Abstract
Acellular nerve allografts have been explored as an alternative to nerve autografting. It has long been recognized that there is a distinct limit to the effective length of conventional acellular nerve grafts, which must be overcome for many grafting applications. In rodent models nerve regeneration fails in acellular nerve grafts greater than two cm in length. In previous studies we found that nerve regeneration is markedly enhanced with acellular nerve grafts in which growth-inhibiting chondroitin sulfate proteoglycan was degraded by pretreatment with chondroitinase ABC (ChABC). Here, we tested if nerve regeneration can be achieved through 4-cm acellular nerve grafts pretreated with ChABC. Adult rats received bilateral sciatic nerve segmental resection and repair with a four-cm, thermally acellularized, nerve graft treated with ChABC (ChABC graft) or vehicle-treated acellularized graft (Control graft). Nerve regeneration was examined 12 weeks after implantation. Our findings confirm that functional axonal regeneration fails in conventional long acellular grafts. In this condition we found very few axons in the distal host nerve, and there were marginal signs of sciatic nerve reinnervation in few (2/9) rats. This was accompanied by extensive structural disintegration of the distal graft and abundant retrograde axonal regeneration in the proximal nerve. In contrast, most (8/9) animals receiving nerve repair with ChABC grafts showed sciatic nerve reinnervation by direct nerve pinch testing. Histological examination revealed much better structural preservation and axonal growth throughout the ChABC grafts. Numerous axons were found in all but one (8/9) of the host distal nerves and many of these regenerated axons were myelinated. In addition, the amount of aberrant retrograde axonal growth (originating near the proximal suture line) was markedly reduced by repair with ChABC grafts. Based on these results we conclude that ChABC treatment substantially increases the effective length of acellular nerve grafts.
Introduction
Nerve grafting is warranted with nerve ablation or transection when the stumps have retracted and cannot be coapted directly without tension (Millesi, 1984). Presently, autologous nerve grafts are the first choice for interpositional grafting. However, alternatives to nerve autografts remain a goal of neurosurgeons to avoid the concurrent functional deficits associated with procuring autografts, which also preclude this approach for management of major or extensive nerve defects. Allogenic nerve grafting overcomes these concerns but remains experimental and is limited by the need for long-term systemic immunosuppression. On the other hand, freeze-killed or decellularized allogenic nerve grafts greatly reduce the concerns of host-graft immunorejection (Evans et al., 1994; 1998). In addition, acellular nerve grafts can be stored frozen for extended periods without losing their growth-promoting potential (Ide et al., 1990; Evans et al., 1998). The nerve sheath structure contains the essential scaffolding and adhesive cues to promote axonal regeneration and significant regeneration has been achieved in acellular nerve grafts (Ide et al., 1983; Hall, 1986; Gulati, 1988; Nadim et al., 1990; Sondell et al., 1998; Hudson et al., 2004). However, there is a significant delay associated with axonal growth into acellular grafts and results are usually inferior to those obtained using cellular grafts (e.g., in syngraft animal models). In addition, it has long been known that there is a distinct limit to the effective length of acellular nerve grafts, which may vary for different species. In rat, axons from transected nerves will regenerate into freeze-killed, normal nerve grafts for a maximum distance of about two cm (Gulati, 1988; Nadim et al., 1990). The number of axons and the rate of ingress are improved using freeze-killed predegenerated nerve grafts, but the maximum distance of axonal growth has a similar limit (Gulati, 1988; Daneilsen et al., 1995; Krekoski et al., 2002). The reason for this limitation remains controversial, however, it is generally accepted that maximum axonal regeneration into acellular nerve grafts is achieved by 6–8 weeks after implantation and the distal graft degrades soon thereafter with inadequate axonal and cellular infiltration.
Although injured peripheral nerve is generally viewed as supportive to axonal growth, numerous studies indicate that normal adult peripheral nerves are not permissive and either fail to encourage or actively inhibit axon growth (Langley and Anderson, 1904; Bedi et al., 1992; Brown et al., 1994). Recent findings indicate the activity of endoneurial laminin, that might otherwise promote axonal regeneration, is suppressed in a normal nerve environment (Zuo et al., 1998; Ferguson and Muir, 2000). Peripheral nerve contains inhibitory chondroitin sulfate proteoglycan (CSPG) that binds laminin and blocks its growth promoting activity (Muir et al., 1989; Zuo et al., 1998). A preponderance of inhibitory CSPGs has a direct impact on the properties of donor nerves and the outcome of nerve grafting.
We reported previously that nerve regeneration is markedly enhanced by ChABC pretreatment of one-cm freeze-killed nerve grafts (Krekoski et al., 2001). Here, we tested if the maximum distance of axonal growth in acellular grafts is increased by ChABC treatment. Our findings show that substantial nerve regeneration and reinnervation in the rat can be achieved through 4-cm freeze-killed nerve grafts pretreated with ChABC.
Materials and Methods
Graft preparation
All surgical procedures were performed according to approved Institutional Animal Care and Use Committee protocols. Adult male Fischer F344 rats (250g) were used as nerve donors and graft recipients. Donor rats were deeply anesthetized with isoflurane and decapitated. Sciatic/tibial nerves were exposed, isolated free of underlying fascia and 5-cm segments were removed. The segments were rinsed with cold, sterile Ringer’s solution, pinned onto a thin plastic support and placed in a cryogenic vial. The nerve segments were frozen in liquid nitrogen for 2 min and then thawed in a 37°C water bath for 2 min. This freeze-thaw cycle was repeated 2 more times so as to yield acellular nerves. The nerve segments were stored in liquid nitrogen until needed. Nerves were thawed the day before engraftment and incubated in PBS containing 2 U/ml chondroitinase ABC (affinity purified; Sigma Chemical Co., St. Louis, MO) or in PBS only for 16 hr at 37°C. The segments were rinsed twice with cold, sterile Ringers and kept on ice until engrafted.
