excluding incidental tumors.
Indications: the cause of graft failure for the preceding liver allograft.
Cause of retransplanted graft failure:
Intraoperative–cardiac arrest of any cause.
Neurological–including, but not limited to, hemorrhagic or ischemic cerebrovascular accident, central pontine myelinolysis, anoxic encephalopathy, and brain herniation.
Cancer, de novo–including lymphoproliferative disease.
Cancer, recurrent–recurrent hepatobiliary or extrahepatic cancer.
Ischemic injury–damage of the allograft, either before revascularization or afterward, that did not have a demonstrable immunologic etiology.
Primary nonfunction (PNF)–a graft with such poor initial function that an additional retransplantation or death occurred within two weeks, without identifiable technical or immunological cause of failure.
Cardiac–including, but not limited to, congestive heart failure, arrhythmias, and acute myocardial infarction.
Multiple organ dysfunction syndrome (MODS) (15)–without concomitant documented sepsis and when the liver dysfunction cannot be attributed to identifiable primary hepatic processes.
Sepsis–MODS from a documented bacterial, viral, or fungal infection (also known as secondary MODS (15)). When a patient died in the early posttransplant period with a documented infection, the assignment to sepsis, PNF (item 6) or ischemic injury (item 5) was made based on whether there was poor function from the beginning. Thus, a death from sepsis in a graft that never functioned was coded as PNF or ischemic injury. On the other hand, in a patient whose transplantation was performed during an unrecognized infection (e.g., positive blood cultures that are not reported until after the surgery), failure was attributed to sepsis without regard for the degree of initial dysfunction.
Hepatitis, de novo–including hepatitis B, hepatitis C, cytomegalovirus, and adenovirus.
Hepatitis, recurrent (self-explanatory)
Technical, HAT–hepatic artery thrombosis and severe hepatic artery stenosis.
Technical, other–including, but not limited to, portal vein thrombosis, caval stenosis, ruptured pseudoaneurysms, hemorrhage after liver biopsy, biliary strictures (whether single or multiple), bile leaks, and bile cast syndrome.
Rejection, acute–including acute cellular and humoral rejections (1).
Rejection, chronic–occlusive arteriopathy or vanishing bile duct syndrome (1).
Unknown–lost to follow-up, unclassifiable.
Other (self-explanatory).
Because multiple processes were operating simultaneously in most cases of failure, the assignment was made to the one considered to be the starting point of the morbidity cascade, or, if this was not identifiable, the most severe of the problems. Thus, when the allograft dysfunction was the primary identifiable process (e.g., chronic rejection), this was the designated cause of failure rather than sepsis or MODS that occurred secondarily. Similarly, when a patient died of sepsis in the face of ongoing acute rejection, the cause of failure was assigned to rejection. The rationale in this circumstance was that the increased immunosuppression required to treat the acute rejection was the primary mortality factor whether or not this resulted in restoration of adequate liver function. Conversely, sepsis was considered the primary event in a patient whose infection led to discontinuance of immunosuppression followed by rejection.
Statistical analysis
Continuous variables are presented as the mean ± standard deviation (SD), and categorical variables as fractions. A two-tailed t test was used to test for differences between means. Pearson's chi-square was used to test for differences between categorical variables. Unadjusted graft failure probabilities, as a function of time to retransplantation, were calculated by determining the graft failure rate for retransplants performed at each time point.
Survival analysis was performed by means of the Kaplan-Meier method (16), with the log-rank test to compare strata. A Bonferroni adjustment was used for multiple comparisons.
