Abstract
Retinal ischemia arises from circulatory failure. As the retinal blood vessels are key organs in circulatory failure, our aim was to study the retinal vasculature separately from the neuroretina to elucidate the role of hypoxia-inducible factor (HIF) 1α and 1β and vascular endothelial growth factor (VEGF) in retinal ischemia. Retinal ischemia was induced in porcine eyes by applying an intraocular pressure, followed by 12 h of reperfusion. HIF-1α mRNA expression was not affected by ischemia, while immunofluorescence staining was higher after ischemia in the neuroretina. HIF-1β immunoreactivity and mRNA expression were unaffected. VEGF protein levels in the vitreous humor and VEGF staining in the neuroretina were more pronounced in eyes subjected to ischemia than in the sham eyes. VEGF may be activated downstream of HIF-1 and is known to stimulate retinal neovascularization, which causes sight-threatening complications. These results emphasize the need for pharmacological treatment to block the HIF and VEGF signaling pathways in retinal ischemia.
Keywords: Blood vessels, Hypoxia-inducible factor, Ischemia, Porcine, Retina, Vascular endothelial growth factor
Introduction
Retinal ischemia due to local circulatory failure in diabetes, vein occlusion, and arterial occlusion is a major cause of sight-threatening complications and blindness [1]. As a result of retinal ischemia, new blood vessels are formed to meet the metabolic demands of the ischemic tissue. These newly formed blood vessels malfunction and are unable to supply the tissue with the necessary nutrients. They are not included in the blood–retina barrier and will thus leak and bleed. This leads to sight-threatening complications such as tractional retinal detachment, vitreous hemorrhage, neovascular glaucoma, and macular edema [1–3]. Today, retinal ischemia is treated by laser photocoagulation, which is effective in saving vision but at the expense of large portions of the retina and its photoreceptors. Although numerous studies have been performed on possible ways of limiting the extent of retinal injury after ischemia, there is still no effective pharmacological treatment for this condition [3, 4].
Most studies performed to date have focused on identifying neuroprotective agents for the treatment of retinal ischemia-reperfusion injury [1]. The blood vessels of the retina are key organs in local circulatory failure, and it may, therefore, be important not only to examine the neuroretina but also the retinal vasculature. For this purpose, we have recently set up and evaluated a porcine model of pressure-induced retinal ischemia in which the retinal blood vessels can be studied separately from the neuroretina. The porcine eye has previously been proven to be suitable for experimental analysis of the retinal blood vessels [5–7].
Hypoxia-inducible factor-1 (HIF-1) is a well-known complex involved in the intracellular signaling in response to hypoxia and ischemia. HIF-1 has been studied extensively and is known to play a key role in the development of cancer and angiogenesis [8]. Only a few groups have investigated the contribution of HIF in connection with retinal ischemia, and those studies have mainly involved small animals such as rodents [9, 10]. HIF-1 is a basic helix-loop-helix protein and acts as a transcription factor. It consists of one HIF-1α subunit and one HIF-1β subunit. Oxygen regulates the function, subcellular localization, and level of the HIF-1α units, while HIF-1β is expressed continuously [11]. HIF-1α is stabilized during hypoxia and enters the nucleus, regulating the expression of the target genes that are involved in, for example, metabolism, apoptosis, and angiogenesis [12, 13]. HIF-1α binds to HIF-1β, and together, they form a complex with a hypoxia-response element. This complex stimulates the transcription of around 70 genes that are upregulated by hypoxia, including vascular endothelial growth factor (VEGF) [12].
VEGF is known to promote angiogenesis in the retina and to stimulate the breakdown of the blood–retina barrier [12]. VEGF plays an important role in diabetic retinopathy during neovascularization [14]. Previous studies in mice have shown an increase in VEGF mRNA expression during a state of hypoxia [15]. Furthermore, increasing levels of HIF-1α in ischemic retinas in mice have been linked both temporally and spatially to increasing expression of VEGF in such a way that HIF-1α preceded the upregulation of VEGF [9].
The aim of the present study was to conduct a detailed study of the roles of HIF-1α, HIF-1β, and VEGF during and after retinal ischemia in porcine eyes. Ischemia was induced in the eyes of large pigs (70 kg) by increasing the intraocular pressure (IOP) above the systolic blood pressure for 1 h. Thereafter, the IOP was normalized, and reperfusion was allowed to proceed for 12 h. This is an established technique used to induce ischemia reperfusion in rodents and other smaller mammals [1]. The porcine eye closely resembles the human eyes since it has a primate-like structure and is, therefore, suitable for the separate analysis of the retinal arteries and the neuroretina [16, 17]. To determine the extent of retinal injury following ischemia reperfusion, retinal sections were examined histologically, and the glial reactivity was assessed by quantification of glial fibrillary acidic protein (GFAP) mRNA. The expression HIF-1α and HIF-1β mRNA levels were assessed in the neuroretina and retinal blood vessels using real-time PCR, and HIF-1α and HIF-1β protein expression levels were evaluated in the neuroretina using immunohistology (Table 1). VEGF protein expression was assessed in the neuroretina, retinal blood vessels, and vitreous humor, using immunohistology and an angiogenesis antibody array test.
Table 1.