Interpositional nerve grafting
Host rats received bilateral sciatic nerve segmental resection. Graft repair was performed with a four-cm, thermally acellularized, nerve graft treated with ChABC (ChABC graft) in the right leg and a vehicle-treated acellularized graft (Control graft) in the left leg. Host rats were anesthetized with isoflurane and the sciatic nerve exposed between the tendon of the internal obdurator muscle and the trifurcation. A plastic support was placed under the nerve and fibrin glue (fibrinogen and thrombin) was applied. Using Biemer scissors, a 10-mm segment was removed and replaced with a freshly trimmed 40-mm acellular nerve graft. The proximal and distal host stumps were coapted with the graft by epineural neurorraphy using one 9-0 Ethilon nylon suture at each end. More fibrin glue was applied to stabilize the coaptations and reduce endoneurial mushrooming. Muscle incisions were sutured and the skin closed with metal wound clips. After recovery from the anesthetic, animals were returned to standard housing with shredded paper bedding and two animals per cage. Ten animals received nerve grafts and 1 was eliminated from the study because of autophagia.
Nerve pinch test
The sciatic nerve was exposed after 12 weeks under the same anesthetic conditions as described above. The tibial and peroneal branches of the host nerve were isolated 1 cm distal to the distal sutures at the graft-nerve coaptation. The isoflurane level was decreased until eye-blink response to touch was observed. The tibial nerve was held with a pair of forceps and the nerve pinched firmly 10mm distal to the graft. Most pinch responses were clearly distinguished as positive or negative. A forceful, reflexive withdrawal of the leg was considered a positive response. Otherwise, a slight leg movement (e.g. twitch or ankle flex) was considered a marginal response. Assessments of the nerve pinch response were made by an observer blinded to the treatment conditions and were validated by a second observer. The initial observation was confirmed by a second nerve pinch made 5mm proximal to the first. Consistent results were obtained with both trials in all nerves.
Histology and Immunocytochemistry
Immediately after nerve pinch testing, animals were then deeply anesthetized and decapitated. Nerves were excised, mounted taut on a plastic support and fixed by immersion in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, overnight at 4°C. The specimens were washed with PBS and immersed in 30% sucrose in phosphate buffer for 3 days at 4°C. Using a dissecting microscope and the epineurial sutures as landmarks, each specimen was subdivided into segments to include the proximal graft, medial graft, distal graft and host nerve as indicated. Specimens were rapidly frozen, sectioned on a cryostat and 14 µm sections were collected. Tissue sections mounted on slides were rinsed with PBS and then 0.5% Triton X-100 in PBS. Sections were treated with blocking buffer (10% serum in PBS + 0.1% Triton X-100) and then incubated overnight at 4°C with primary antibodies (diluted in blocking buffer). Bound primary antibodies were labeled with swine anti-rabbit IgGs (Dako, Carpinteria, CA) or goat anti-mouse IgGs (Sigma) conjugated with fluorescein for 1 hr at room temperature in darkness. The antimouse secondary antibody was preadsorbed with rat serum before use. Sections were washed, post-fixed with 4% paraformaldehyde in PBS, rinsed and coverslipped in a fluorophore-stabilizing mounting medium. Antibodies used were as follows: affinity-purified anti-GAP-43 (Ferguson and Muir, 2000)(Novus Biologicals, Littleton, CO) to label extrafascicular axons; anti-neurofilament IgG (NAP4; Harris et al., 1993) to label all axons; and anti-S-100 (Dako, Carpinteria, CA) to label Schwann cells.
Photomicrographs were captured using an Axioscope II (Carl Zeiss, Jena, Germany) equipped with a Spot II digital camera (Diagnostic Instruments, Sterling Heights, MI). Axon counting image analysis was performed with ImagePro Plus (Media Cybernetics, Inc., Silver Springs, MD). Neurofilament-positive axons were counted in the distal host tibial nerve (transgraft antegrade regeneration, 3 mm distal to the graft), within the proximal host nerve (intrafascicular retrograde regeneration, 3 mm proximal to the graft) and outside the proximal host nerve (extrafascicular retrograde regeneration, 3 mm proximal to the graft). Immunofluorescence micrographs were flattened ten times (background-Dark, 20 pixels) and Best Fit brightness and contrast was applied. Area of interest (intrafascicular or extrafascicular) was selected and HiGauss Filter (7×7, Pass-1, strength-10) applied. Count range was set to 28–255 (gray level) with no filter and Watershed split applied twice. For antegrade regeneration and extrafascicular retrograde regeneration (ERR) counts were obtained directly (without sampling). To assess intrafascicular retrograde regeneration (IRR) counts were obtained from 18 distinct areas of interest (samples) selected at random for each nerve as described previously (Graham et al., 2007). Data were exported and analyzed using Prism Software (GraphPad Software, San Diego, CA). Photographic images were contrast-enhanced and sharpened (unsharp mask) in Photoshop (Adobe Systems, San Jose, CA) for reproduction.
Myelination was examined by Toluidine blue staining of semi-thin plastic sections. Briefly, a 4-mm segment of distal host nerve was excised 4 mm past distal sutures and immersed in a 2% glutaraldehyde solution. The nerve segments were dehydrated in graded acetone solutions and embedded in Epon plastic resin. Semi-thin (500 nm) sections were obtained using a glass knife and stained with 1% toluidine blue/1% sodium borate on a warming plate.
Results
The effective length of acellular nerve grafts is increased by treatment with ChABC
All nerve grafts used in this study were acellularized by thermal methods prior to any treatments. Thermal acellularization is minimally disruptive to nerve structure and has the added benefit of increasing permeability of nerve sheaths for lateral diffusion of subsequent enzyme treatment. We previously found that CSPG was thoroughly degraded throughout a 1-cm long, thermally acellularized rat sciatic nerve graft after ChABC treatment (Krekoski et al., 2001). The same held true in the present study for 4-cm grafts using the same ChABC treatment (results not shown). Sprague Dawley rats were used in our previous short-term studies with 1-cm grafts. Isogenic Fisher rats were used in this study to reduce variability and concerns of immunorejection. In addition, adult male Fisher rats grow larger and have longer sciatic nerves with less fasciculation, which is advantageous for long grafting.
