Abstract
To carry out tasks with the highest possible efficiency we have developed executive mechanisms which monitor task performance and optimize cognitive processing. It has been hypothesized that these executive mechanisms operate even without conscious awareness to maximize their sensitivity to task-relevant outcomes. To test this hypothesis the present study examined the error-related negativity (ERN), an electrophysiological index of the performance monitoring neural circuitry, during masked visual search. The findings show that representations of target objects that are processed perceptually but not to the level of awareness fail to elicit an ERN despite the ability of these targets to elicit a shift of attention. These findings indicate that the performance monitoring mechanism indexed by the ERN requires target information to be processed to the level of awareness for a mismatch between stimulus and response to be detected.
Our dynamic environment requires that we adjust how our brain processes information while we are performing tasks vital to our survival, such as hunting, avoiding predators, or operating vehicles. In the laboratory, the error-related negativity (ERN) of the event-related potential (ERP) waveform is used to measure when the brain detects errors or evaluates feedback (Gehring, Gross, Coles, Meyer, & Donchin, 1993; Gehring & Willoughby, 2002; Holroyd & Coles, 2002). The theories explaining the processes underlying the ERN have proposed that it measures error detection (Gehring et al., 1993), response conflict (Botvinick, Braver, Barch, Carter, & Cohen, 2001), and learning (Brown & Braver, 2005; Holroyd & Coles, 2002). Empirically, several studies suggest that the ERN can be found even when subjects are unaware that they have made an error. Evidence for this hypothesis has been provided using paradigms in which observers make a behavioral response at the end of the trial in a saccadic response paradigm (Endrass, Franke, & Kathmann, 2005; Endrass, Reuter, & Kathmann, 2007; Nieuwenhuis, Ridderinkhof, Blom, Band, & Kok, 2001) or on the next trial of a go/no-go paradigm (O'Connell et al., 2007) to indicate that they made an error. Using these paradigms, researchers have found similar ERNs whether or not subjects explicitly reported that they made an error. Thus, it has been proposed that the mechanism indexed by the ERN may operate beyond awareness. The question addressed here is whether the error-detection mechanism indexed by the ERN can operate on the preliminary products of visual scene processing which are sufficient for visual attention mechanisms to shift attention to target objects. If this is the case and the visual system responds as if a target is present although the subject is not aware that it was present, then a target-absent response by the subject should elicit an ERN. To test this hypothesis the study utilizes a specific type of visual masking.
Object-substitution masking occurs when a visual search target is rendered unreportable by the continued presence of four-dot stimuli surrounding the target location (Di Lollo, Enns, & Rensink, 2000). Target discrimination or detection is accurate when the four-dots surrounding the possible targets offset simultaneously with the objects in the search array (see Figure 1A). However, when the four-dot masks remain visible after the search array offsets, observers approach chance at discriminating or detecting search targets in cluttered visual scenes. Previously, Woodman & Luck (2003a) showed that despite being unreportable, substitution-masked targets elicit shifts of attention as indexed by the N2pc component of the visual ERP waveform. The N2pc is a negative going potential typically elicited 200 ms following the onset of a search array, contralateral to the target or attended item (N2-posterior-contralateral Luck & Hillyard, 1994a, 1994b; Woodman & Luck, 1999, 2003b). The N2pc appears to index a mechanism of spatially focused visual attention in human and nonhuman primates (Luck, Girelli, McDermott, & Ford, 1997; Woodman, Kang, Rossi, & Schall, 2007). The previous findings indicate that during a substitution-masking paradigm the target representation is sufficient to trigger a normal shift of perceptual attention as indexed by the N2pc component (Woodman & Luck, 2003a). The present study uses the same substitution-masking paradigm to test the hypothesis that the error-detection mechanism indexed by the ERN operates prior to awareness and is as sensitive to the target information in a masked array as the N2pc component. If the mechanism indexed by the ERN operates beyond perceptual awareness, then it should be observed even when subjects fail to detect substitution-masked targets. In contrast, if the mechanism indexed by the ERN requires representations to be encoded into working memory resulting in awareness of the task-relevant information, then masking the targets by object substitution should eliminate the ERN.
