Abstract
Tumor necrosis factor-alpha (TNF-α) is a pleiotropic inflammatory cytokine. Tumor necrosis factor-alpha was evaluated in the serum samples of patients with idiopathic retinal periphlebitis in young adults (Eales’ disease). Retinal periphlebitis was graded according to a new grading system based on severity of inflammation (grade 1–4). Quantification of the TNF-α levels was carried out using ELISA kit in the serum samples of young adults with idiopathic retinal periphlebitis (n = 17) and healthy controls (n = 17) of similar age. Tumor necrosis factor-α level was found to be significantly raised in cases with retinal periphlebitis as compared with controls (p < 0.001). Higher levels of TNF-α were found to be associated with increased severity of retinal periphlebitis. Tumor necrosis factor-α represents a novel target for controlling inflammatory activity in idiopathic retinal periphlebitis. Higher levels of TNF-α, in association with the increased severity of retinal periphlebitis, have implications for early anti-TNF-α therapy.
Keywords: Cytokine, Idiopathic retinal periphlebitis, Eales’ disease, Tumor necrosis factor-α, Anti-TNF-α therapy
Introduction
Idiopathic retinal periphlebitis (Eales’ disease) primarily affects the peripheral retina of young adult males [1]. Retinal changes are characterized by periphlebitis, peripheral non-perfusion, and neovascularization [2, 3]. Idiopathic retinal periphlebitis is an immunologic reaction triggered by the exposure of an exogenous agent. Autoimmunity to retinal antigens plays an important role in the etiopathogenesis. An extraneous agent results in the exposure of normally sequestered uveitopathogenic antigens of the immune system leading to an immune response in the eye, which may initiate the disease process [4]. Resulting oxidative stress leads to alterations in membrane fluidity and thereby retinal photoreceptors dysfunction [5].
Idiopathic retinal periphlebitis is distinctively characterized by stage of inflammation as well as stage of proliferation [6]. Hypoxia-induced expression of vascular endothelial growth factor is an aspect of the complicated processes involved in intraocular neovascularization [7]. Chemokines have also been found to play a role in intraocular neovascularization [8]. Further, cytokines play an important role in intraocular inflammation [9, 10]. Additionally, the cascade of multiple angiogenic cytokines induced by oxidative damage may interact to promote sustained retinal neovascularization [11].
Cytokines, low-MW protein mediators that usually act at short range between neighboring cells, are involved in essentially every important biological process. Tumor necrosis factor-α is a pleiotropic inflammatory cytokine which is produced by various types of cells, but especially by macrophages. It is an acute phase protein which initiates a cascade of cytokines and increases vascular permeability [12]. Our recent study found that Interleukin-1 and TNF-α play a significant role in causation of retinal periphlebitis and neovascularization in idiopathic retinal periphlebitis [13]. In the present study, we have evaluated TNF-α levels in the serum samples of cases with idiopathic retinal periphlebitis in young adults (Eales’ disease) and studied its association with increased severity of periphlebitis.
Material and methods
Patients
The study group consisted of 17 consecutive new patients of idiopathic retinal periphlebitis, presenting in the retina clinic of our tertiary care center. All the cases were males. Seventeen healthy controls (all males of similar age) presenting for refraction in the outpatient department were included in this study. They were not on any medication and did not show tuberculin skin test reactivity. Informed consent was obtained from all patients and controls prior to their inclusion in this study. Systemic disorders such as tuberculosis, diabetes mellitus, sickle cell hemoglobinopathy, blood dyscrasias, sarcoidosis, and collagen vascular diseases were ruled out after proper history examination and investigations (chest X-ray, fasting and postprandial blood sugar, sickle cell preparation, hemoglobin, hematocrit, total red and white blood cell count, differential count, erythrocyte sedimentation rate, serum angiotensin converting enzyme, antinuclear antibody, and ELISPOT). A complete ophthalmological assessment (including LogMAR visual acuity assessment, slit lamp biomicroscopy, indirect ophthalmoscopy, and fluorescein angiography) of the cases was done. Cases were eligible for this study, if they: (a) had not undergone laser photocoagulation, (b) had not received oral steroids within the last 6 months, (c) had not been on any immunosuppressive therapy, and (d) were non-smoker and non-alcoholic.