Change in HIF-1α, HIF-1β, and VEGF mRNA and protein expression levels following retinal ischemia
| mRNA | ||
| HIF-1α mRNA | ±0 | Neuroretina |
| HIF-1β mRNA | ±0 | Neuroretina |
| HIF-1α mRNA | ±0 | Retinal blood vessels |
| HIF-1α mRNA | ±0 | Retinal blood vessels |
| Protein | ||
| HIF-1α protein | ↑ | Neuroretina |
| HIF-1β protein | ±0 | Neuroretina |
| VEGF protein | ↑ | Neuroretina |
| VEGF protein | ↑ | Vitreous |
Comprehensive table to illustrate the results from immunohistology, real-time PCR and an angiogenesis antibody array for evaluating the expression levels of HIF-1α, HIF-1β, and VEGF in the neuroretina, retinal blood vessels, and vitreous humor. The results are shown as change in ischemia-reperfusion versus sham-operated eyes
Material and methods
Animals and anesthesia
A total of 14 healthy landrace pigs with a mean body weight of 70 kg, of both genders, were used for this study (obtained from a conventional pig breeder, Lund, Southern Sweden). Before surgery, the animals were fasted overnight with free access to water. An intramuscular injection of ketamine (Ketaminol vetTM; Farmaceutici Gellini S.p.A, Aprilia, Italy), 100 mg/ml per 15 mg/kg bodyweight, was used in combination with xylazine (Rompun vetTM; Bayer AG, Leverkusen, Germany), 20 mg/ml per 2 mg/kg bodyweight, for premedication. After the induction of anesthesia with thiopental, 12.5 mg/kg bodyweight (Pentothal, Abbott, Stockholm, Sweden), the animals were orally intubated with cuffed endotracheal tubes. Anesthesia was maintained by continuous intravenous infusion of 20 mg/ml propofol (DiprivanTM; Astra Zeneca, Södertälje, Sweden) at a dose of 0.1–0.2 mg/kg/min, in combination with intermittent fentanyl (Fentanyl B. Braun Melsungen AG, Melsungen, Germany) at approximately 3.5 μg/kg/h.
Mechanical ventilation was established with a Siemens-Elema 900B ventilator in the volume-controlled mode and adjusted to obtain normocapnia. The animals were ventilated with a mixture of oxygen (70%) and dinitrous oxide (30%). During the procedure, the mean arterial blood pressure of the pigs was 92 ± 7 mmHg. After completion of the experiments, the animals were euthanized by a lethal injection of potassium, 2 mmol/kg (ADDEX potassium chloride, Fresenius KABI SE, Uppsala, Sweden). All procedures and animal treatment took place in accordance with the guidelines of the Ethics Committee of Lund University, the Institute for Laboratory Animal Research (Guide for the Care and Use of Laboratory Animals), and the ARVO statement on the Use of Animals in Ophthalmic and Vision Research. The study was approved by the County Administrative Court under the auspices of the Swedish Department of Agriculture.
Ischemia reperfusion
Ischemia was induced in the right eye of each pig by increasing the IOP above the systolic blood pressure. The posterior chamber of both eyes was cannulated with a 30-gauge needle, and the IOP was increased in the right eye to 80 mmHg by continuous infusion of a balanced salt solution for ophthalmic irrigation (AmoTM EndosolTM; AMO Groningen BV, Groningen, The Netherlands), verified by the use of a Tono-Pen®XL tonometer (Medtronic, Jacksonville, FL). The other eye served as a control, and the same surgical procedure was performed, except for elevation of the IOP (this is referred to as the “sham” eye in the text and figures). After 60 min, the cannulation needles were removed, and reperfusion of the retinal vasculature was allowed to take place for 12 h. Ischemia was confirmed by indirect ophthalmoscopic examination, during which interrupted blood flow was observed in the retinal arteries. This was confirmed directly after elevating the IOP, during ischemia (after 30 min), and at the end of the ischemic period (60 min).
Tissue preparation
After 12 h of reperfusion, both eyes of each pig were enucleated, including the optic nerve, under anesthesia. The eyes were dissected, the anterior segment was removed, and the vitreous humor collected. This was homogenized by aspiration through a Pasteur pipette and then centrifuged for 30 min at 15,000 rpm, at 4°C, to precipitate insoluble tissue. The vitreous homogenate was divided into 200 μl samples, snap frozen in liquid nitrogen, and stored at −80°C until further analysis. A slice along the superior–inferior axis containing the head of the optic nerve was excised and prepared for later use in immunofluorescence and histological studies (see below). The rest of the retina was dissected free from the sclera, retinal pigment epithelium and the choroid, and the neuroretina and blood vessels were isolated separately. The neuroretina was isolated by dissecting pieces lacking major blood vessels, and frozen at −80°C. Dissection was performed at 4°C in balanced salt solution (see above). After removing pieces of the neuroretina, the rest of the retina was placed in distilled water for 1 h on ice. During this time, the tissue was gently and repeatedly aspirated through a Pasteur pipette under magnification, taking care not to damage the blood vessels. The blood vessels were collected, frozen, and stored at −80°C until used in real-time PCR experiments.
Histology
Slices from both eyes were fixed in 4% paraformaldehyde for 4 h. After fixation, the tissue was rinsed in 0.1 M Sørensen’s phosphate buffer (28 mM NaH2PO4 and 72 mM Na2HPO4; pH 7.4) and then washed in the same solution with increasing concentrations of sucrose (10% to 25%). The specimens were embedded in 30% egg albumin and 3% gelatin and were stored at −80°C until sectioning. The specimens were sectioned at 12 µm in a cryostat (Microm HM500M; Thermo Scientific, Walldorf, Germany) and placed on microscope slides (Menzel, Braunschweig, Germany), three sections on each slide. The slides were allowed to dry at room temperature for 30 to 60 min and were then stored at −20°C until used. For the morphological analysis, sections taken within 1 mm of the edge of the optic nerve head were stained with hematoxylin and eosin, examined in a light microscope (Olympus BX60, Tokyo, Japan), and photographed using a digital camera (Pixera Pro 600ES, Los Gatos, CA).