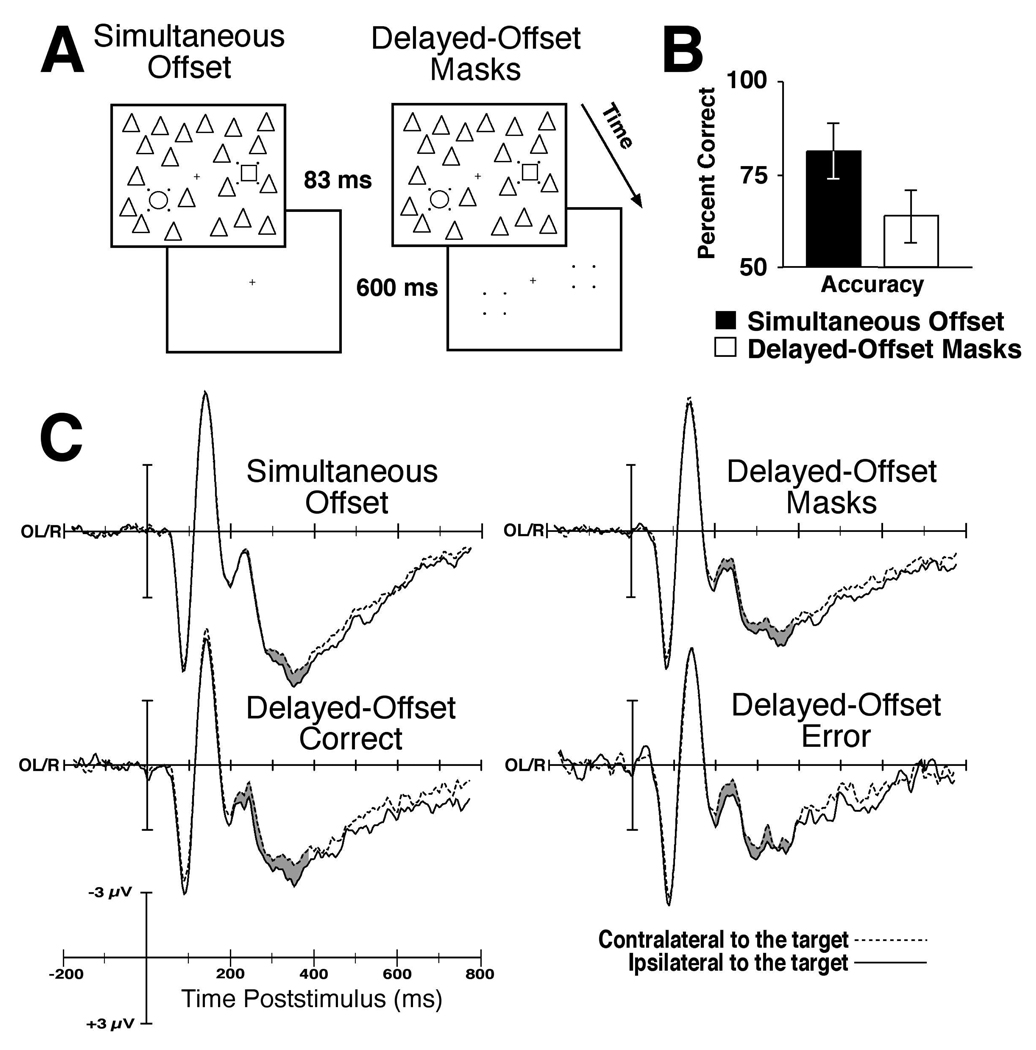
Figure 1.
Examples of the stimuli, the behavioral performance and the stimulus-locked waveforms. A. Example stimuli for both trial types. B. The behavioral results with 95% confidence intervals shown. C. The grand average ERPs time-locked to the onset of the array for simultaneous-offset trials, upper left, delayed-offset trials, upper right, correct delayed-offset trials, lower left, and delayed-offset trials with error responses, lower right. The waveforms recorded over sites contralateral to the target are dashed and the ipsilateral waveforms are the solid lines. Electrode sites OL and OR are shown as this is where the N2pc component is maximal.