Rats received bilateral, 4-cm sciatic nerve grafts, one vehicle control (Control graft) and one treated with ChABC (ChABC graft). Twelve weeks after grafting and just prior to termination, reinnervation of the distal host nerve was assessed by direct nerve pinch testing in live, lightly anesthetized animals. Two (of nine) nerves repaired with a Control graft showed a marginal pinch response and the other seven gave no response. In contrast, 8 of 9 nerves repaired with a ChABC graft showed strong signs of sciatic nerve reinnervation by direct nerve pinch (eliciting a pain reflex).
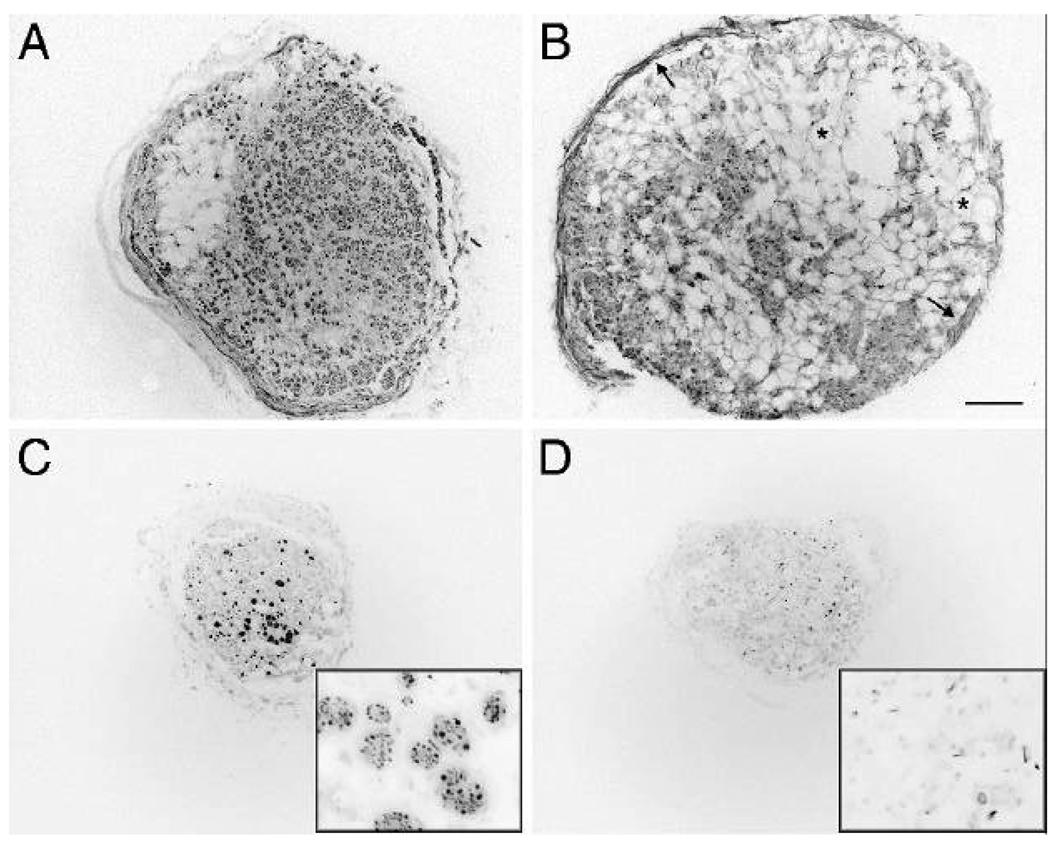
Engrafted nerves were excised 12 weeks after implantation and examined for axonal regeneration and graft integrity. At the proximal nerve-graft interface, abundant regeneration of axons from the host nerve into the grafts was evident in both Control and ChABC graft conditions at 12 weeks (not shown). Sustained axonal growth appeared in transverse sections as dense, round, clusters of neurofilament-immunopositive axons (Fig 1A and C). At higher magnification the neurofilament-rich clusters were visible as “regenerating units” containing numerous axons within a common endoneurial sheath (Fig. C, inset). Marked differences in axonal growth and graft integrity between the two graft conditions was evident one cm into the grafts. Numerous and widespread regenerating units were found only in the ChABC grafts (Fig. 1A). Failing regeneration was associated with extensive vacuolization indicative of basal lamina and overall graft deterioration causing axons to grow diffusely in random orientation or along the inner perineurium (Fig. 1B). These features precluded reliable scoring of axonal regeneration within the grafts at 12 weeks. Nevertheless, the extent of axonal regeneration in the grafts was indicated by neurofilament immunolabeling. In control grafts, the disorganized growth rapidly diminished and very few or no axons were seen at the midpoint of the majority (7/9) of these grafts. This failed axonal regeneration was associated with pervasive vacuolization and deterioration of the distal graft (Fig. 1D). In contrast, substantial axonal growth was observed at the midpoint and well beyond in all but one (8/9) of the ChABC grafts. Nerve regeneration within the distal graft occurred mainly in organized, tightly packed regenerating units containing numerous axons and was associated with overall better preservation of the graft structure (Fig 1C). Despite substantial compaction, remodeling of ChABC grafts by infiltrating host cells was evident and axons were encased by Schwann cells and newly formed basal laminae. Neurofilament-immunolabeled axons were always accompanied by S-100-immunolabeled Schwann cells surrounded by a laminin-immunopositive endoneurial sheath (not shown).
Figure 1.