To calculate the probability that a graft will fail within one year of retransplantation, a stepwise logistic regression analysis (17) was carried out, starting with variables that achieved a significance level of ≤ 0.3 in the screening univariate analysis. For categorical variables, preliminary univariate logistic regression models were fit to determine what subcategories could be properly grouped together. Models were fit using forward inclusion and backward elimination, with a likelihood ratio test. The presence of an interaction between variables was tested by introducing appropriate multiplicative terms. A significance level of 0.1 was used in the stepwise procedure. After a preliminary model was obtained, the functional relation between the continuous variables and the outcome was explored by fitting generalized additive models (18) in which the continuous variables were introduced as smooth terms, using a local regression procedure (19). A final logistic regression model was then fit, incorporating the appropriate functional form for the continuous variables.
The generalized additive models and local regression procedures were done in S-Plus (StatSci Inc., Seattle WA). All other procedures were performed using SPSS (SPSS Inc., Chicago IL).
RESULTS
There were 2376 transplants performed on 2019 patients; 1958 (82.4%) were primary grafts and 418 (17.6%) were retransplantations. Of the retransplantations, 341 (81.6%) were second grafts, 65 (15.6%) were third grafts, and 12 (2.8%) were fourth grafts or higher. The leading indication for retransplantation was ischemic injury-PNF (40%), followed by technical complications (26.5%, Table 2). There were 6 ABO mismatches, 3 of which failed (P = 0.54).
Table 2.
Indications for retransplantation
| Indication | Counta | Percentage |
|---|---|---|
| Ischemic injury–PNF | 167 | 40.0 |
| Technical, HAT | 75 | 17.9 |
| Technical, other | 36 | 8.6 |
| Rejection, chronic | 69 | 16.5 |
| Rejection, acute | 14 | 3.3 |
| Hepatitis, recurrent | 23 | 5.5 |
| Hepatitis, de novo | 12 | 2.9 |
| Other | 22 | 5.3 |
Total=418.
Graft survival
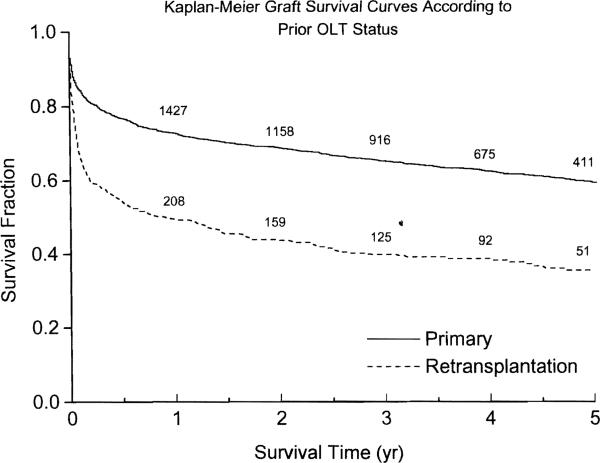
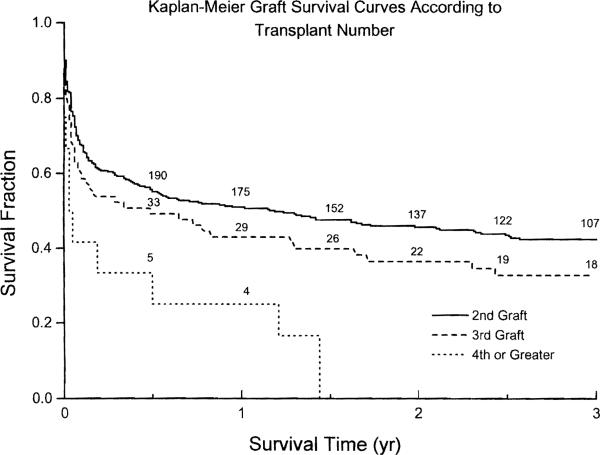
Figure 1 shows the Kaplan-Meier graft survival curves stratified according to their prior transplant (OLT) status. At the end of each of the first 12 months, graft survival after retransplantation was 68–80% that of primary grafting (Table 3). The five-year graft survival was 59.5% (95% CI 57.1 to 61.9) for primary grafts and 35.5% (95% CI 32.9 to 38.1) for retransplantations (P < 0.0005 for the overall survival distributions). Among the failed grafts, 62.4% were due to, or led to, patient death (58% of the primaries and 75% of the retransplantations, P < 0.00005). A total of 74% of the graft failures occurred within the first year (72% of the primaries and 81% of the retransplantations, P = 0.004). Figure 2 shows the Kaplan-Meier graft survival curves for retransplantations, stratified according to the transplant number. The only significant difference was that between second transplants and fourth or higher (P = 0.026).