Method
Ten subjects participated after informed consent was obtained. As the present analysis focused on error trials, subjects were excluded from the analysis if they did not have at least 30 artifact-free error responses on both simultaneous offset and delayed-offset trials. This led to the exclusion of three of the ten participants from the analysis.
The methods used have been reported previously (Woodman & Luck, 2003a) so a brief summary is provided presently. ERPs were recorded while observers viewed two types of visual search arrays that were randomly interleaved (see Figure 1A). On simultaneous offset trials, the four dots surrounding the possible target objects disappeared at the same time as the rest of the search array. On delayed-offset trials the four dots remained visible for approximately 500 ms following the offset of the search array. Observers were instructed to respond as fast and accurately regardless of trial type by pressing one button on a gamepad with their right index finger to indicate that the target was present and another button with their right middle finger to indicate that the target was absent. Subjects alternated between blocks of searching for square, circle, or diamond target shapes while distractor triangles were always present. Two possible targets were presented surrounded by four dots on each trial (one in the left and right hemifield) meaning the target shape was present on 66% of trials in each block and the subjects were informed of this target probability structure.
Visual ERPs were time locked to the onset of the search array and baselined to the activity in the 200-ms interval before onset (i.e., for the N2pc analyses). The response-locked ERP averages were time locked to the response onset and baselined to the activity in the 200-ms interval prior to the response. Trials were rejected due to ocular or muscle artifacts according to a two-step procedure described previously (Woodman & Luck, 2003b). Trials were also excluded when the measured RT was shorter than 200 ms and longer than 2000 ms.
The data were analyzed using an analysis of variance (ANOVA) with the within subjects factors of trial type (simultaneous offset vs. delayed-offset masks) response accuracy (correct vs. incorrect response to target presence) and electrode sites (F3/4, C3/4, P3/4 for the ERN and O1/2, OL/R, and T5/6 for the N2pc) using the Greenhouse-Geyser correction. The ERN is measured using the lateral sites that flank those typically used in ERN experiments because midline electrodes were not recorded in this study. The amplitude of the N2pc component was measured from 200–350 ms poststimulus array onset and the ERN was measured using a time window of 0–100ms after the onset of the manual response.
Results
Observers’ behavioral response accuracies are shown in Figure 1B. Responses were significantly more accurate on simultaneous offset trials (85.2% correct) than delayed-offset masking trials (72.3% correct), F(1,6) = 17.25, p < .01. A follow-up analysis showed that performance on delayed-offset masking trails was not significantly different than the 66% chance level, p > .20. I also measured target detection using the A’ measure of sensitivity and found that performance was significantly better on simultaneous-offset trials than delayed-offset masking trials (.89 versus .77, respectively), F(1,6) = 6.90, p < .05. Mean reaction times (RTs) showed that the responses were faster on simultaneous offset trials (799 ms and 1003 ms, for target present and absent, respectively) than delayed-offset trials (947 ms and 1047 ms, for target present and absent, respectively). These RTs lead to a significant effect of target presence, F(1,6) = 30.84, p < .01. However, neither the main effect of trial type nor the interaction of trial type with target presence was significant (ps > .10). Mean RTs did not significantly differ as a function of the correctness of response (949 ms and 934 ms for correct and incorrect responses, respectively) nor did this response correctness factor interact with trial type (simultaneous versus delayed offset), ps > .25. These behavioral findings demonstrate that the delayed-offset masks impaired target detection performance relative to targets that were not trailed by masks.