Sustained axonal growth within ChABC treated, 4-cm acellular nerve grafts. Axonal growth in acellular nerve grafts was examined 12 weeks after implantation by neurofilament immunocytochemistry. One cm into the grafts, ChABC treated grafts contained abundant and widespread axons growing in dense clusters (A). These grafts appeared mostly remodeled by cellular infiltration although localized vacuolization was often observed. The distal portion of ChABC treated grafts remained intact although compacted and most (8/9) grafts contained numerous axon clusters (C). Sustained axonal growth appeared in axons clusters or “regenerating units” (C inset). In contrast, the proximal portions of control grafts were highly vacuolized and swollen (asterisks) indicative of basal lamina and overall graft deterioration (B). Signs of failed regeneration were evident even in the proximal grafts which contained low numbers of axons growing diffusely in random orientation and along the inner perineurium (B, arrows). Very few or no axons were seen beyond the midpoint of the majority of the control grafts (D). Regenerating units of axons were rarely observed and failed regeneration was associated with axonal degeneration and deterioration of the distal graft (D inset). The graft shown in D depicts one of the better control grafts; most were deteriorated badly and showed little histological detail. A, B and C, D are sections of graft 10.2 and 20.2 mm from the proximal coaptation, respectively. Scale bar: 200 µm (insets: 25 µm)
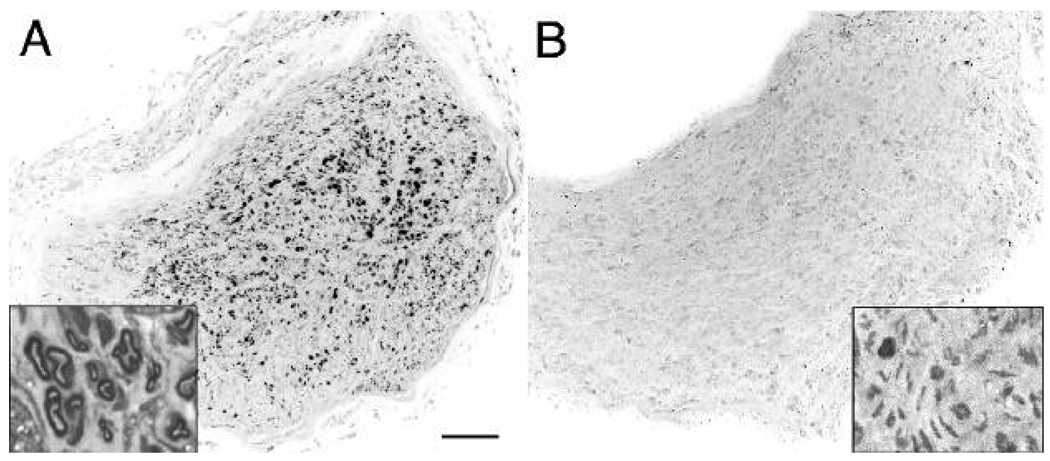
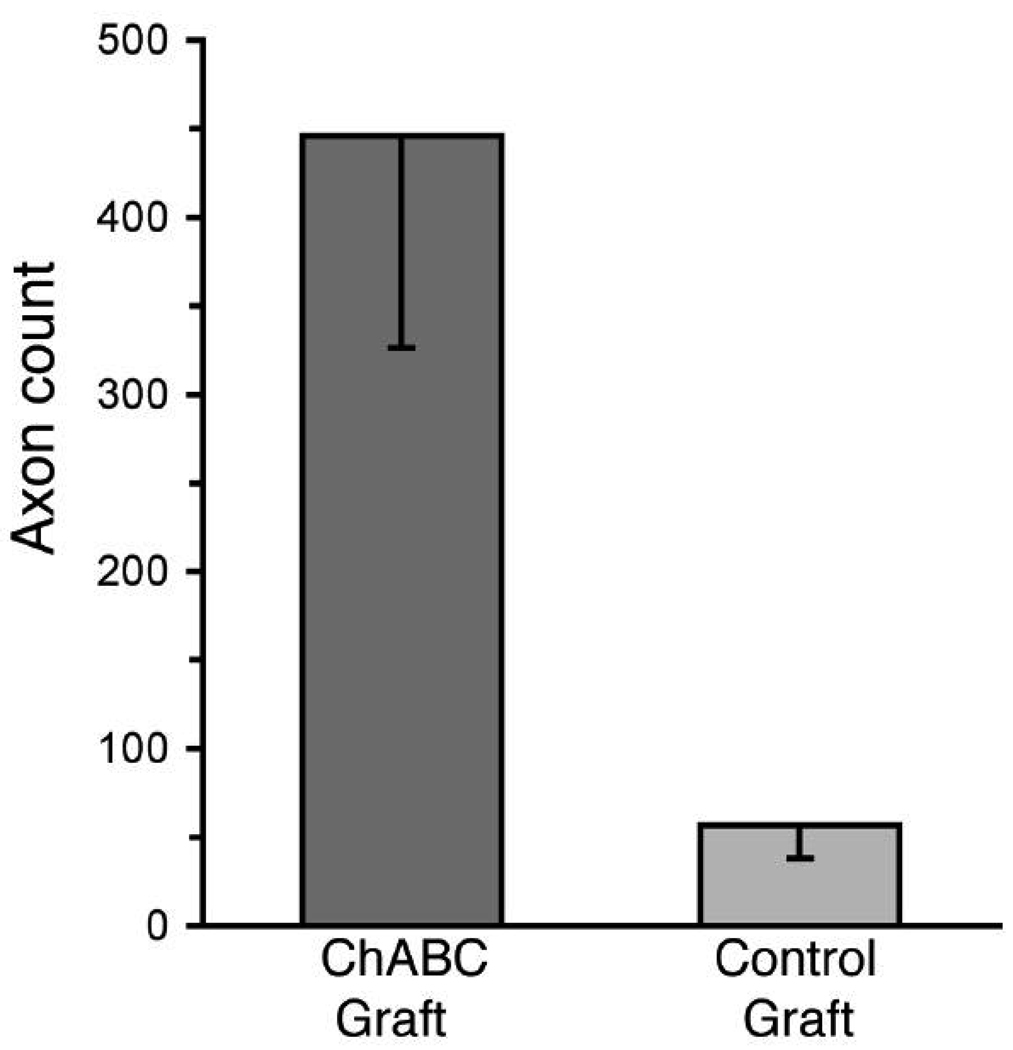
Successful nerve regeneration conducted through the grafts was scored by counting neurofilament-immunopositive profiles within the distal host nerve (Fig. 2). The mean number of axons found distal to the Control grafts was 57 (SEM ±19) and none were myelinated (Fig. 3). Some neurofilament-positive axons were found in all distal nerves, even when there was clear histological evidence that a graft had deteriorated and no axons were found within the distal graft. Therefore, in these cases it is likely that the few neurofilament-positive profiles observed in the distal host nerve were axons that had not traverse the graft. These unmyelinated axons (Fig. 2B, inset) might be collaterals of sympathetic neurons (e.g., associated with blood vessels). In contrast, numerous axons had traversed all but one of the ChABC grafts (Fig. 2A). The mean axon count distal to the treated grafts was 446 (SEM ±120) (P < 0.01, t-test) (Fig. 3). There was substantial variability in the ChABC condition counts because two of the distal nerves contained more than twice the mean number of axons. In contrast to the control condition, many of the axons found distal to ChABC treated grafts were myelinated (Fig. 2A, inset).
