Abstract
Dramatic advances in the care of patients with advanced renal cell carcinoma have occurred over the last ten years, including insights into the molecular pathogenesis of this disease, that have now been translated into paradigm-changing therapeutic strategies. Elucidating the importance of signaling cascades related to angiogenesis is notable among these achievements. Pazopanib is a novel small molecule tyrosine kinase inhibitor that targets VEGFR-1, -2, and -3; PDGFR-α, PDGFR-β; and c-kit tyrosine kinases. This agent exhibits a distinct pharmacokinetic profile as well as toxicity profile compared to other agents in the class of VEGF signaling pathway inhibitors. This review will discuss the scientific rationale for the development of pazopanib, as well as preclinical and clinical trials that led to approval of pazopanib for patients with advanced renal cell carcinoma. The most recent information, including data from 2010 national meeting of the American Society of Clinical Oncology, and the design of ongoing Phase III trials, will be discussed. Finally, an algorithm utilizing Level I evidence for the treatment of patients with this disease will be proposed.
Keywords: pazopanib, renal cell carcinoma, VEGF, VEGFR TKI, tyrosine kinase inhibitor, GW786034
Introduction
Renal cell carcinoma
Renal cell carcinoma (RCC) was the ninth most common cancer diagnosed in the United States during 2009, with nearly 58,000 new diagnoses and almost 13,000 deaths.1 Sporadic RCC has numerous histologic subtypes that occur with varying incidences, including clear cell carcinoma (75%), papillary (12%, chromophobe (4%), oncocytoma (4%), collecting duct (<1%) and unclassified (3%–5%).2,3 The histologic classifications of clear cell and predominant clear cell carcinoma are the most commonly identified subtypes and thus the best studied to date. The mainstay of treatment is radical nephrectomy for localized disease, and until recently, the treatment options for patients with either unresectable or metastatic renal cell carcinoma were limited.
Prior to the advent of targeted therapy, the prognosis of patients with metastatic renal cell carcinoma was very poor, with a median survival of one year and a five-year survival of 0%–20%.4 Historically, treatment of metastatic renal cell carcinoma involved immunotherapeutic agents such as interferon (IFN-α) or interleukin-2 (IL-2).5 IFN-α has been shown to provide a benefit in terms of overall survival compared to inactive therapy but with an average response rate between 10%–15% and few durable responses. High-dose IL-2 (infusion therapy requiring hospitalization) has a higher overall and complete response (CR) rate compared with low-dose cytokines (subcutaneous, outpatient), with the real benefit realized in the small percentage (5% to 7%) of patients who experience a durable CR.6 The patient most likely to obtain a durable CR with high-dose IL-2 includes the young, previously untreated patient with clear-cell histology RCC, ECOG performance status 0, and limited volume metastatic disease to lung. The morbidity and lack of applicability of high dose IL-2 to the broad RCC population has dampened enthusiasm for this approach, although it remains a valid treatment option in a very limited subset of patients.
The von Hippel Lindau gene in RCC
Of critical importance in the pathogenesis of clear cell RCC is the von Hippel-Lindau (VHL) tumor suppressor gene,7 the understanding of which has revolutionized RCC treatment. The VHL gene encodes the VHL protein, which is composed of an E3 ubiquitin ligase involved in the degradation of the transcription factor hypoxia-inducible factor (HIF). In RCC, one allele of the VHL gene is inactivated through a deletion event that can be observed in >90% of sporadic clear cell RCC.8 The remaining VHL allele is also commonly affected, with inactivation in up to 50% of clear cell RCC through gene mutation and an additional 5%–10% via altered methylation patterns.9,10 This biallelic gene inactivation leads to intracellular accumulation of the HIF transcription factor. Dimerization of the HIF isoforms, HIF-α and HIF-β, leads to upregulation of hypoxia-inducible genes such as VEGF, platelet-derived growth factor (PDGF), transforming growth factor (TGF) and others.5 This overabundance of HIF thus results in a cascade of overexpression of multiple growth factors that are normally reserved for cellular responses to hypoxia.