The stimulus-locked ERP results are shown in Figure 1C. An N2pc component was observed on both simultaneous offset and delayed-offset trials. In addition, an N2pc was evident on target-present delayed-offset trials whether or not the subjects correctly reported its presence. The omnibus ANOVA of the ERP waveforms during the N2pc measurement window found no significant interaction of condition (delayed-offset masks versus simultaneous offset) with the contralaterality factor (ipsi versus contralateral to the target hemifield), F < 1.0. Planned comparisons confirmed that masked targets elicited a significant N2pc just as did the randomly interleaved unmasked targets, ps < .05. The amplitude and factional-area latency of the N2pc on masking trials was nonsignificantly larger and earlier than the N2pc measured on simultaneous-offset trials, ps > .30. This was due to physical presence of the masks after the offset of the 80-ms array containing the search target providing a physical stimulus on which attention could be focused. The amplitude of the N2pc to masked targets did not significantly differ between correctly detected masked targets (−0.46 µV) and missed masked targets (−0.31 µV, F < 1.0), and a significant N2pc was found on both trial types (ps < .05). These stimulus-locked ERP results confirmed that masked targets elicited significant N2pcs whether or not the behavioral response indicated they were detected, consistent with a previous report (Woodman & Luck, 2003a). The N2pc component findings indicate that the focusing of attention on the target occurred even when the object-substitution masks were presented and subjects reported being unaware of its presence1.
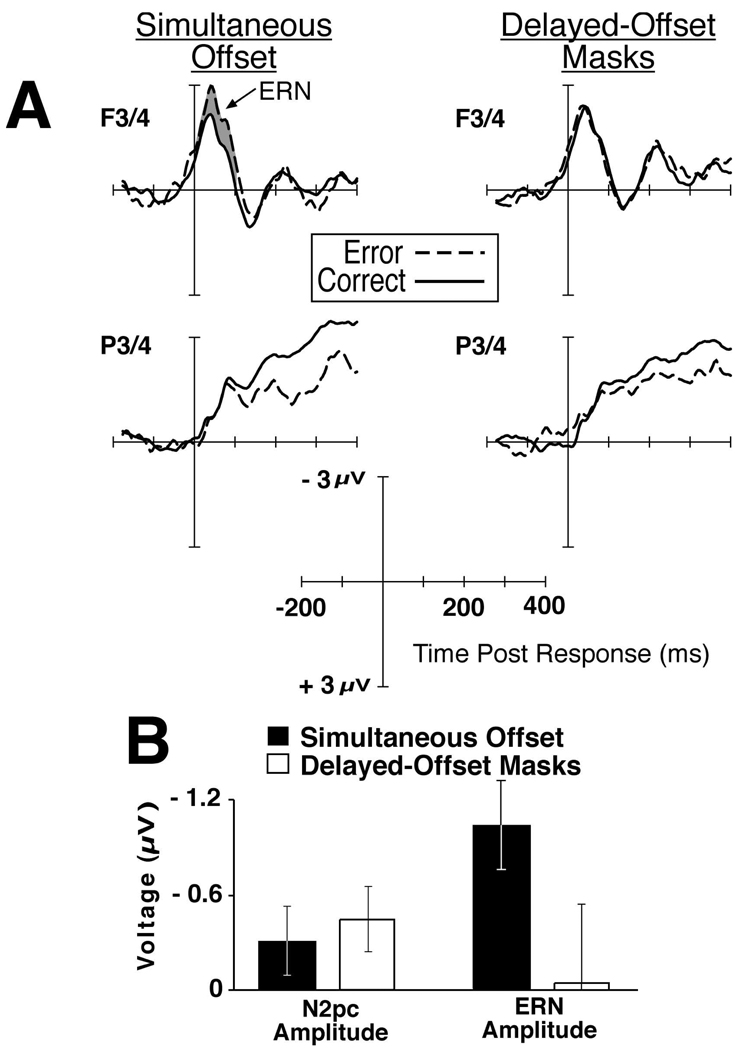
The waveforms time locked to correct and incorrect target detection responses are shown in Figure 2A. The amplitude of the response-locked waveforms was significantly larger on trials in which errors were made on the simultaneous offset trials (mean amplitude = −3.3 µV) than simultaneous offset trials with correct responses (mean = −2.3 µV), F(1,6) = 3.10, p < .01. In contrast, the mean amplitude of the waveform on trials with error responses was essentially identical to correct response trials in the delayed-offset mask condition (−2.7 µV on correct and −2.7 µV on error trials, F < 1.0) 2. The omnibus ANOVA yielded a significant main effect of electrode site, F(5,30) = 4.36, p < .01, a significant interaction of electrode site X response accuracy, F(5,30) = 2.36, p < .05, and an interaction of trial type (simultaneous offset versus delayed-offset masks) X response accuracy (correct versus incorrect) X electrode site (F3/4, C3/4 versus P3/4), F(5,30) = 2.62, p < .05. Thus, the findings from the analysis of the ERN between simultaneous offset and delayed-offset trials show a qualitatively different pattern than those of the perceptual attention-related N2pc which was found on both trial types and whether a target was correctly detected or not (see Figure 2B)3.