Figure 1.
Kaplan Meier graft survival curves, stratified according to prior transplant status (whether they were primary grafts or retransplantations). The numbers along the curves denote the grafts at risk.
Table 3.
Monthly graft survival probabilities for first yeara
| Month | Primary OLT (n=1958) | Retransplant (n=418) |
|---|---|---|
| First | 0.852 | 0.684 |
| Second | 0.823 | 0.619 |
| Third | 0.806 | 0.595 |
| Fourth | 0.789 | 0.581 |
| Fifth | 0.777 | 0.562 |
| Sixth | 0.768 | 0.550 |
| Seventh | 0.760 | 0.528 |
| Eighth | 0.749 | 0.519 |
| Ninth | 0.743 | 0.514 |
| Tenth | 0.738 | 0.504 |
| Eleventh | 0.732 | 0.500 |
| Twelfth | 0.728 | 0.497 |
The numbers denote the Kaplan-Meier estimator of the probability of surviving to the end of the month.
Figure 2.
Kaplan-Meier graft survival curves for retransplantations, stratified according to graft number. The numbers along the curves denote the grafts at risk.
Causes of failure and timing of retransplantation
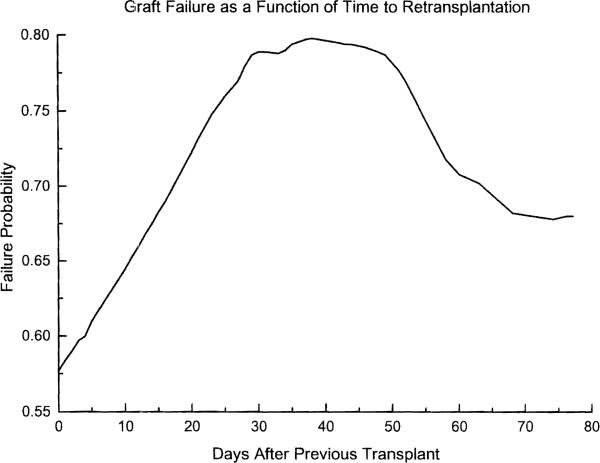
The leading cause of graft failure was sepsis-MODS, fonowed by technical complications, ischemic injury–PNF, and hepatitis (Table 4). Table 5 shows the distribution of the retransplants according to their timing (relative to the preceding transplant). Slightly over half (51.7%) of the retransplants were performed within the first month of the preceding transplant, and almost 70% were carried out within the first six months. Figure 3 shows a plot of the probability of graft failure as a function of time to retransplantation (without adjusting for the effect of covariates). The probability of failure increases from 0.58 at day zero to 0.8 at day 38, decreasing slowly after that.
Table 4.
Cause of graft failure after retransplantation
| Cause | Counta | Percentage |
|---|---|---|
| Cancer, de novo | 4 | 1.5 |
| Cancer, recurrent | 8 | 3.1 |
| Cardiac | 7 | 2.7 |
| Hepatitis, recurrent | 12 | 4.6 |
| Hepatitis, de novo | 6 | 2.3 |
| Intraoperative | 6 | 2.3 |
| Ischemic injury–PNF | 31 | 12.0 |
| Neurological | 4 | 1.6 |
| Rejection, chronic | 14 | 5.4 |
| Rejection, acute | 2 | 0.8 |
| Sepsis-MODS | 114 | 44.0 |
| Technical, HAT | 20 | 7.7 |
| Technical, other | 22 | 8.5 |
| Other | 6 | 2.3 |
| Unknown | 3 | 1.2 |
Total=259.