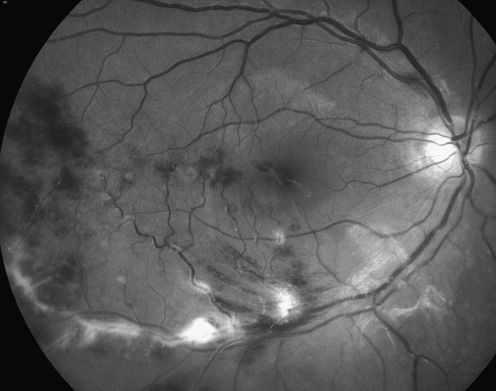
Retinal periphlebitis was graded according to a new grading system, based on the severity of inflammation, into grades 1–4 (Table 1). Retinal periphlebitis in all the cases was graded according to this new grading system by two independent observers (Fig. 1).
Table 1.
Grades of idiopathic retinal periphlebitis in young adults (n = 17) and association with tumor necrosis factor-α levels (pg/ml)
| Grades | Description | Tumor necrosis factor-α |
|---|---|---|
| Grade 1 (n = 4) | Periphlebitis of small caliber vessels with superficial retinal hemorrhages | 14.3–16.2 |
| Grade 2 (n = 5) | Periphlebitis of large caliber vessels with superficial retinal hemorrhages | 15.1–19.8 |
| Grade 3 (n = 8) | Periphlebitis of large caliber vessels with macular edema | 19.2–22.4 |
| Grade 4 | Central periphlebitis | – |
Fig. 1.
Red-free fundus photograph shows grade 3 idiopathic retinal periphlebitis in a young adult
TNF-α estimation
ELISA kits for TNF-α were purchased from eBioscience, San Diego, CA, USA.
Serum samples Five milliliters of venous blood was drawn in a disposable syringe. Blood samples were processed for serum separation by centrifugation under sterile conditions. Serum samples were stored at −80°C until used for TNF-α analysis.
Methodology For the quantification of the levels of TNF-α in the serum samples of patients, sandwich ELISA was performed as per the protocol provided by the manufacturer. Briefly, 96-well plates were coated with coating buffer (100 μl/well) and incubated overnight at 4°C. Following incubation, wells were washed thrice with wash buffer, and the plates were dried by inverting them on to the absorbent paper. Blocking was carried out with sample diluent and incubated for 1 h at room temperature. Plates were again washed as stated earlier. After blocking, cytokine standard (100 μl/well) or test samples (100 μl/well) were added to wells and incubated at room temperature for 2 h. Then the contents of the wells were aspirated followed by three washings as detailed above. Detection antibody (100 μl/well) was then added, and plates were further incubated for 1 h at room temperature. The contents were aspirated again, and plate was washed. Avidin-HRP conjugate was added (100 μl/well) to each well and incubated for 30 min at room temperature. It was then aspirated, and wells were washed seven times with wash buffer. Finally, substrate solution (100 μl/well) was added to each well and incubated for 15 min at room temperature. Following the incubation, stop solution (50 μl/well) was added to each well and plates were read at 570 and 450 nm wavelengths, and the OD values at 570 nm were subtracted from the OD values at 450 nm and the data was analyzed with the help of standard curve plotted for this purpose to calculate quantity of TNF-α (pg/ml).
Statistical analysis
Data of TNF-α are presented as mean ± SD. STATA 9.2 statistical software package (StataCorp, College Station, TX, USA) was used to analyze the data. Two-tailed t test was used to test the level of significance between the two groups. Differences were considered significant if “p” value was found to be <0.05. Interobserver correlation, for grading retinal periphlebitis, was computed using kappa statistics.
Results
The mean age of the cases and the controls were 21.9 ± 2.3 and 22.3 ± 1.4 years, respectively. The cases and the controls did not differ in age (p > 0.05). None of the cases or the controls had undetectable levels of TNF-α, and we were able to detect TNF-α in all samples tested. Tumor necrosis factor-α level were found to be significantly increased in the serum samples of the cases as compared to controls (p < 0.001; Table 2). Grade 1–3 retinal periphlebitis was observed in our cases. Central periphlebitis (grade 4) was not observed in any of the cases. Interobserver correlation was computed as 0.8. Higher levels of TNF-α were found to be associated with increased severity of retinal periphlebitis (Table 1).
Table 2.