Figure 2.
The response locked waveforms for correct and incorrect trials and summary bar graphs of the ERP findings. A. The grand average ERPs time locked to the manual response for simultaneous offset (no mask) trials, left, and delayed-offset trials, right, for correct (solid lines) and incorrect responses (dashed lines). Electrode pairs F3/4 and P3/4 are shown to illustrate the typical ERN distribution was observed. B. Bar graphs showing the stimulus-locked N2pc amplitude (contralateral minus ipsilateral amplitude, 200–375 ms poststimulus) and the response-locked ERN amplitude (incorrect minus correct amplitude, 0–100 ms postresponse) for simultaneous offset and delayed-offset masking trials. The error bars show the standard error of the mean amplitude.
Discussion
This study found that targets that were masked by object substitution elicited an essentially normal N2pc component indexing the focusing of perceptual attention on the masked target but when observer’s behavioral report was that no target was present the ERN component was absent. These results show that although a masked target can be sufficiently processed to elicit a shift of attention this is not sufficient to elicit an ERN. The present findings suggest that task-relevant information needs to be processed to the level of consciousness for performance monitoring mechanisms to detect that an error was made.
The present study shows that the N2pc component is not only more sensitive than behavioral report to the presence of a target item but is also more sensitive to task-relevant information than the ERN. Both ERP components have been proposed to index mechanisms that can operate without awareness. This demonstrates a dissociation between the information available to mechanisms that operate at the perceptual level and cognitive mechanisms that oversee the processing of information across the brain. These results have implications for theories of executive control given the centrality of the ERN component in testing such cognitive models (e.g., Holroyd & Coles, 2002; Yeung, Botvinick, & Cohen, 2004). In addition, the ERN has become an important tool in understanding clinical disorders (Liotti, Pliszka, Perez, Kothmann, & Woldorff, 2005; Riba, Rodriquez-Fornells, Munte, & Barbanoj, 2005; Ruchsow et al., 2005). The present results show that abnormal processing indexed by the ERN is due to processing that occurs with awareness of the processed information and response.
The absence of the ERN for targets that are perceptually processed but made unavailable to awareness by substitution masking suggest that previous evidence for the presence of the ERN when subjects fail to report that they made an error may need to be reinterpreted. Specifically, these failures to report errors that were just made may have been due to memory failures or task-switching costs and not a complete lack of awareness regarding what stimuli were shown and the response that was made. Nieuwenhuis and colleagues (2001) pointed out that most of the studies of the relationship between the ERN and awareness required subjects to explicitly report that they made an error at the end of the trial or even on the next trial. The evidence presented here is consistent with the interpretation that in these previous studies executive mechanisms did detect the incompatibility of the response given the stimulus at the time the response was made. However, the dual-task nature of the explicit error reporting paradigm lead subjects to fail to report that they made an error after it occurred. The present study cannot rule out that some of these failures to self-report errors were due to limited metacognitive capabilities or social biases to not report one’s own errors. Finally, it is also possible that we are not aware of certain errors we make during saccadic behavior due to the habitual nature of such responses and this possibility remains a topic of study (e.g., Belopolsky, Kramer, & Theeuwes, 2008).