Table 5.
Time to retransplantation
| Month after prior OLT | Number of OLTsa | Percent | Cumulative percent |
|---|---|---|---|
| First | 216 | 51.7 | 51.7 |
| Second | 32 | 7.7 | 59.3 |
| Third | 11 | 2.6 | 62.0 |
| Fourth | 15 | 3.6 | 65.6 |
| Fifth | 12 | 2.9 | 68.4 |
| Sixth | 4 | 1 | 69.4 |
| After six months | 128 | 30.6 | 100 |
Total=418.
Figure 3.
Probability of graft failure as a function of time elapsed since the previous transplant. These are based on the gross failure rates (i.e., unadjusted for the effect of covariatesl for grafts performed at each time point.
Recipient factors
Table 6 shows the results of the screening univariate analysis of recipient characteristics, according to whether the graft survived more than one year (group I) or failed within this time (group II). There were no differences in terms of the original diagnosis or preoperative prothrombin time. All other recipient variables studied showed a significant statistical association with outcome.
Table 6.
Recipient characteristics according to retransplant outcomea
| Group I (n=208) | Group II (n=210) | Significance | |
|---|---|---|---|
| Age (yr) | 43.7±12.8 | 46.9±13.1 | P=0.01 |
| Sex (m/f) | 107/101 | 132/78 | P=0.018 |
| Time to ReTx (days) | 369±690 | 218±416 | P=0.007 |
| Preoperative bilirubin (mg/dl) | 15.6±12.6 | 20.0±14.9 | P=0.002 |
| Preoperative creatinine (mg/dl) | 2.1±1.5 | 2.7±1.9 | P=0.001 |
| Preoperative prothrombin time (s) | 17.5±7.3 | 17.6±5.9 | P=0.88 |
| UNOS 4 status (%) | 80.8 | 89.0 | P=0.018 |
| Preoperative mechanical ventilation (%) | 47.2 | 62.5 | P=0.002 |
| Primary immunosuppressant (%): | |||
| Tacrolimus | 60.1 | 58.4 | |
| Cyclosporine | 19.2 | 36.4 | |
| Tacrolimus rescue | 20.7 | 5.2 | P<0.0005 |
| Indication for ReTx (%): | |||
| Hepatitic | 4.8 | 11.9 | |
| PNF-ischemia | 39.4 | 40.5 | |
| Technical | 30.3 | 22.9 | |
| Acute rejection | 2.4 | 4.3 | |
| Chronic rejection | 21.2 | 11.9 | |
| Other | 1.9 | 8.5 | P=0.002 |
| Original diagnosis (%) | |||
| Alcoholic | 15.4 | 14.8 | |
| Autoimmune | 5.3 | 2.9 | |
| Cholestatic | 25.5 | 19.0 | |
| Cryptogenic | 12.5 | 9.0 | |
| FHF | 4.3 | 4.3 | |
| HCC-cholangio. | 3.4 | 7.6 | |
| Hepatitic | 25.0 | 30.5 | |
| Other malignancy | 0.5 | 1.9 | |
| Metabolic | 3.4 | 4.8 | |
| Other | 4.7 | 5.2 | P=0.24 |
Group I=grafts surviving more than one year; group II=grafts that failed within 1 year.
Donor factors
Table 7 shows the donor characteristics according to outcome. Failed grafts (group II) were more likely to come from females and older donors.
Table 7.
Donor characteristics according to retransplant outcomea
| Group I (n=208) | Group II (n=210) | Significance | |
|---|---|---|---|
| Age (yr) | 30.1±14.3 | 35.0±14.7 | P=0.001 |
| Female sex (%) | 35.6 | 46.2 | P=0.03 |
| Ischemia time (hr) | 12.2±4.6 | 12.9±4.7 | P=0.14 |
Group I=grafts surviving more than one year; group II=grafts that failed within 1 year.