Figure 2.
ChABC treated, 4-cm acellular nerve grafts support growth of axons into the distal host nerve. Twelve weeks after nerve graft repair axons in the distal host nerve were examined by neurofilament immunocytochemistry. (A) Numerous axons were found in distal host nerves repaired with ChABC treated, 4-cm acellular nerve grafts. Toluidine blue stained semi-thin sections revealed many of these axons were myelinated (inset). (B) In contrast, very few axons were found distal to control grafts and rarely were any of these myelinated (inset). Scale bar: 150 µm (insets: 10 µm)
Figure 3.
Axon counts in the distal host nerve after repair with 4-cm acellular nerve grafts. Axons were labeled as described in Fig. 2. Digital micrographs were taken of the distal host tibial nerve 3 mm distal to the grafts and axons counted by image analysis software. The mean number of axons in the distal host nerves resulting from repair with ChABC treated, 4-cm acellular nerve grafts was significantly greater than that in the control graft condition (P < 0.01, t-test). Data represent the means (±SE) of 9 nerves in each condition.
Aberrant retrograde axonal regeneration is reduced by ChABC
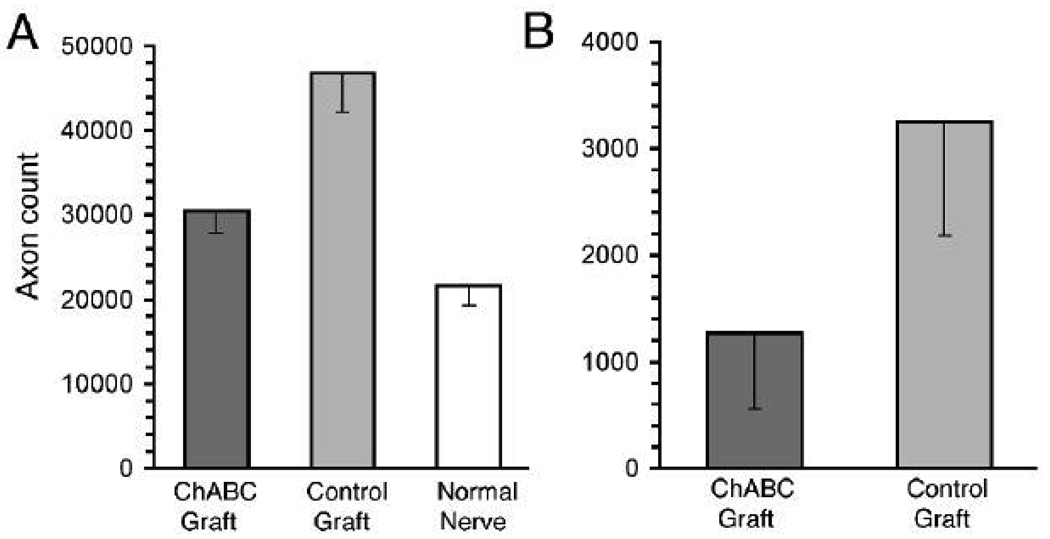
After nerve resection and graft repair, axonal sprouts arise in the proximal stump and advance distally toward the graft coaptation. Some axons fail to traverse the coaptation, become misdirected, and grow in the opposite direction. This retrograde axonal regeneration was classified as intrafascicular and extrafascicular. Many axonal sprouts turn back and grow in association with established basal laminae in the proximal nerve and remain within the original perineurium (intrafascicular). On the other hand, other axonal sprouts deviate laterally at the repair site, exit the nerve proper and grow outside the epineurium (extrafascicular). In a previous study we developed methods to identify and score intrafascicular retrograde regeneration (IRR) and extrafascicular retrograde regeneration (ERR) (Graham et al., 2007). IRR was calculated as the difference between the number of axons in a normal nerve and the number of axons in a nerve several mm proximal to nerve repair. Quantitative light microscopic image analysis of neurofilament-immunopositive profiles in transverse nerve sections detected 21,589 (SEM ±2243) axons in the normal (uninjured) rat sciatic nerve at midthigh (Fig. 4A). Twelve weeks after nerve repair with Control grafts the mean axon count in the proximal host nerve (3 mm proximal to the coaptation) was 46,778 (SEM ±4650), representing a 117% increase in axons over that in the average normal nerve (P < 0.01, Dunnett’s multiple comparison test). This increase was mainly attributed to a distinct population of small axon clusters observed proximal to the repair site that were not found in normal nerves (see Graham et al., 2007). The clusters of small caliber axons most likely represent multiple sprouts arising from axons that failed to enter the graft and turned back to grow in the retrograde direction. Remarkably, IRR was dramatically reduced in nerves repaired with a ChABC graft. The mean axon count in the proximal host nerve in this condition was 30,494 (SEM ±2,494), representing a 41% increase in axons over that in the average normal nerve (P > 0.05, Dunnett’s multiple comparison test). In other words, nerve repair with a ChABC graft eliminated approximately 2/3 of the dysfunctional IRR associated with acellular nerve grafting (P < 0.0001, one-way ANOVA).
Figure 4.