The findings of this study have implications for a number of models of cognitive control. First, several models hold the view that the ERN indexes a mechanism of reinforcement learning or reward likelihood (Brown & Braver, 2005; Holroyd & Coles, 2002). In such a framework, the absence of the ERN when subjects are not aware that the target was processed perceptually suggest that it is representations in working memory that have reached the level of awareness that are used to guide learning and reinforce correct behavior. This is generally consistent with neurophysiological models of prefrontal activity and cognitive control (e.g., Miller & Cohen, 2001). Second, the present findings are relevant for the conflict-monitoring theory of executive control and the ERN. This theory might seem to predict that the ERN would be largest on masked target trials in this study because these would be the trials in which there is conflict between the perceptual evidence that a target is present (i.e., as indexed by the N2pc) and the lack of a working memory level representation of the target to guide behavior. However, Cohen, Botvinick and colleagues (Botvinick, Nystrom, Fissell, Carter, & Cohen, 1999; Botvinick et al., 2001; Yeung et al., 2004) have proposed that the activity in the anterior cingulate cortex that generates the ERN is specifically sensitive to response conflict at later stages of processing and not ‘perceptual conflict’(van Veen, Cohen, Botvinick, Stenger, & Carter, 2001). The findings of the present study suggest an elaboration of this proposal in that that the neural circuitry generating the ERN is also not sensitive to conflict between perceptual and working memory level representations but instead measure conflict only at the late stage of processing suggested in the conflict-monitoring models.
Acknowledgments
This study was made possible by a National Research Service Award from the National Institute of Health to G.F.W. (F31 MH12995) and the benevolent support of Steve Luck.
Footnotes
To verify that the baseline corrected ERPs time locked to the response did not remove evidence of an ERN generated prior to the manual response I analyzed the uncorrected waveforms in a 300 ms baseline period prior to the response. The ANOVA of this epoch showed that there was no significant effect of trial type (simultaneous versus delayed offset), response correctness (correct versus error response), nor an interaction of these terms (Fs < 1.0).
Data were also analyzed from an experiment in which exactly the same task and visual search arrays were presented to subjects but simultaneous-masking noise was presented on a subset of trials to impair target detection performance (for details see Woodman & Luck, 2003a). This type of masking should interfere with perceptual processing unlike the object-substitution masking which appears to operate at a relatively late stage of visual processing. Analyses of these data showed that simultaneous masking noise impaired perceptual processing as measured by the elimination of the N2pc component relative to unmasked trials and this type of masking eliminated the ERN in planned comparisons (F < 1.0). This shows that masks which disrupt processing at either perceptual or post-perceptual stages of processing prevent the mechanism that is indexed by the ERN from detecting that an erroneous response was made.
The response-locked waveforms also show evidence of a parietal error-related positivity (or Pe, see Falkenstein, Hoormann, Christ, & Hohnsbein, 2000). To assess this I used a mean amplitude measurement window of 200–400 ms postresponse. An ANOVA with factors of trial type (simultaneous offset versus delayed-offset masks), response accuracy (correct versus incorrect), and electrode site (P3, P4, C3, C4, F3, F4) showed a significant main effect of electrode, F(5,30) = 10.99, p < .001, and a significant interaction of trial type X electrode, F(5,30) = 8.64, p < .001, due to the negativity of the posterior waveforms being greater in the simultaneous offset than the delayed-offset masking trials. However, the ANOVA did not yield a significant three-way interaction, p > .30. Separate planned comparisons of the waveforms within each trial type showed significant interactions of electrode pair X response accuracy (F(5,30) = 6.32, p < .001, F(5,30) = 4.35, p < .01, for simultaneous offset and delayed-offset masking trials, respectively) due to more positive potentials following errors at the parietal electrodes. Thus, significant Pe components were found during both simultaneous offset and delayed-offset masking trials although this effect was larger on the trials without masks. This finding is generally consistent with the hypothesis that the Pe reflects the subjective or emotional assessment of a response (Falkenstein et al., 2000). The present finding of a reduction of the Pe on masking trials is generally consistent with the subjective-assessment hypothesis because pure guesses were more likely on masking trials which should be less arousing than errors the performance monitoring system knows to be wrong based on accurate perception.