Logistic regression analysis
The results are presented in Table 8. Donor age, donor sex, recipient age, need for preoperative mechanical ventilation, preoperative serum creatinine and bilirubin, and primary immunosuppression were all significantly associated with graft failure within one year. There was no evidence of interaction between the covariates.
Table 8.
Variables independently associated with graft failure
| β a | Odds ratio | 95% CI | |
|---|---|---|---|
| Donor age | 0.08 | 2.2b | 1.3 to 3.7b |
| Female donor sex | 0.52 | 1.7 | 1.05 to 2.7 |
| Recipient age | 0.05 | 1.6b | 1.2 to 2.3b |
| Preoperative mechanical ventilation | 0.58 | 1.8 | 1.1 to 2.9 |
| Preoperative creatinine | 0.214 | 1.24c | 1.1 to 1.4c |
| Preoperative bilirubin | 0.033 | 1.4d | 1.1 to 1.8d |
| Primary immunosuppression: | |||
| Tacrolimusf | Reference | ||
| Cyclosporine | 1.37 | 3.9 | 2.3 to 6.8 |
β=regression coefficient; the model also includes a constant=–2.019.
For each 10-year increase over age 45.
For each increase of 1 mg/dl.
For each 10-mg increase over 15 mg/dl.
Includes tacrolimus-rescue cases.
Mode and cause of failure
Males were more likely to die than females (77.4% of graft failures in male recipients were due to, or led to, patient death, as opposed to 68.1% in females), but this was only of borderline significance (P = 0.1). There were no differences in the causes of graft failure when stratified according to recipient sex.
Predicted probability of graft failure
Table 9 shows the predicted probabilities that a graft will fail within the first year of a retransplant, for selected hypothetical scenarios. These are calculated on the basis of the logistic regression model shown in Table 8. To perform the calculation, dichotomous variables are entered into the logistic equation as either zero (male donor, nonintubated, tacrolimus) or one (female donor, on mechanical ventilation, cyclosporine). For donor and recipient age, we use the number of years in excess of 45. For preoperative bilirubin, we use a bilirubin level in excess of 15. Finally, the serum creatinine is entered unmodified.
Table 9.
Probability of graft failure after retransplantation under selected hypothetical scenarios
| Scenario 1 | Scenario 2 | Scenario 3 | Scenario 4 | |
|---|---|---|---|---|
| Donor age (yr) | 30 | 55 | 30 | 65 |
| Donor sex | Male | Female | Male | Female |
| Recipient age | 45 | 65 | 60 | 45 |
| Pretransplant mechanical ventilation | No | Yes | No | Yes |
| Pretransplant creatinine (mg/dl) | 2 | 5 | 2 | 5 |
| Pretransplant bilirubin (mg/dl) | 15 | 30 | 15 | 30 |
| Immunosuppressant | Tacrolimus | Tacrolimus | Tacrolimus | Cyclosporine |
| Probability of graft failurea | 0.17 | 0.92 | 0.30 | 0.99 |
Calculated probability, using the logistic regression model of Table 8.
DISCUSSION
A significant fraction of hepatic grafts continue to fail, especially in the early posttransplant period. In the present series, 27% of primary grafts and fully half of those retransplanted failed within one year (Figure 1 and Table 3), consistent with what has been reported from other centers (8). Five-year graft survival following a primary transplant was 59.5%, but only 35.5% after retransplantation. Most (62.4%) of the failures, primary as well as retransplants, were due to, or led to, patient death. However, there was still a substantial number of patients whose lives were significantly prolonged by retransplantation (Fig. 2).