Retrograde axonal regeneration was reduced after repair with ChABC treated 4-cm acellular nerve grafts. Twelve weeks after nerve repair, neurofilament-immunopositive axons were counted within (intrafascicular) and surrounding (extrafascicular) the host nerve 3mm proximal to the grafts. (A) Intrafascicular retrograde regeneration was evident by an increase in axon count within repaired nerves when compared to normal nerves. Results indicate 25,189 of the axons found within the host nerve proximal to the control grafts (i.e., the difference between mean counts in the control condition and normal nerve) were attributed to retrograde axonal growth, whereas in the ChABC graft condition 8,905 of axons were attributed to retrograde growth. Therefore, nerve repair with a ChABC graft eliminated 65% of the dysfunctional IRR associated with acellular nerve grafting (P < 0.0001) (One-way ANOVA). (B) Extrafasciclular retrograde regeneration was scored by direct count of neurofilament-immunopositive axons found outside the nerve proper 3mm proximal to the graft repair. Extrafascicular retrograde regeneration was lower in the ChABC graft condition compared to the control graft condition (P = 0.06, t-test). Data represent the means (±SE) of 9 nerves in each condition.
Next, we scored the number of axons that had exited the nerves at the proximal nerve-graft suture line and were growing in the retrograde direction outside the nerve proper. These axons were scored by GAP-43 immunolabeling as previously described (Graham et al., 2007). In the Control graft condition the mean number of ERR axons was 3,248 (SEM ±1067). For nerves repaired with a ChABC graft the mean number of ERR axons was reduced to 1,268 (SEM ±710) (P = 0.06, t-test) (Fig. 4B). The reduction in ERR associated with ChABC grafts was observed for all animals (n=9, subject to each condition in bilateral repair).
Discussion
Acellular peripheral nerve grafts have been studied extensively to assess the role of the nerve extracellular matrix in axonal regeneration and for their potential in clinical nerve repair. It is generally accepted that Schwann cell basal laminae serve as pathways for and promote axonal regeneration even after the cellular components of nerve grafts have been killed or extracted (Fawcett and Keynes, 1990; Ide et al., 1990). However, there may be a limit to the extent of nerve regeneration that can occur in acellular grafts. Zalewski and Gulati (1982) found that axons do not regenerate all the way through a 4-cm acellular nerve graft in the rat. Most studies in rat indicate that axon regeneration through thermally acellularized grafts is limited to 1–2 cm (Gulati, 1988; Nadim et al., 1990; Ochi et al., 1994; Chong et al., 1994). This apparent regenerative limit does not appear to be the result of an intrinsic deficiency in neurons and outcomes have been improved by using grafts modified by various preservation and predegeneration schemes (Osawa et al., 1990; Chong et al., 1994; Evans et al., 1999).
The present study tested the hypothesis that nerve regeneration can be achieved through 4-cm acellular nerve grafts pretreated with ChABC. Sciatic nerve segments were thermally acellularized, treated with ChABC or vehicle alone, and then implanted as orthotopic interpositional grafts. Our findings confirm that nerve regeneration mostly fails before reaching the midpoint of conventional (Control) 4-cm acellular grafts. Axonal and Schwann cell infiltration was scarce beyond the midpoint of the control grafts and the distal portions showed extensive deterioration of all structural components including basal lamina tubes. Progressive fragmentation and dispersion of basal laminae in long grafts is commonly found and likely attributed to phagocytotic activity associated with failed nerve regeneration (Giannini and Dyck, 1990; Nadim et al., 1990). Correspondingly, the number of axons in the host nerves distal to these grafts was uniformly low. Also, 12 weeks after grafting, there were few and marginal signs of sciatic nerve reinnervation. By comparison, ChABC grafts supported markedly better nerve regeneration. This was evident in both histological and functional assessments. Although sufficient to achieve functional distal nerve reinnervation, the amount of axonal regeneration through these grafts was fractional after only 12 weeks. Yet, graft integrity was much better preserved in the ChABC graft condition and therefore the repair remained capable of conducting continued regeneration. Nerve regeneration is progressive after graft repair and axonal regrowth can continue for up to a year in the rat. The abundance of growing axons found in the proximal portion of the ChABC grafts suggests that continued regeneration through the grafts may be anticipated over time.
Several factors might contribute to the failure of nerve regeneration through long acellular grafts. Inceptive reports suggest that Schwann cell migration and proliferation may be a limiting factor in regeneration through long acellular grafts (Gulati, 1988; Nadim et al., 1990). Findings in the present study indicate that axonal growth can be achieved through long acellular grafts that have been treated with ChABC. In addition, similar to our previous observations, axonal advance in ChABC-treated acellular grafts is closely accompanied by Schwann cell migration (Krekoski et al., 2001). Therefore, long graft failure is not limited by Schwann cell capabilities per se, but rather by an inability of axons and Schwann cells to overcome deficiencies in normal acellular grafts. ChABC treatment restores the adhesive properties of the nerve basal lamina that promote nerve regeneration (Zuo et al., 1998). It is most likely that degradation of CSPG deinhibits laminin activity and potentiates the acellular graft scaffolding (Krekoski et al., 2001).
Numerous studies implicate Wallerian degeneration, involving Schwann cells and infiltrating phagocytic cells, as a key process in the progression of nerve regeneration (Fernandez-Valle et al., 1995; Sorensen et al., 2001). Acellular grafts are inherently incapable of Wallerian degeneration. Schwann cells and inflammatory cells from the host nerve will migrate into and remodel the acellular graft, promoting regeneration. The importance of the degenerative process has been demonstrated in several nerve grafting models and grafts prepared from nerves allowed to degenerate in vivo before excision can be more effective than normal nerve grafts in promoting regeneration of peripheral nerve axons (Danielsen et al., 1994; Ochi et al., 1994). Furthermore, predegeneration reduces the initial delay of axon penetration and enhances regeneration into freeze-killed nerves as well (Danielsen et al., 1995; Krekoski et al., 2002). This indicates that, in degeneration, cellular mechanisms act to enhance the growth-promoting properties of the basal lamina which then retains the ability to stimulate nerve regeneration after the cellular elements have been killed. Despite these enhancements, axonal growth does not progress more than two cm in acellular predegenerated grafts. On the other hand, axons will grow through fresh cellular grafts and into the distal nerve of isogenic hosts in the same time period (Gulati, 1988, Ochi et al., 1994).