References
- Belopolsky AV, Kramer AF, Theeuwes J. The role of awareness in processing of oculomotor capture: Evidence from event-related potentials. Journal of Cognitive Neuroscience. 2008;20:2285–2297. doi: 10.1162/jocn.2008.20161. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402(6758):179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307:1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Di Lollo V, Enns JT, Rensink RA. Competition for consciousness among visual events: The psychophysics of reentrant visual processes. Journal of Experimental Psychology: General. 2000;129(4):481–507. doi: 10.1037//0096-3445.129.4.481. [DOI] [PubMed] [Google Scholar]
- Endrass T, Franke C, Kathmann N. Error awareness in a saccade countermanding task. Jorunal of Psychophysiology. 2005;19:275–280. [Google Scholar]
- Endrass T, Reuter B, Kathmann N. ERP correlates of conscious error recognition: aware and unaware errors in an antisaccade task. European Journal of Neuroscience. 2007;26:1714–1729. doi: 10.1111/j.1460-9568.2007.05785.x. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components of reaction errors and their functional significance: A tutorial. Biological Psychology. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Gross B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Liotti M, Pliszka SR, Perez R, Kothmann D, Woldorff MG. Abnormal brain activity related to performance monitoring and error detection in children with ADHD. Cortex. 2005;41:377–388. doi: 10.1016/s0010-9452(08)70274-0. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Girelli M, McDermott MT, Ford MA. Bridging the gap between monkey neurophysiology and human perception: An ambiguity resolution theory of visual selective attention. Cognitive Psychology. 1997;33:64–87. doi: 10.1006/cogp.1997.0660. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA. Electrophysiological correlates of feature analysis during visual search. Psychophysiology. 1994a;31:291–308. doi: 10.1111/j.1469-8986.1994.tb02218.x. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA. Spatial filtering during visual search: Evidence from human electrophysiology. Journal of Experimental Psychology: Human Perception and Performance. 1994b;20:1000–1014. doi: 10.1037//0096-1523.20.5.1000. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GPH, Kok A. Error-related brain potentials are differentially related to awareness of response errors: Evidence for an antisaccade task. Psychophysiology. 2001;38:752–760. [PubMed] [Google Scholar]
- O'Connell RG, Dockree PM, Bellgrove MA, Kelly SP, Hester R, Garavan H, et al. The role of cingulate cortex in the detection of errors with and without awarness: a high-density electrical mapping study. European Journal of Neuroscience. 2007;25:2571–2579. doi: 10.1111/j.1460-9568.2007.05477.x. [DOI] [PubMed] [Google Scholar]
- Riba J, Rodriquez-Fornells A, Munte TF, Barbanoj MJ. A neurophysiological study of the detrimental effects of alprazolam on human action monitoring. Cognitive Brain Resarch. 2005;25:554–565. doi: 10.1016/j.cogbrainres.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Ruchsow M, Gron G, Reuter K, Spitzer M, Harmle L, Kiefer M. Error-related brain activity in patients with obsessive-compulsive disorder and in healthy controls. Journal of Psychophysiology. 2005;19:298–304. [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior cingulate cortex, conflict monitoring, and levels of processing. Neuroimage. 2001;14(6):1302–1308. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Kang M-K, Rossi AF, Schall JD. Nonhuman primate event-related potentials indexing covert shifts of attention. Proceedings of the National Academy of Sciences. 2007;104:15111–15116. doi: 10.1073/pnas.0703477104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Electrophysiological measurement of rapid shifts of attention during visual search. Nature. 1999;400:867–869. doi: 10.1038/23698. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Dissociations among attention, perception, and awareness during object-substitution masking. Psychological Science. 2003a;14:605–611. doi: 10.1046/j.0956-7976.2003.psci_1472.x. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Serial deployment of attention during visual search. Journal of Experimental Psychology: Human Perception and Performance. 2003b;29:121–138. doi: 10.1037//0096-1523.29.1.121. [DOI] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: Conflict monitoring and the error-related negativity. Psychological Review. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]