The VEGF signaling pathway and VHL
Extensive work has further clarified the VEGF axis as it relates to tumorogenesis in clear cell RCC.11 Increased levels of circulating angiogenesis factors like basic fibroblast growth factor (bFGF), VEGF, and angiogenin can be found in the serum of renal cancer patients, consistent with the hypervascular nature of these tumors.4,12–14 The VEGF ligand family includes four protein isoforms, VEGF-A, -B, -C, -D, that differentially interact with cell surface receptors to initiate a cascade of downstream signaling events.15 The number of receptors able to bind VEGF isoforms as well as the complexity of the interactions between various signaling cascades in angiogenic pathways has both complicated drug development in this field and simultaneously provided new targets for therapeutic agents.
VEGF receptors are members of a family of transmembrane tyrosine kinase receptors that mediate signal transduction from extracellular signaling ligands, like VEGF, to intracellular signaling cascades. Members of this receptor family include VEGFR-1 (Flt-1), VEGFR-2 (KDR/Flk-1), and VEGFR-3 (Flt-4). Similar to Kit and platelet-derived growth factor (PDGF) receptors, VEGF receptors are activated upon ligand-mediated receptor dimerization.16 VEGF-A binds to VEGFR-1 and -2, while VEGF-C and -D bind to VEGFR-2 and -3. Each receptor subtype interacts with different signaling molecules at the plasma membrane to activate different cellular processes. For example, activation of VEGFR-2 by VEGF-A leads to activation of Raf and the mitogen-activated protein kinase (MAPK) pathway via phospholipase C-γ (PLC-γ) in the endothelial cell. Activation of these intracellular signaling cascades promote tumor angiogenesis via multiple mechanisms that include endothelial cell survival, proliferation, and migration.16 These molecular findings are consistent with historical descriptions of increased vascularity of renal cell tumors and correlate with elevated VEGF protein in tumor and serum samples.
Therapy targeting the VEGF ligand
The first VEGF targeted therapy to receive FDA approval in solid tumors was bevacizumab. This well-studied agent is a monoclonal antibody that binds VEGF-A and has been shown to improve overall survival in multiple solid tumors when combined with chemotherapy. It has also demonstrated single-agent activity in a randomized Phase II trial in renal cell carcinoma.17 More recently, the FDA has approved bevacizumab in combination with IFN-α for the treatment of metastatic renal cell carcinoma on the basis of improvements in progression free survival (PFS) compared to IFN-α alone in the AVOREN (median PFS 10.2 versus 5.4 months, HR = 0.63, P < 0.0001) and CALBG 90206 (median PFS 8.5 months versus 5.2 months, HR = 0.71 P < 0.0001) trials.18,19 While neither trial met the primary endpoint of overall survival, both showed nonsignificant trends toward increased median survival in the bevacizumab-containing arms. The dramatic improvements in PFS resulted in FDA approval in this setting. Of note, with multiple therapies now approved and widely available, many patients received second and even third line therapies (including additional VEGF signaling pathway targeted agents). These findings have contributed to the significant debate in the field regarding the impact of post protocol therapies on overall survival data.
Therapy targeting the VEGF receptor
The VEGF Receptor Tyrosine Kinase Inhibitor (VEGFR TKI) family of drugs continues to grow and includes agents such as Sorafenib, Sunitinib, Axitinib and Pazopanib. This class is broadly defined as small molecule inhibitors of the VEGF signaling cascade that exert their mechanism via blockade of one or more VEGFR tyrosine kinases. These four drugs all exhibit the ability to inhibit VEGF receptor 1, 2 and 3, PDGFR and c-kit. They differ in other off target effects including Raf kinase (sorafenib), RET (sorafenib and sunitinib), and FLT3 (sunitinib). They further differ in pharmacokinetic properties such as kinase IC50, terminal half life, and Cmax.20–23 As clinical trials with these varied agents reach maturity, we are beginning to discriminate differences in both efficacy and toxicity profiles among these agents. The first of the VEGFR TKIs to receive FDA approval was sorafenib, based on the randomized, placebo controlled Phase III trial by Escudier et al showing an improved PFS of 5.5 months in the sorafenib group versus 2.8 months in the placebo group in a cytokine-refractory population (hazard ratio, 0.44; 95% confidence interval [CI], 0.35 to 0.55; P < 0.01).24 Sunitinib was later approved for treatment of mRCC based on the randomized Phase III trial showing improved PFS of 11 months for sunitinib compared with 5 months for IFN-α in a treatment-naïve population (P < 0.001).25,26 Recent Phase II and Phase III trials with other agents in the VEGFR TKI family of drugs have recently been reported for treatment of mRCC. This article will review the clinical trials conducted with the VEGFR TKI pazopanib to date (as summarized in Table 1) and discuss the evidence-based role of pazopanib for the treatment of advanced renal cell carcinoma with predominant clear cell histology.