Fluorescent immunohistochemistry
The slides were dried at room temperature for 15 min and permeabilized in phosphate-buffered saline (PBS) + 0.25% Triton X-100. Blocking solution, containing PBS + 0.25% Triton X-100 + 1% bovine serum albumin and an additional 5% normal serum, was added to the slides to avoid unspecific binding of antibodies. The slides were incubated for 1 h at room temperature. Specimens were incubated overnight at 4°C with the same blocking solution but with 2% normal serum, and the primary antibody of interest: 1:300 mouse monoclonal HIF-1α antibody (H9807-02; Biosite, San Diego, CA), 1:100 rabbit polyclonal HIF-1β antibody (#3718; Cell signaling Technology®, Boston, MA), 1:100 rabbit polyclonal VEGF (A-20) antibody (sc-152; Santa Cruz Biotechnology, Santa Cruz, CA), or/and 1:400 mouse monoclonal smooth muscle actin (SMA) antibody (sc-53015; Santa Cruz Biotechnology).
The slides were rinsed in PBS (3 × 15 min), incubated in blocking solution with the appropriate secondary antibody: 1:300 donkey anti-mouse Texas red (715-076-150; Jackson ImmunoResearch, West Grove, PA) or 1:100 swine anti-rabbit (F 0205; DakoCytomation, Glostrup, Denmark) at room temperature for 1 h. Then slides were washed in PBS (3 × 15 min) and mounted in anti-fading mounting medium (Vectashield; Vector Laboratories Inc., Burlingame, CA). Sections incubated without the primary or secondary antibody were used as negative controls to verify the lack of autofluorescence and unspecific secondary antibody staining. The staining intensity and location were analyzed using a light microscope equipped for fluorescence microscopy (Olympus BX60, Tokyo, Japan) and photographed with a digital camera mounted on the microscope (Pixera Pro 600ES, Los Gatos, CA). For the purpose of comparison, sections from ischemia-reperfusion eyes and the corresponding sham-operated eyes were processed at the same time to minimize variability.
RNA extraction
An Allprep DNA/RNA/Protein Mini Kit (Qiagen, Valencia, CA) was used for the extraction of RNA. Briefly, the tissue was homogenized in buffer RLT including β-mercaptoethanol (600 µl for neuroretina specimens and 350 µl for blood vessels), using a stainless steel bead and a TissueLyser (Qiagen). The stainless steel bead was removed, and the samples were centrifuged. The supernatant was then transferred to an Allprep DNA spin column and centrifuged. Ethanol (99.5%) was added to the flow-through (430 µl for neuroretina and 250 µl for blood vessels) and the samples transferred to an RNeasy spin column and centrifuged. On-column DNase digestion was performed according to the manufacturer’s instructions (RNase-Free DNase Set, Qiagen). The column was washed with RW1 buffer and RPE buffer, and the RNA was eluted in RNase-free water: 50 µl for neuroretina specimens and 30 µl for the blood vessels. Following extraction, the RNA concentration and purity (A260/A280 ratio) was measured at 260 nm using a spectrophotometer (TECAN, Männedorf, Switzerland). All samples were stored at −80°C until further analysis.
cDNA transcription and real-time polymerase chain reaction
Reverse transcription of total RNA to cDNA was carried out using the Taqman GeneAmp RNA polymerase chain reaction kit (N808-0234; Applied Biosystems, Foster City, CA) in a Perkin–Elmer DNA Thermal Cycler (Perkin–Elmer, Applied Biosystems). cDNA was synthesized from 1 µg total RNA from neuroretina and 0.5 µg total RNA from blood vessels in a 50-µl reaction with a master mix containing a reverse transcriptase and random hexamers that act as primers. The reaction was run at 25°C for 10 min, 37°C for 60 min, and 95°C for 5 min. Following reverse transcription, 50 µl ddH2O was added to neuroretina specimens (but not to the blood vessel specimens) to a final volume of 100 µl, and all the cDNA samples were divided into aliquots of 10 µl and stored at −80°C.
Real-time PCR was performed in a GeneAmp 7300 Real-Time PCR system, using the Gene Amp Power SYBR® Green kit (Applied Biosystems). The cDNA obtained as described above was used as a template in a 25-µl reaction. A control without template was included in all experiments. Forward and reverse primers were designed as follows, using the Primer3 Input 0.4.0 software [18].