Our previous studies indicate that ChABC treatment mimics a key degenerative process by enhancing the growth-promoting properties of the basal lamina scaffold in acellular nerve grafts. Like in vivo nerve predegeneration, modification of acellular nerve grafts by ChABC markedly hastens the rate of ingress and extent of axonal regeneration (Krekoski et al., 2001). In addition, we now find that ChABC treatment also increases the effective length of acellular nerve grafts. It therefore seems that selective degradation of CSPG has distinct advantages compared to in vivo nerve predegeneration. For instance, predegeneration involves extensive remodeling and may compromise basal lamina integrity, which then is exacerbated in acellular nerve grafting (Ochi et al., 1994; Sorensen et al., 2001). In addition, inhibitory CSPGs are highly expressed after nerve injury which might interfere with the regenerative potential of predegenerated grafts (Zuo et al., 1998; Rezajooi et al., 2004).
In injury and repair, misalignment of CSPG-containing structures can misdirect regenerating axons. We recently reported that ChABC applied to the site of direct nerve transection repair reduced the loss of axons at the suture line, decreased retrograde axonal regeneration and improved recovery of function (Graham et al., 2007). Aberrant retrograde axonal growth occurs with all nerve repair methods and can be expected to increase with the complexity of the injury and repair. This expectation is confirmed by our studies. Nerve repair with a control acellular nerve graft resulted in a more than two-fold increase in axons proximal to the repair (i.e., in the proximal host nerve). This increase is the result of multiple sprouts arising from axons at the nerve-graft interface that fail to enter the graft and instead are misdirected in the retrograde direction. Importantly, the amount of retrograde axonal growth was greatly reduced by repair with a ChABC graft. These findings confirm our contention that degradation of CSPG dramatically enhances axonal ingress through nerve coaptations and, consequently, improves antegrade, target-oriented axonal regeneration.
Inactivation of inhibitory CSPGs by ChABC allows for a robust regenerative response into acellular nerve grafts. It appears that rapid and extensive axonal ingress is required for regeneration to precede sufficiently and negate deterioration of long acellular grafts. Moreover, our findings are consistent with the conclusion that inhibitory CSPG, as an extant component the graft basal lamina, contributes to the limited regeneration achieved in acellular nerve grafts. The success of repair with ChABC treated long acellular grafts was clearly associated with sustained axonal ingrowth and graft remodeling resulting in preservation of the distal graft. Future study is warranted to determine the full potential for increasing effective graft length and recovery of function in nerve repair with ChABC treated acellular grafts.
Acknowledgements
This work was funded by a grant from the National Institutes of Health (NS37901). We thank Elizabeth Baldwin for excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bedi KS, Winter J, Berry M, Cohen J. Adult rat dorsal root ganglion neurons extend neurites on predegenerated but not on normal peripheral nerves in vitro. Eur J Neurosci. 1992;4:193–200. doi: 10.1111/j.1460-9568.1992.tb00867.x. [DOI] [PubMed] [Google Scholar]
- Brown MC, Perry VH, Hunt SP, Lapper SR. Further studies on motor and sensory nerve regeneration in mice with delayed Wallerian degeneration. Eur J Neurosci. 1994;6:420–428. doi: 10.1111/j.1460-9568.1994.tb00285.x. [DOI] [PubMed] [Google Scholar]
- Chong MS, Woolf CJ, Andrews P, Turmaine M, Schreyer DJ, Anderson PN. The downregulation of GAP-43 is not responsible for the failure of regeneration in freeze-killed nerve grafts in the rat. Exp Neurol. 1994;129:311–320. doi: 10.1006/exnr.1994.1173. [DOI] [PubMed] [Google Scholar]
- Danielsen N, Kerns JM, Holmquist B, Zhao Q, Lundborg G, Kanje M. Predegenerated nerve grafts enhance regeneration by shortening the initial delay period. Brain Res. 1994;666:250–254. doi: 10.1016/0006-8993(94)90779-x. [DOI] [PubMed] [Google Scholar]
- Danielsen N, Kerns JM, Holmquist B, Zhao Q, Lundborg G, Kanje M. Predegeneration enhances regeneration into acellular nerve grafts. Brain Res. 1995;681:105–108. doi: 10.1016/0006-8993(95)00300-f. [DOI] [PubMed] [Google Scholar]
- Evans PJ, Mackinnon SE, Levi AD, Wade JA, Hunter DA, Nakao Y, Midha R. Cold preserved nerve allografts: changes in basement membrane, viability, immunogenicity, and regeneration. Muscle Nerve. 1998;21:1507–1522. doi: 10.1002/(sici)1097-4598(199811)21:11<1507::aid-mus21>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Evans PJ, MacKinnon SE, Midha R, Wade JA, Hunter DA, Nakao Y, Hare GM. Regeneration across cold preserved peripheral nerve allografts. Microsurgery. 1999;19:115–127. doi: 10.1002/(sici)1098-2752(1999)19:3<115::aid-micr1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Evans PJ, Midha R, Mackinnon SE. The peripheral nerve allograft: a comprehensive review of regeneration and neuroimmunology. Prog Neurobiol. 1994;43:187–233. doi: 10.1016/0301-0082(94)90001-9. [published erratum appears in Prog Neurobiol 1995 Feb;45(3):iii] [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Keynes RJ. Peripheral nerve regeneration. Annu Rev Neurosci. 1990;13:43–60. doi: 10.1146/annurev.ne.13.030190.000355. [DOI] [PubMed] [Google Scholar]