Table 1.
Clinical trials of pazopanib in patients with renal cell carcinoma.
| Study | Dose | Setting | No. of pts with mRCC | MSKCC risk category37,38 (F/I/P/U§) | Prior nephrectomy | Clear cell |
|---|---|---|---|---|---|---|
| Phase I23 |
|
Dose escalation | 12/63 | NR | NR | NR |
| Phase II34 | 800 mg once daily versus placebo | First-line, cytokine-naïve | 225 | 43/41/2/14% | 91% | Yes† |
| Phase III36 | 800 mg once daily versus placebo | First-line, pre- or post- cytokine | 290* | 39/55/3/3%* | 89%* | Yes† |
Notes:
Pazopanib arm only;
Clear cell or predominantly clear cell histology required.
Abbreviations: §F/I/P/U, favorable, intermediate, poor, or unknown risk status; NR, not reported.
Mechanism of Action, Pharmacokinetic, and Metabolism Profile of Pazopanib
Pazopanib (GW786034, Votrient®; GlaxoSmithKline) is a potent and selective, orally available, small molecule inhibitor of VEGFR-1, -2, and -3; PDGFR-α, PDGFR-β; and c-kit tyrosine kinases.27,28 The agent selectively inhibits proliferation of endothelial cells stimulated with VEGF but not with basic fibroblast growth factor. In preclinical angiogenesis models, pazopanib inhibited VEGF-dependent angiogenesis in a dose-dependent manner, and in xenograft tumor models twice-daily administration of pazopanib significantly inhibited tumor growth in mice implanted with various human tumor cells.29 Pharmacokinetic and pharmacodynamic studies showed that a pazopanib concentration of ≥40 μmol/L inhibited VEGFR-2 in mice. These data differed from the IC50 of 0.02 μmol/L based on VEGF-stimulated proliferation in cell culture models and was attributed to in vivo protein binding of pazopanib.29 A target steady-state concentration of ≥40 μmol/L was thus selected for the Phase I trial and achieved in patients receiving either 800 mg daily or 300 mg BID.23
Pazopanib is absorbed orally with median time to peak plasma concentrations of 2 to 4 hours and a mean half-life of 30.9 hours after administration of an 800 mg dose.23 Daily dosing at 800 mg resulted a in mean AUC of 1,037 hr μg/mL and Cmax of 58.1 μg/mL with no consistent increase in AUC or Cmax at pazopanib doses above 800 mg.23 Administration of a single pazopanib 400 mg crushed tablet increased Cmax approximately 2 fold and decreased tmax by approximately 2 hours compared to administration of the whole tablet, indicating increased bioavailability and rate of oral absorption after administration of a crushed tablet. Systemic exposure to pazopanib was increased with a high-fat or low-fat meal resulting in an approximately 2-fold increase in AUC and Cmax leading to the recommendation that pazopanib be administered at least 1 hour before or 2 hours after a meal.30 Further pharmacokinetic data from patients with normal hepatic function (n = 12) and moderate (n = 7) hepatic impairment indicate that pazopanib clearance was decreased by 50% in those with moderate hepatic impairment.31 The pazopanib dose in patients with moderate hepatic impairment is recommended at 200 mg once daily.30
In vitro studies demonstrated that pazopanib is metabolized by CYP3A4 with a minor contribution from CYP1A2 and CYP2C8. Co-administration of oral pazopanib with CYP3A4 inhibitors has resulted in increased plasma pazopanib concentrations. For example, administration of 1,500 mg lapatinib, a substrate and weak inhibitor of CYP3A4, with 800 mg pazopanib resulted in an approximately 50% to 60% increase in mean pazopanib AUC(0–24) and Cmax compared to administration of 800 mg pazopanib alone.32 Clinical pharmacology studies using pazopanib 800 mg once daily have demonstrated that pazopanib does not have a clinically relevant effect on the pharmacokinetics of caffeine (CYP1A2 probe substrate),30 warfarin (CYP2C9 probe substrate), or omeprazole (CYP2C19 probe substrate). Co-administration of pazopanib 800 mg once daily and paclitaxel 80 mg/m2 (CYP3A4 and CYP2C8 substrate) once weekly resulted in a mean increase of 26% and 31% in paclitaxel AUC and Cmax, respectively.33