- HIF-1α (GenBank number: NM001123124)
- Forward: 5′-GCCAGAACCTCCTGTAACCA-3′
- Reverse: 5′-AGGCTGTCCGACTTCCAGTA-3′
- HIF-1β (GenBank number: AY485671)
- Forward: 5′-GCCATTGTTCAGAGGGCTAT-3′
- Reverse: 5′-CCGCCGTTCAATTTCACATAT-3′
- VEGF (GenBank number: X81380)
- Forward: 5′-TGCAGATTATGCGGATCAAA-3′
- Reverse: 5′-AAATGCTTTCTCCGCTCTGA-3′
- GFAP (GenBank number: AJ551395)
- Forward: 5′-CAGCGGCCCTGAGAGAGAT-3′
- Reverse: 5′-TGTTAGGTCCGCAAACTTGGA-3′
The results were calculated relative to the amount of the housekeeping gene β-actin and elongation factor-1α (EF-1α), since these are believed to be continuously expressed at constant amounts in cells [19]. The primers were designed as follows:
- EF-1α (GenBank number: AM040195)
- Forward: 5′-GCTGACTGTGCTGTCCTGATT-3′
- Reverse: 5′-TGTAGGCCAGAAGAGCATGCT-3′
- β-actin (GenBank number: U07786)
- Forward: 5′-CCTTCAACTCGATCATGAAGTGC-3′
- Reverse: 5′-AGAGGTCCTTCCTGATGTCC-3′
The forward and reverse primers were dissolved in water according to the manufacturer’s instructions, and a mixture of forward and reverse primers was made. A master mix containing RNase-free water, Power SYBR® Green, and the chosen forward and reverse primers (at a final concentration of 200 nM) was added to the wells. One microliter cDNA from specimens containing neuroretina (sham-operated and ischemia-reperfusion eyes) or retinal blood vessels (sham-operated and ischemia-reperfusion eyes) was added to the wells. The real-time PCR was performed with the following profile: 1 cycle at 50°C for 2 min and 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. This was followed by a dissociation step: 95°C for 15 s, 60°C for 30 s, and 95°C for 15 s. One standard curve was constructed for each pair of primers to confirm that the primers were optimal and that the cDNA had been amplified with the same efficiency during the real-time PCR process, and the values of CT were plotted against the cDNA concentration on the basis of the following equation:
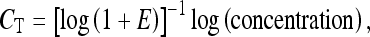 |
where E is the amplification efficiency, with the optimal value of 1. The amount of HIF-1 and VEGF mRNA in the specimens was calculated relative to the amount of β-actin and EF-1α mRNA in the same sample using the relation:
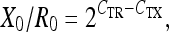 |
where X0 is the original amount of HIF-1α and HIF-1β mRNA separately, R0 is the original amount of β-actin/EF-1α mRNA, CTR is the CT value for β-actin/EF-1α and CTX is the CT value for HIF-1α and HIF-1β.
Angiogenesis antibody array
VEGF protein levels were determined in vitreous extracts from sham-operated and ischemia-reperfusion eyes using angiogenesis antibody array membranes (MA6310; Panomics, Fremont, CA). The assay was performed according to the manufacturer’s instructions. Protein spots on membranes were visualized using a Fujifilm LAS-1000 Luminescent Image Analyzer (Fujifilm, Stamford, CT, USA). Quantification of the spot intensity was performed using Image J software [20].
Calculations and statistics
Statistical analysis was performed using paired Student’s ratio t test. Calculations and statistical analysis were performed using GraphPad Prism 5.0 software. Results are presented as means ± the standard error of the mean (SEM).
Results
Gross histology and GFAP expression
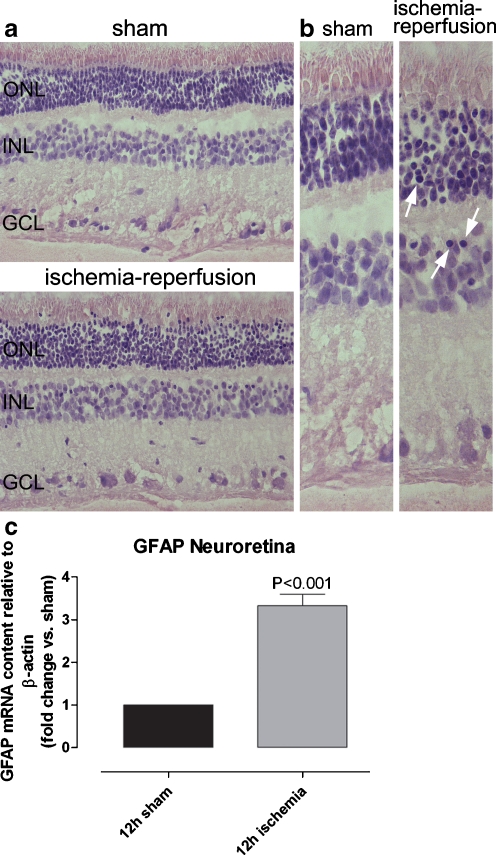
In the hematoxylin–eosin-stained sections of retina, pyknotic cell nuclei were observed in both the neuroretina and in the retinal blood vessels from ischemia-reperfusion eyes, suggesting retinal injury (Fig. 1). No pyknotic cell nuclei were seen in the retinas from the sham-operated eyes. Pyknotic cell nuclei were observed in the retinal outer nuclear layer (ONL), in the inner nuclear layer (INL), and in the ganglion cell layer (GCL). No other apparent morphological differences were observed between the sham-operated and ischemia-reperfusion eyes.
Fig. 1.
Hematoxylin-eosin-stained sections of neuroretina and GFAP levels in the neuroretina. a, b Histopathological examination of hematoxylin–eosin-stained sections of porcine neuroretina from the eye exposed to ischemia-reperfusion and the corresponding sham-operated eye using light microscopy. Pyknotic cell nuclei can be seen throughout the retinal sections, including the ONL, INL, and GCL of the ischemia-reperfusion eyes (arrows), but not in the corresponding sham-operated eyes. b Enlargements of pictures in (a). c GFAP mRNA expression in neuroretina from sham-operated and ischemia-reperfusion eyes, assessed using real-time PCR (n = 8). The mRNA levels were calculated relative to the housekeeping gene β-actin. Results are expressed as mean ± SEM of the fold changes in the neuroretina from ischemia-reperfusion eyes versus the sham-operated eyes. Statistical analysis was performed using paired Student’s ratio t test. Note the presence of pyknotic cell nuclei and the high GFAP mRNA levels after ischemia reperfusion
The levels of GFAP mRNA were higher (P < 0.001) in the neuroretinas from ischemia-reperfusion eyes than in those from the sham-operated eyes (Fig. 1), indicating glial reactivity. Similar patterns of GFAP mRNA expression were observed when using β-actin and EF-1α as reference genes, indicating that both these genes were reliable references.