Several factors were independently associated with graft failure within one year after retransplantation (Table 8), and were generally the same as previously reported in series with a preponderance of primary grafts: older and female donors (20, 21), renal failure (22, 23), and extreme hyperbilirubinemia (22, 23). Interestingly, prolonged graft ischemia, a well-known risk factor (14, 21, 24), failed to achieve significance in this analysis. There are several possible explanations for this discrepancy, the most simple being that variables such as preoperative creatinine and need for mechanical ventilation, which were not included in some of our previous modeling attempts (21, 24), have more explanatory power than ischemia time. Another possibility is that this a reflection of the limitations inherent in trying to model patient death and retransplantation as a single outcome, as it is likely that some risk factors are more strongly associated with one or the other. In the present example, we find that our retransplantation cohort is a group at a higher risk for death (75% of the graft failures were caused by patient death, versus 58% after primary transplantation), and this may obscure the effects of ischemia time if this variable is more strongly associated with need for retransplantation than with patient death. We have found support for this notion by modeling both endpoints separately, as competing risks, in a large series of primary transplants (unpublished observations). The two other factors that emerged from this analysis were the choice of immunosuppressant and recipient age. The odds of failing were about four times greater in patients treated exclusively with cyclosporine (versus tacrolimus), which is consistent with previous reports from our center (21, 25–27) and elsewhere (28–30). The last finding, recipient age, is in contrast to our previous observations (20, 21, 31), and will require further studies of larger samples for confirmation.
The timing played an important role in the outcome of retransplantation, the probability of eventual graft failure increasing from 0.58 to 0.8 between days 0 and 38, and decreasing slowly thereafter (Fig. 3). Presumably, this reflected worsening multiple organ dysfunction as a result of a poorly functioning liver allograft, the recovery of which was awaited in vain; the slowly diminishing risk after the fifth week is most likely simply a reflection of natural selection. Similarly, Powelson et al. (8), found that patients retransplanted within the first three days had a 57% graft survival, versus 24% for those retransplanted between days 4 to 30 (8). The need for early recognition of patients who require retransplantation was one of the earliest lessons in this field (2, 3), and the motivation for our previous work in outcome prediction (23, 32). Other ways of improving the outcome of retransplantation are listed in Table 8: younger, male donors and optimum immunosuppression.
Some might argue that the preceding paragraph begs an important question: given the severe organ shortage, should we offer retransplantation as an option? Saying that it is unfair to allow a patient to receive multiple transplants, while others wait for their first one, does not hold up to careful scrutiny, as even critics of the present system point out (11). Aside from producing an ethical quagmire of patient abandonment, a “one organ, one recipient” mandate would inhibit current efforts to expand the organ pool by acceptance of marginal donors. The safety net of retransplantation is implicit in these initiatives.
In addition, grouping all retransplantations together is a dangerous oversimplification. All authors who have investigated outcome variables have found differential risk categories. With the information already available, individualized prognoses can be formulated. For example, a 45-year-old patient with a bilirubin of 15 mg/dl and a creatinine of 2 mg/dl, who is not intubated, has a calculated probability of graft failure (within one year) of only 0.17 if tacrolimus is used along with a liver from a male donor less than 45 years of age. On the other hand, if the same patient has a bilirubin of 50 mg/dl and a creatinine of 5 mg/dl, and requires mechanical ventilation; receives a liver from a 65 year old female; and is given cyclosporine-based immunosuppression, the calculated probability of failure is 0.99 (Table 9). The calculated risk under the first scenario would be acceptable to most people, even for a primary transplant, whereas the second would not.
We should emphasize that unless the model is validated prospectively these probability estimates cannot be accepted at face value. However, they agree with the clinical impression of most experienced liver transplant surgeons, and illustrate the point that not all retransplant procedures are created equal. We agree that the very liberal retransplantation policies of the past cannot be justified any longer, and one of the challenges is to decide which patients should be offered retransplantation, and which should not. Predictive models such as the one described in this report provide a reasonable starting point for this endeavor.