Tumor necrosis factor-α levels (pg/ml) in the inflammatory stage of idiopathic retinal periphlebitis in young adults
| Groups | Number | (Mean ± SD) |
|---|---|---|
| Cases | 17 | 19.63 ± 2.75 |
| Controls | 17 | 11.41 ± 1.88 |
Discussion
In our study, increased levels of TNF-α were found to be associated with increased severity of idiopathic retinal periphlebitis in young adults, for the first time. Tumor necrosis factor-α was found to represent a novel target for controlling inflammatory activity in idiopathic retinal periphlebitis.
Tumor necrosis factor is primarily produced as a 212-amino acid-long type II transmembrane protein arranged in stable homotrimers [14, 15]. From this membrane-integrated form, the soluble homotrimeric cytokine (sTNF) is released via proteolytic cleavage by the metalloprotease TNF-α converting enzyme [16]. The soluble 51 kDa trimeric sTNF tends to dissociate at concentrations below the nanomolar range, thereby losing its bioactivity. Dysregulation and, in particular, overproduction of TNF have been implicated in a variety of human diseases [17]. Two receptors, TNF-R1 (p55 receptor) and TNF-R2 (p75 receptor), bind to TNF. Upon contact with their ligand, TNF receptors form trimers. This binding causes a conformational change in the receptor, and three pathways may be initiated [18, 19]. Firstly, activation of nuclear factor-kB mediates the transcription of a vast array of proteins involved in cell survival and proliferation, inflammatory response, and anti-apoptotic factors. Tumor necrosis factor-α, by targeting the endothelium, initiates the cascade of inflammatory mediator production. Secondly, activation of the mitogen-activated protein kinases pathways is involved in cell differentiation, proliferation, and is generally pro-apoptotic. Thirdly, like all death-domain-containing members of the TNFR superfamily, TNF-R1 is involved in cell death signaling [20]. Tumor necrosis factor-induced cell death plays only a minor role compared to its overwhelming contribution to the inflammatory process [21].
Our data suggests that, clinically, higher grade of idiopathic retinal periphlebitis in young adults is associated with higher level of TNF-α. Based upon the knowledge of the pathogenic pathways for TNF-α [18, 19], this cytokine initiates production of a cascade of inflammatory mediators during the inflammatory stage of idiopathic retinal periphlebitis in young adults. Our earlier study had highlighted that persistent increased levels of TNF-α were associated with the proliferative stage of idiopathic retinal periphlebitis in young adults [13]. Higher levels of TNF-α may convert inflammatory stage of the disease into proliferative stage due to TNF-α-induced pro-apoptotic process and cell death signaling. Hence, increasing severity of retinal periphlebitis has implications for early anti-TNF-α therapy to control inflammation and associated long-term sequelae.
The TNF monoclonal antibody is widely used for the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, spondyloarthropathies, Crohn’s disease, and psoriasis with an acceptable safety profile [22]. A pathogenetic role of TNF in ocular inflammatory conditions has recently emerged from small trials reporting preliminary results on the efficacy of these agents in patients with noninfectious uveitis, regardless of the origin of the disease [23]. Strategies for preventing TNF-α activity include neutralization of the cytokine via anti-TNF antibodies, soluble receptors, or receptor fusion proteins. This inhibition can be achieved with a monoclonal antibody such as infliximab (murine binding Fab domains and human constant Fc domains chimeric molecule) or adalimumab (fully human monoclonal antibody), or with a circulating receptor fusion protein such as etanercept (a fusion protein linking human soluble TNF receptor to the Fc component of human immunoglobulin G1). Suppression of TNF-α synthesis may be achieved by drugs, such as cyclosporine A and glucocorticoids, or cytokine IL-10.