HIF-1α and HIF-1β
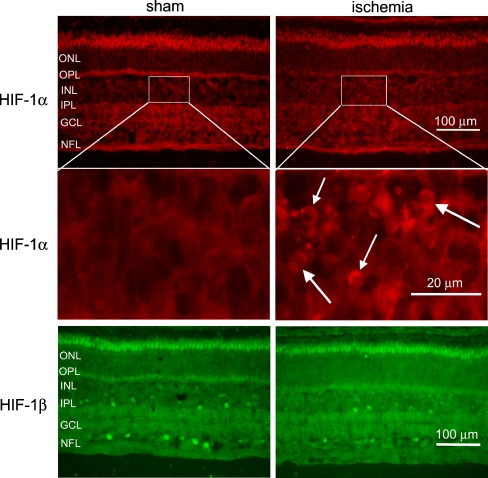
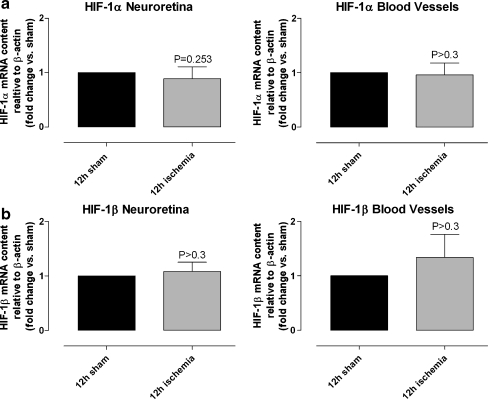
In the immunohistological examinations, HIF-1α was expressed in the cell nuclei in the INL of the neuroretinas from ischemia-reperfusion eyes, while no such staining was observed in the neuroretinas from the corresponding sham-operated eye (Fig. 2). Real-time PCR experiments demonstrated similar levels of HIF-1α mRNA expression in the sham-operated and ischemia-reperfusion eyes, in both the neuroretina (P = 0.253) and the blood vessel (P > 0.3) specimens (Fig. 3).
Fig. 2.
HIF-1α and HIF-1β immunoreactivity in the neuroretina. The top panels show representative examples of immunohistological images from eight pigs, showing HIF-1α staining in the neuroretina from sham-operated and ischemia-reperfusion eyes. The middle panels show enlargements of the top panels. HIF-1α staining was observed in the INL in retinas from ischemia-reperfusion eyes (arrows), while no such staining was seen in the neuroretinas from the corresponding sham-operated eyes. Similar results were found in all the pigs studied. The bottom panels show staining for HIF-1β in the neuroretina from sham-operated and ischemia-reperfusion eyes. Similar HIF-1β staining intensities and distributions were observed in the INL and GCL in the neuroretinas from sham-operated and ischemia-reperfusion eyes. ONL outer nuclear layer, OPL outer plexiform layer, INL inner nuclear layer, IPL inner plexiform layer, GCL ganglion cell layer, and NFL nerve fiber layer
Fig. 3.
HIF-1α and HIF-1β mRNA levels in the neuroretina and in the retinal blood vessels. a HIF-1α mRNA and b HIF-1β mRNA expression in the neuroretina and the retinal blood vessels from sham-operated and ischemia-reperfusion eyes, assessed using real-time PCR (n = 5 in all cases). The mRNA levels were calculated relative to the housekeeping gene β-actin. Results are expressed as the mean ± SEM of the fold changes in the neuroretina and in the retinal blood vessels from ischemia-reperfusion versus sham-operated eyes. Statistical analysis was performed using paired Student’s ratio t test. No difference was found in the levels of HIF-1α mRNA and HIF-1β mRNA expression between sham-operated and ischemia-reperfusion eyes
No difference was observed in the level of HIF-1β mRNA expression between retinas from sham-operated and ischemia-reperfusion eyes, in either the neuroretina (P > 0.3) or the retinal blood vessel (P > 0.3) specimens (Fig. 3). Furthermore, HIF-1β staining intensities were similar in the neuroretinas from sham-operated and ischemia-reperfusion eyes (Fig. 2). The staining of HIF-1β was observed in the cell nuclei of a small population of cells in the GCL and a small population of cells in the INL (Fig. 2).