- Ferguson TA, Muir D. MMP-2 and MMP-9 increase the neurite-promoting potential of Schwann cell basal laminae and are upregulated in degenerated nerve. Mol Cell Neurosci. 2000;16:157–167. doi: 10.1006/mcne.2000.0859. [DOI] [PubMed] [Google Scholar]
- Fernandez-Valle C, Bunge RP, Bunge MB. Schwann cells degrade myelin and proliferate in the absence of macrophages: Evidence from in vitro studies of Wallerian degeneration. J Neurocytol. 1995;24:667–679. doi: 10.1007/BF01179817. [DOI] [PubMed] [Google Scholar]
- Giannini C, Dyck PJ. The fate of Schwann cell basement membranes in permanently transected nerves. J Neuropathol Exp Neurol. 1990;49:550–563. doi: 10.1097/00005072-199011000-00002. [DOI] [PubMed] [Google Scholar]
- Graham JB, Neubauer D, Xue QS, Muir D. Chondroitinase applied to peripheral nerve repair averts retrograde axonal regeneration. Exp Neurol. 2007;203:185–195. doi: 10.1016/j.expneurol.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati AK. Evaluation of acellular and cellular nerve grafts in repair of rat peripheral nerve. J Neurosurg. 1988;68:117–123. doi: 10.3171/jns.1988.68.1.0117. [DOI] [PubMed] [Google Scholar]
- Hall SM. The effect of inhibiting Schwann cell mitosis on the re-innervation of acellular autografts in the peripheral nervous system of the mouse. Neuropathol Appl Neurobiol. 1986;12:401–414. doi: 10.1111/j.1365-2990.1986.tb00151.x. [DOI] [PubMed] [Google Scholar]
- Harris J, Moreno S, Shaw G, Mugnaini E. Unusual neurofilament composition in cerebellar unipolar brush neurons. J Neurocytol. 1993;22:1039–1059. doi: 10.1007/BF01235748. [DOI] [PubMed] [Google Scholar]
- Hudson TW, Zawko S, Deister C, Lundy S, Hu CY, Lee K, Schmidt CE. Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng. 2004;10:1641–1651. doi: 10.1089/ten.2004.10.1641. [DOI] [PubMed] [Google Scholar]
- Ide C, Osawa T, Tohyama K. Nerve regeneration through allogeneic nerve grafts, with special reference to the role of the Schwann cell basal lamina. Prog Neurobiol. 1990;34:1–38. doi: 10.1016/0301-0082(90)90024-b. [DOI] [PubMed] [Google Scholar]
- Ide C, Tohyama K, Yokota R, Nitatori T, Onodera S. Schwann cell basal lamina and nerve regeneration. Brain Res. 1983;288:61–75. doi: 10.1016/0006-8993(83)90081-1. [DOI] [PubMed] [Google Scholar]
- Krekoski CA, Neubauer D, Graham JB, Muir D. Metalloproteinase-dependent predegeneration in vitro enhances axonal regeneration within acellular peripheral nerve grafts. J. Neurosci. 2002;22:10408–10415. doi: 10.1523/JNEUROSCI.22-23-10408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krekoski CA, Neubauer D, Zuo J, Muir D. Axonal regeneration into acellular nerve grafts is enhanced by degradation of chondroitin sulfate proteoglycan. J Neurosci. 2001;21:6206–6213. doi: 10.1523/JNEUROSCI.21-16-06206.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley JN, Anderson HK. The union of different kinds of nerve fibres. J Physiol. 1904;31:365–391. doi: 10.1113/jphysiol.1904.sp001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millesi H. Nerve grafting. Clin Plast Surg. 1984;11:105–113. [PubMed] [Google Scholar]
- Muir D, Engvall E, Varon S, Manthorpe M. Schwannoma cell-derived inhibitor of the neurite-promoting activity of laminin. J Cell Biol. 1989;109:2353–2362. doi: 10.1083/jcb.109.5.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadim W, Anderson PN, Turmaine M. The role of Schwann cells and basal lamina tubes in the regeneration of axons through long lengths of freeze-killed nerve grafts. Neuropathol Appl Neurobiol. 1990;16:411–421. doi: 10.1111/j.1365-2990.1990.tb01277.x. [DOI] [PubMed] [Google Scholar]
- Ochi M, Wakasa M, Ikuta Y, Kwong WH. Nerve regeneration in predegenerated basal lamina graft: The effect of duration of predegeneration on axonal extension. Exp Neurol. 1994;128:216–225. doi: 10.1006/exnr.1994.1130. [DOI] [PubMed] [Google Scholar]
- Osawa T, Tohyama K, Ide C. Allogeneic nerve grafts in the rat, with special reference to the role of Schwann cell basal laminae in nerve regeneration. J Neurocytol. 1990;19:833–849. doi: 10.1007/BF01186814. [DOI] [PubMed] [Google Scholar]
- Rezajooi K, Pavlides M, Winterbottom J, Stallcup WB, Hamlyn PJ, Lieberman AR, Anderson PN. NG2 proteoglycan expression in the peripheral nervous system: upregulation following injury and comparison with CNS lesions. Mol Cell Neurosci. 2004;25:572–584. doi: 10.1016/j.mcn.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Sondell M, Lundborg G, Kanje M. Regeneration of the rat sciatic nerve into allografts made acellular through chemical extraction. Brain Res. 1998;795:44–54. doi: 10.1016/s0006-8993(98)00251-0. [DOI] [PubMed] [Google Scholar]
- Sorensen J, Fugleholm K, Moldovan M, Schmalbruch H, Krarup C. Axonal elongation through long acellular nerve segments depends on recruitment of phagocytic cells from the near-nerve environment. Electrophysiological and morphological studies in the cat. Brain Res. 2001;903:185–197. doi: 10.1016/s0006-8993(01)02441-6. [DOI] [PubMed] [Google Scholar]
- Zalewski AA, Gulati AK. Evaluation of histocompatibility as a factor in the repair of nerve with a frozen nerve allograft. J Neurosurg. 1982;56:550–554. doi: 10.3171/jns.1982.56.4.0550. [DOI] [PubMed] [Google Scholar]
- Zuo J, Hernandez YJ, Muir D. Chondroitin sulfate proteoglycan with neurite-inhibiting activity is up-regulated following peripheral nerve injury. J Neurobiol. 1998;34:41–54. [PubMed] [Google Scholar]