References
- 1.Gieser SC, Murphy RP. Eales' disease. In: Ryan SJ, editor. Retina Vol II. Medical retina. St. Louis: CV Mosby; 1994. [Google Scholar]
- 2.Atmaca LS, Idil A, Gunduz K. Visualization of retinal vasculitis in Eales disease. Ocul Immunol Inflamm. 1993;1:41–48. doi: 10.3109/09273949309086536. [DOI] [PubMed] [Google Scholar]
- 3.Bali T, Saxena S, Kumar D, Nath R. Response time and efficacy of oral methotrexate pulsed therapy in idiopathic retinal periphlebitis. Eur J Ophthalmol. 2005;15:374–378. doi: 10.1177/112067210501500310. [DOI] [PubMed] [Google Scholar]
- 4.Saxena S, Rajasingh J, Biswas S, Kumar D, Shinohara T, Singh VK. Cellular response to retinal S-antigen and interphotoreceptor retinoid binding protein fragments in patients with Eales' disease. Pathobiology. 1999;67:37–44. doi: 10.1159/000028049. [DOI] [PubMed] [Google Scholar]
- 5.Saxena S, Srivastava P, Kumar D, Khanna VK, Seth PK. Decreased platelet membrane fluidity in retinal periphlebitis in Eales’ disease. Ocul Immunol Inflamm. 2006;14:113–116. doi: 10.1080/09273940600557043. [DOI] [PubMed] [Google Scholar]
- 6.Saxena S, Kumar D. A new staging system for idiopathic retinal periphlebitis. Eur J Ophthalmol. 2004;14:236–239. doi: 10.1177/112067210401400308. [DOI] [PubMed] [Google Scholar]
- 7.Inomata H, Ishibashi T, Murata T, Iwasaki M, Tahara A, Hata K, et al. Intraocular neovascularization. Nippon Ganka Gakkai Zasshi. 1997;101:906–926. [PubMed] [Google Scholar]
- 8.Kawashima M, Shoji J, Kamura Y, Sato Y. Role of chemokines in the vitreous of proliferative diabetic retinopathy. Nippon Ganka Gakkai Zasshi. 2005;109:596–602. [PubMed] [Google Scholar]
- 9.Rai G, Saxena S, Kumar H, Singh VK. Human retinal S-antigen: T cell epitope mapping in posterior uveitis patients. Exp Mol Pathol. 2001;70:140–145. doi: 10.1006/exmp.2000.2338. [DOI] [PubMed] [Google Scholar]
- 10.Tripathi P, Saxena S, Yadav VS, Naik S, Singh VK. Human S-antigen: peptide determinant recognition in uveitis patients. Exp Mol Pathol. 2004;76:122–128. doi: 10.1016/j.yexmp.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong D, Ueda T, Ueda T, et al. Lipid hydroperoxide stimulates retinal neovascularization in rabbit retina through expression of tumor necrosis factor-alpha, vascular endothelial growth factor and platelet-derived growth factor. Angiogenesis. 1998;2:93–104. doi: 10.1023/A:1009010628371. [DOI] [PubMed] [Google Scholar]
- 12.Dinarello CA. Proinflammatory cytokines. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- 13.Saxena S, Pant AB, Khanna VK, Singh K, Kumar D, Singh VK. Interleukin-1 and tumor necrosis factor-alpha: novel targets for immunotherapy in Eales’ disease. Ocul Immunol Inflamm. 2009;17:201–206. doi: 10.1080/09273940902731015. [DOI] [PubMed] [Google Scholar]
- 14.Kriegler M, Perez C, DeFay K, Albert I, Lu SD. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988;53:45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- 15.Tang P, Hung MC, Klostergaard J. Human pro-tumor necrosis factor is a homotrimer. Biochemistry. 1996;35:8216–8225. doi: 10.1021/bi952182t. [DOI] [PubMed] [Google Scholar]
- 16.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 17.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/S0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 18.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 19.Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–1635. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- 20.Gaur U, Aggarwal BB. Regulation of proliferation, survival and apoptosis by members of the TNF superfamily. Biochem Pharmacol. 2003;66:1403–1408. doi: 10.1016/S0006-2952(03)00490-8. [DOI] [PubMed] [Google Scholar]
- 21.Hunter CA, Timans J, Pisacane P, Menon S, Cai G, Walker W, et al. Comparison of the effects of interleukin-1 alpha interleukin-1 beta and interferon-gamma inducing factor on the production of interferon-gamma by natural killer. Eur J Immunol. 1997;27:2787–2792. doi: 10.1002/eji.1830271107. [DOI] [PubMed] [Google Scholar]
- 22.Theodossiadis PG, Markomichelakis NN, Sfikakis PP. Tumor necrosis factor antagonists: preliminary evidence for an emerging approach in the treatment of ocular inflammation. Retina. 2007;27:399–413. doi: 10.1097/MAJ.0b013e3180318fbc. [DOI] [PubMed] [Google Scholar]
- 23.Thi HD, Cassoux N, Lebrun-Vignes B, Wechsler B, Bodaghi B, Lehoang P, et al. Therapy of chronic non infectious uveitis. Rev Med Intern. 2007;28:232–241. doi: 10.1016/j.revmed.2006.10.326. [DOI] [PubMed] [Google Scholar]