VEGF
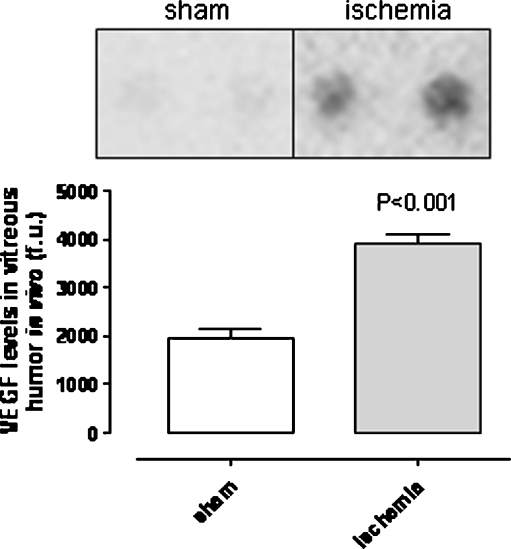
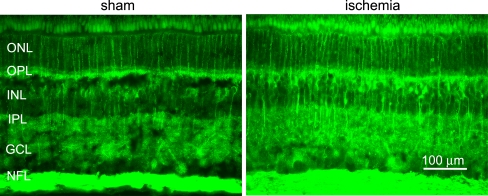
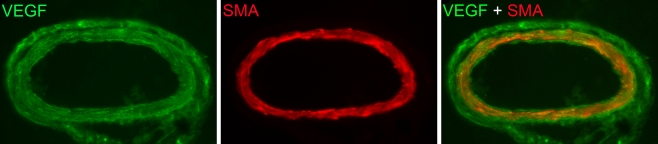
The angiogenesis antibody array showed that VEGF protein levels were higher in vitreous humor from ischemia-reperfusion eyes than in that from sham-operated eyes (Fig. 4). Immunohistology showed higher VEGF staining intensities in sections of the retina from ischemia-reperfusion than from sham-operated eyes. VEGF staining was restricted to the radial processes of Müller cells and the inner retina, probably corresponding to Müller cell endfeet and astrocytes (Fig. 5). VEGF staining of the retinal blood vessels showed localization to the smooth muscle cell layer of the retinal arteries (Fig. 6). This was verified by double staining with a SMA antibody, which is known to be a marker of smooth muscle cells.
Fig. 4.
Protein levels of VEGF in the vitreous humor. VEGF protein levels in vitreous humor from sham-operated and ischemia-reperfusion eyes. Extracts of vitreous humor were incubated on an angiogenesis antibody array membrane (see “Materials and Methods”). The upper panel shows a representative example of the array membrane after incubation with vitreous humor. The bottom panel shows the mean values ± SEM obtained from the quantification of the spot intensity (n = 6). Statistical analysis was performed using paired Student’s ratio t test. Note that VEGF protein levels in the vitreous humor are higher following ischemia reperfusion
Fig. 5.
VEGF immunoreactivity in the neuroretina. Immunohistological images showing VEGF staining in the neuroretina from sham-operated and ischemia-reperfusion eyes. VEGF staining was detected in the radial processes of Müller cells and in the inner retina, probably corresponding to Müller cell endfeet and astrocytes. VEGF staining was more intense in retinas from eyes subject to ischemia and reperfusion than sham-operated eyes. Similar results were seen in all pigs. ONL outer nuclear layer, OPL outer plexiform layer, INL inner nuclear layer, IPL inner plexiform layer, GCL ganglion cell layer, and NFL nerve fiber layer
Fig. 6.
VEGF immunoreactivity in the retinal blood vessels. Immunohistological images showing VEGF staining in the smooth muscle cell layer of a retinal artery, verified by double staining with SMA
Discussion
Most studies on retinal ischemia performed to date have focused on treatment using neuroprotective agents. The aim of this study, however, was to separately investigate the retinal vasculature and neuroretina in order to elucidate the role of HIF-1α, HIF-1β, and VEGF in retinal ischemia.
The results presented here indicate that the retinal ischemia induced in the porcine model used resulted in retinal injury. Ischemia was induced by raising the IOP to 80 mmHg by continuous infusion of a balanced salt solution for ophthalmic irrigation. GFAP mRNA levels were higher in neuroretinas from ischemia-reperfusion eyes than in sham-operated eyes, indicating that an insult triggering a molecular response had taken place. It is well known that the expression of intermediate filaments such as GFAP is increased in retinal glial cells in reaction to injury, including ischemia [21–23]. Histopathological analysis revealed large numbers of pyknotic cell nuclei indicating cell death in retinas from eyes subjected to ischemia and reperfusion, while none was observed in the sham-operated eyes. These findings show that the intervention was sufficient to produce retinal ischemic injury, and we proceeded with further analyses of the cellular events involved.
The intensity of HIF-1α immunofluorescence staining was greater in the neuroretinas from ischemia-reperfusion eyes than in the corresponding sham-operated eyes. HIF-1α is known to increase under hypoxic conditions, which has hitherto mainly been studied in other parts of the body [24, 25]. In cardiac ischemia, HIF-1 stimulates angiogenesis and blood flow and is believed to prevent cardiac damage as a result of increased oxygenation [24]. HIF-1 also plays a key role in stimulating the vascularization of tumors in response to hypoxia. Inhibition of the HIF-1 has been found to suppress the progression of cancer [8]. It has also been shown to play a role in inflammation and the overexpression of HIF-1α in vivo results in increased inflammation [26].