Although on average retransplantations are undoubtedly more costly than the primary procedure (33, 34), D'Allesandro et al. found that retransplantations carried out during a separate admission had a cost and length of stay similar to that of an initial liver replacement (9). As with other medical procedures, cost benefit analyses of retransplantation must take into account the differences in severity of illness, all factors associated with such efforts, and–most important–the degree of rehabilitation of patients who survive by virtue of these efforts and return to a meaningful role in society.
Acknowledgments
The authors thank Dr. Richard Simmons for reviewing the manuscript.
Footnotes
Supported in part by Research grants from the Veterans Administration and Project Grant DK 29961 from the National Institutes of Health, Bethesda, MD.
Abbreviations: HAT, hepatic artery thrombosis; MODS, multiple organ dysfunction syndrome; OLT, orthotopic liver transplant; PNF, primary non-function; ReTx, retransplantation; UNOS, United Network for Organ Sharing; UW, University of Wisconsin.
REFERENCES
- 1.Starzl TE, Demetris AJ. Liver transplantation. A 31-year perspective. Year Book; Chicago: 1990. [DOI] [PubMed] [Google Scholar]
- 2.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Evolution of liver transplantation. Hepatology. 1982;2:614. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw BW, Gordon RD, Iwatsuki S, Starzl TE. Retransplantation of the liver. Semin Liver Dis. 1985;5:394. doi: 10.1055/s-2008-1040638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ringe B, Neuhaus P, Lauchart W, Pichlmayr R. Experience with hepatic retransplantation. Transplant Proc. 1986;18:1207. [Google Scholar]
- 5.Shaw BW, Wood RP. Improved results with retransplantation of the liver. Transplant Proc. 1989;21:2407. [PubMed] [Google Scholar]
- 6.Morel P, Rilo HLR, Tzakis AG, Todo S, Gordon RD, Starzl TE. Liver retransplantation in adults: overall results and determinant factors affecting outcome. Transplant Proc. 1991;23:3029. [PMC free article] [PubMed] [Google Scholar]
- 7.Mora NP, Klintmalm JB, Cofer SS, et al. Results after liver retransplantation (RETx): a comparative study between “elective” vs. “nonelective” RETx. Transplant Proc. 1990;22:1509. [PubMed] [Google Scholar]
- 8.Powelson JA, Cosimi AB, Lewis WD, et al. Hepatic retransplantation in New England–A regional experience and survival model. Transplantation. 1993;55:802. doi: 10.1097/00007890-199304000-00023. [DOI] [PubMed] [Google Scholar]
- 9.D'Alessandro AM, Ploeg RJ, Knechtle SJ, et al. Retransplantation of the liver–A seven-year experience. Transplantation. 1993;55:1083. doi: 10.1097/00007890-199305000-00028. [DOI] [PubMed] [Google Scholar]
- 10.Mora NP, Klintmalm GB, Cofer JB, et al. Results after liver transplantation in a group of 50 regrafted patients: two different concepts of elective versus emergency retransplantation. Transplant Int. 1991;4:23l. doi: 10.1007/BF00649109. [DOI] [PubMed] [Google Scholar]
- 11.Ubel PA, Arnold RM, Caplan AL. Rationing failure: the ethical lessons of the retransplantation of scarce vital organs. JAMA. 1993;270:2469. doi: 10.1001/jama.270.20.2469. [DOI] [PubMed] [Google Scholar]
- 12.Starzl TE. Ethical problems in organ transplantation: a clinician's point of view. Ann Intern Med. 1967;67(suppl 7):32. doi: 10.7326/0003-4819-67-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.La Puma J, Cassel CK, Humphrey H. Ethics, economics, and endocarditis: the physician's role in resource allocation. Arch Intern Med. 1988;148:1809. [PubMed] [Google Scholar]
- 14.Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation. 1988;45:673. doi: 10.1097/00007890-198804000-00001. [DOI] [PubMed] [Google Scholar]