The role of HIF in connection with retinal ischemia has been little studied, and the studies conducted have mainly involved small animals and rodents. In a study using mice [9], immunohistochemical analysis showed increased staining for HIF-1α throughout the hypoxic inner retina, while HIF-1β levels remained unchanged. In a study using cultured ARPE-19 cells [27], HIF-1α protein levels also seemed to increase during the initial phase of hypoxia. These findings are in accordance with the results obtained in the present study. In our study, HIF-1α protein levels were higher in neuroretinas from ischemia-reperfusion eyes than from sham-operated eyes. On the other hand, no difference was found in the HIF-1α mRNA expression between ischemia-reperfusion and sham-operated eyes. These results suggest increased translation to HIF-1α protein, while the transcription of HIF-1α mRNA remains unchanged. This is in line with the general belief that HIF-1α protein is produced during both normoxic and hypoxic conditions, and the rate of degradation is controlled by the oxygen tension in the tissue [12]. Proline hydroxylase enzymes hydroxylate HIF-1α and facilitate the interaction between HIF-1α and von Hippel–Lindau protein. This interaction results in HIF-1α protein being tagged for ubiquitination and is degraded via the proteasomal pathway. When hypoxia is induced, the activity of proline hydroxylase enzymes decreases, which stabilizes HIF-1α, and the levels thus increase. HIF-1α is then transported from the cytoplasm into the nucleus where it forms a dimer with HIF-1β. This complex then binds to hypoxia-response elements in the promoter or enhancer regions of different genes [12].
HIF-1β staining intensities were similar in the neuroretinas from sham-operated and ischemia-reperfusion eyes. Furthermore, HIF-1β mRNA levels were similar in sham-operated and ischemia-reperfusion eyes, in both the neuroretina and the retinal blood vessels. This is in line with previous work showing that oxygen regulates the expression of HIF-1α, while HIF-1β is expressed continuously [11].
The results of the present study show that the VEGF protein levels are higher in vitreous humor from ischemia-reperfusion than sham-operated eyes. This is in accordance with previous studies of VEGF in retinal ischemia. In a study using mice, VEGF mRNA expression was found to increase during a state of hypoxia [15]. VEGF is known to promote angiogenesis in the retina and to stimulate the breakdown of the blood–retina barrier [12]. VEGF has also been shown to play an important role in diabetic retinopathy during the development of neovascularization [14].
HIF-1 is known to upregulate VEGF following hypoxia, and the expression of VEGF has been shown to be increased in regions of tumors with poor blood flow [9]. This has caused attention to be focused on VEGF as a potential mediator of retinal neovascularization. Indeed, the levels of HIF-1α protein are increased in ischemic retinas, and the expression is linked both temporally and spatially to elevated levels of VEGF mRNA in mice [9]. The findings of the present experiments further confirm that HIF-1α precedes the upregulation of VEGF.
The aim of the present study was to conduct a detailed study of the roles of HIF-1α, HIF-1β, and VEGF during and after retinal ischemia in porcine eyes. Ischemia was induced in the eyes of large pigs (70 kg) by increasing the IOP above the systolic blood pressure for 1 h. Thereafter, the IOP was normalized, and reperfusion was allowed to proceed for 12 h. This is an established technique used to induce ischemia reperfusion in rodents and other smaller mammals [1]. The porcine eye closely resembles the human eyes since it has a primate-like structure and is, therefore, suitable for the separate analysis of the retinal arteries and the neuroretina [16, 17]. To determine the extent of retinal injury following ischemia and reperfusion, retinal sections were examined histologically, and the glial reactivity was assessed by quantification of GFAP mRNA. The expression HIF-1α and HIF-1β mRNA levels were assessed in the neuroretina and retinal blood vessels using real-time PCR, and HIF-1α and HIF-1β protein expression levels were evaluated in the neuroretina using immunohistology. VEGF protein expression was assessed in the neuroretina, retinal blood vessels, and vitreous humor, using immunohistology and an angiogenesis antibody array test.
In conclusion, the results of the present study show that the retinal ischemia induced in this porcine model by high IOP causes substantial injury to the retina, with elevated GFAP levels and evident pyknotic cell nuclei. HIF-1α protein levels increased, while HIF-1β levels remained unchanged following ischemia and reperfusion. Furthermore, VEGF expression was observed increased following ischemia. These results are consistent with the general belief that HIF-1α is stabilized during hypoxia, binds to HIF-1β (which is continuously expressed), and enters the nucleus to regulate the expression of the target genes that are involved, for example, in angiogenesis. The increase in VEGF expression may be from activation downstream of the HIF-1 complex. VEGF is known to stimulate retinal neovascularization, which is known to cause sight-threatening complications. These results emphasize the importance of pharmacological agents that can block the HIF/VEGF signaling pathways in the treatment of retinal injury following ischemia.
Acknowledgements
This study was supported in part by the Swedish Medical Research Council, Lund University Faculty of Medicine, the Swedish Government Grant for Clinical Research, the Lund University Hospital Research Grants, the Swedish Medical Association, the Royal Physiographic Society in Lund, the Åke Wiberg Foundation, the Anders Otto Swärd Foundation/Ulrika Eklund Foundation, the Magn Bergvall Foundation, the Crafoord Foundation, the Anna-Lisa and Sven-Erik Nilsson Foundation, Jeansson’s Foundation, Kronprinsessan Margaretas Arbetsnämnd för synskadade, Synskadade i Malmöhus län, Anna and Edvin Berger’s Foundation, the Märta Lundqvist Foundation, and the Lars Hierta Memorial Foundation.
Competing interests statement The authors declare that they have no competing financial interests.
References
- 1.Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004;23(1):91–147. doi: 10.1016/j.preteyeres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Curtis TM, Scholfield CN. The role of lipids and protein kinase Cs in the pathogenesis of diabetic retinopathy. Diabetes Metab Res Rev. 2004;20(1):28–43. doi: 10.1002/dmrr.431. [DOI] [PubMed] [Google Scholar]
- 3.Dorrell M, Uusitalo-Jarvinen H, Aguilar E, Friedlander M. Ocular neovascularization: basic mechanisms and therapeutic advances. Surv Ophthalmol. 2007;52(Suppl 1):S3–19. doi: 10.1016/j.survophthal.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Comer GM, Ciulla TA. Pharmacotherapy for diabetic retinopathy. Curr Opin Ophthalmol. 2004;15(6):508–18. doi: 10.1097/01.icu.0000143685.60479.3b. [DOI] [PubMed] [Google Scholar]
- 5.Hessellund A, Jeppesen P, Aalkjaer C, Bek T. Characterization of vasomotion in porcine retinal arterioles. Acta Ophthalmol Scand. 2003;81(3):278–82. doi: 10.1034/j.1600-0420.2003.00063.x. [DOI] [PubMed] [Google Scholar]
- 6.Holmgaard K, Aalkjaer C, Lambert JD, Bek T. N-methyl-d-aspartic acid causes relaxation of porcine retinal arterioles through an adenosine receptor dependent mechanism. Invest Ophthalmol Vis Sci. 2008;49(10):4590–4594. doi: 10.1167/iovs.08-1890. [DOI] [PubMed] [Google Scholar]
- 7.Nagaoka T, Hein TW, Yoshida A, Kuo L. Resveratrol, a component of red wine, elicits dilation of isolated porcine retinal arterioles: role of nitric oxide and potassium channels. Invest Ophthalmol Vis Sci. 2007;48(9):4232–9. doi: 10.1167/iovs.07-0094. [DOI] [PubMed] [Google Scholar]
- 8.Chavez A, Miranda LF, Pichiule P, Chavez JC. Mitochondria and hypoxia-induced gene expression mediated by hypoxia-inducible factors. Ann N Y Acad Sci. 2008;1147:312–20. doi: 10.1196/annals.1427.021. [DOI] [PubMed] [Google Scholar]
- 9.Ozaki H, Yu AY, Della N, Ozaki K, Luna JD, Yamada H, et al. Hypoxia inducible factor-1alpha is increased in ischemic retina: temporal and spatial correlation with VEGF expression. Invest Ophthalmol Vis Sci. 1999;40(1):182–9. [PubMed] [Google Scholar]
- 10.Zhu Y, Zhang Y, Ojwang BA, Brantley MA, Jr, Gidday JM. Long-term tolerance to retinal ischemia by repetitive hypoxic preconditioning: role of HIF-1alpha and heme oxygenase-1. Invest Ophthalmol Vis Sci. 2007;48(4):1735–43. doi: 10.1167/iovs.06-1037. [DOI] [PubMed] [Google Scholar]
- 11.Weinberg RA. Biology of cancer. London: Taylor & Francis; 2006. [Google Scholar]
- 12.Arjamaa O, Nikinmaa M. Oxygen-dependent diseases in the retina: role of hypoxia-inducible factors. Exp Eye Res. 2006;83(3):473–83. doi: 10.1016/j.exer.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Thiersch M, Raffelsberger W, Frigg R, Samardzija M, Wenzel A, Poch O, et al. Analysis of the retinal gene expression profile after hypoxic preconditioning identifies candidate genes for neuroprotection. BMC Genomics. 2008;9:73. doi: 10.1186/1471-2164-9-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331(22):1480–7. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 15.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A. 1995;92(3):905–9. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buckley CH, Hadoke PW, O'Brien CJ. Use of isolated ocular arteries in vitro to define the pathology of vascular changes in glaucoma. Br J Ophthalmol. 1997;81(7):599–607. doi: 10.1136/bjo.81.7.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rootman J. Vascular system of the optic nerve head and retina in the pig. Br J Ophthalmol. 1971;55(12):808–19. doi: 10.1136/bjo.55.12.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, editor. Bioinformatics methods and protocols: methods in molecular biology. Totowa: Humana; 2000. pp. 365–86. [DOI] [PubMed] [Google Scholar]
- 19.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25(2):169–93. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 20.Abràmoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics Int. 2004;11(7):36–42. [Google Scholar]
- 21.Bignami A, Dahl D. The radial glia of Muller in the rat retina and their response to injury. An immunofluorescence study with antibodies to the glial fibrillary acidic (GFA) protein. Exp Eye Res. 1979;28(1):63–9. doi: 10.1016/0014-4835(79)90106-4. [DOI] [PubMed] [Google Scholar]
- 22.Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, et al. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25(4):397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Iandiev I, Uckermann O, Pannicke T, Wurm A, Tenckhoff S, Pietsch UC, et al. Glial cell reactivity in a porcine model of retinal detachment. Invest Ophthalmol Vis Sci. 2006;47(5):2161–71. doi: 10.1167/iovs.05-0595. [DOI] [PubMed] [Google Scholar]
- 24.Kim HA, Mahato RI, Lee M. Hypoxia-specific gene expression for ischemic disease gene therapy. Adv Drug Deliv Rev. 2009;61:614–22. doi: 10.1016/j.addr.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Yi JH, Park SW, Kapadia R, Vemuganti R. Role of transcription factors in mediating post-ischemic cerebral inflammation and brain damage. Neurochem Int. 2007;50(7–8):1014–27. doi: 10.1016/j.neuint.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ziello JE, Jovin IS, Huang Y. Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J Biol Med. 2007;80(2):51–60. [PMC free article] [PubMed] [Google Scholar]
- 27.Forooghian F, Razavi R, Timms L. Hypoxia-inducible factor expression in human RPE cells. Br J Ophthalmol. 2007;91(10):1406–10. doi: 10.1136/bjo.2007.123125. [DOI] [PMC free article] [PubMed] [Google Scholar]