- 15.The ACCP/SCCM Consensus Conference Committee Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assoc. 1958;53:457. [Google Scholar]
- 17.Hosmer DW, Lemeshow S. Applied logistic regression. Wiley; New York: 1989. [Google Scholar]
- 18.Hastie TJ, Tibshirani RJ. Generalized additive models. Chapman & Hall; London: 1990. [Google Scholar]
- 19.Cleveland WS, Devlin SJ. Locally weighted regression: an approach to regression analysis by local fitting. J Am Statist Assoc. 1988;83:596. [Google Scholar]
- 20.Marino IR, Doyle HR, Aldrighetti L, et al. Effect of donor age and sex on the outcome of liver transplantation. Hepatology. 1995;22:1754. [PMC free article] [PubMed] [Google Scholar]
- 21.Doyle HR, Marino IR, Morelli F, et al. Assessing risk in liver transplantation–with special reference to the significance of a positive cytotoxic crossmatch. Ann Surg. doi: 10.1097/00000658-199608000-00009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuervas-Mons V, Millan I, Gavalcr JS, Starzl TE, Van Thiel DH. Prognostic value of preoperatively obtained clinical and laboratory data in predicting survival following orthotopic liver transplantation. Hepatology. 1986;6:922. doi: 10.1002/hep.1840060519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doyle HR, Marino IR, Jabbour N, et al. Early death or retransplantation in adults after orthotopic liver transplantation: can outcome be predicted? Transplantation. 1994;57:1028. doi: 10.1097/00007890-199404150-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furukawa H, Todo S, Imventarza O, et al. Effect of cold ischemia time on the early outcome of human hepatic allografts preserved with UW solution. Transplantation. 1991;51:1000. doi: 10.1097/00007890-199105000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Starzl TE, Todo S, Fung J, Demetris AJ, Venkataramman R, Jain A. FK506 for liver, kidney, and pancreas transplantation. Lancet. 1989;2:1000. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fung J, Abu-Elmagd K, Jain A, et al. A randomized trial of primary liver transplantation under immunosuppression with FK 506 vs cyclosporine. Transplant Proc. 1991;23:2977. [PMC free article] [PubMed] [Google Scholar]
- 27.Todo S, Fung JJ, Starzl TE, et al. Single-center experience with primary orthotopic liver transplantation with FK 506 immunosuppression. Ann Surg. 1994;220:297. doi: 10.1097/00000658-199409000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDiarmid SV, Klintmalm GB, Busuttil RW. FK 506 conversion for intractable rejection of the liver allograft. Transplant Int. 1993;6:305. doi: 10.1007/BF00335966. [DOI] [PubMed] [Google Scholar]
- 29.European FK506 Multicentre Liver Study Group Randomised trial comparing tacrolimus (FK506) and cyclosporin in prevention of liver allograft rejection. Lancet. 1994;344:423. [PubMed] [Google Scholar]
- 30.Neuhaus P, Blumhardt G, Bechstein WO, et al. Comparison of FK506- and cyclosporine-based immunosuppression in primary orthotopic liver transplantation: a single center experience. Transplantation. 1995;59:31. doi: 10.1097/00007890-199501150-00007. [DOI] [PubMed] [Google Scholar]
- 31.Stieber AC, Gordon RD, Todo S, et al. Liver transplantation in patients over sixty years of age. Transplantation. 1991;51:271. doi: 10.1097/00007890-199101000-00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doyle HR, Dvorchik I, Mitchell S, et al. Predicting outcomes after liver transplantation: a connectionist approach. Ann Surg. 1994;219:408. doi: 10.1097/00000658-199404000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans RW, Manninen dl, Dong FB. An economic analysis of liver transplantation. Gastroenterol Clin North Am. 1993;22:451. [PubMed] [Google Scholar]
- 34.Evans RW, Manninen dl, Dong FB, McLynne DA. Is retransplantation cost effective? Transplant Proc. 1993;25:1694. [PubMed] [Google Scholar]