Clinical Safety and Efficacy
Pazopanib Phase I experience
A company-sponsored phase I study of orally administered pazopanib enrolled 63 patients with a variety of solid tumor types (dose escalation, n = 43; dose expansion, n = 20).23 Doses administered ranged from 50 mg three times per week to 2000 mg once daily to 400 mg twice daily. Tumor shrinkage was observed in two of twelve renal cell carcinoma patients, with confirmed partial responses. Of the remaining ten renal cell carcinoma patients, stable disease was observed in four patients and progressive disease in four patients. Two other renal cell carcinoma patients were withdrawn from the study due to toxicity before the first disease assessment. Tumor shrinkage was also seen in patients with Hurthel cell and neuroendocrine tumors, as well as chondrosarcoma. In all, 17 patients, including those with RCC, Hurthel cell, carcinoid, GIST, neuroendocrine, sarcoma, melanoma, and lung cancer tumors, remained on study for 6 months or longer. Pazopanib was generally well tolerated up to 2,000 mg. However, the pharmacokinetic analysis demonstrated that a plateau in steady state exposure was observed from 800 mg to 2000 mg doses. On the basis of this finding, a traditional maximum tolerated dose was not established and the recommended phase II dose was set at 800 mg, administered one hour before or two hours after a meal.23
The most common adverse events (AEs) seen in this phase I trial regardless of causality were all grade 1 or 2.23 Forty eight (76%) patients experienced drug-related AEs with the most frequently reported of all grades including hypertension (33%), diarrhea (33%), hair depigmentation (32%), and nausea (32%). One of three patients treated with 2000 mg daily of pazopanib developed dose-limiting toxicity of grade 3 fatigue. Hair depigmentation (indicative of c-kit and, potentially, VEGFR modulation) was seen in 12 patients, all of whom were treated at doses ≥ 800 mg. One of three patients dosed at 2000 mg once daily experienced grade 3 fatigue that resolved upon dose reduction to 800 mg. As seen with many VEGF signaling pathway inhibitors, grade 1–3 hypertension that could be controlled with antihypertensive medication was observed in the phase I study. In addition, there were single events of gastrointestinal bleeding, pulmonary thrombosis, and deep vein thrombosis. Pazopanib did not affect QTc in this phase I trial, nor was hand foot syndrome observed.23,24
Pazopanib Phase II results in metastatic renal cell carcinoma
Upon designation of the recommended phase II dose of pazopanib, a randomized, discontinuation phase 2 trial was designed for patients with metastatic or locally recurrent RCC.34 Study patients were required to have predominantly clear cell histology with measurable disease who had received either no systemic therapy or 1 prior systemic therapy, that may have included bevacizumab. Pazopanib was administered at 800 mg PO daily until progression of disease. The primary end point was progressive disease rate at 16 weeks post randomization. Two hundred twenty-five patients with metastatic RCC were treated (69% were previously untreated and 84% had favorable or intermediate risk). Interestingly, after a planned interim analysis showing indication of activity, the study was changed to an open-label trial design. At that time, the primary endpoint was changed to overall response rate according to RECIST guidelines. Accrual was completed by this interim analysis and the amendment allowed patients with stable disease who had been randomized to placebo to restart the study drug (n = 28). The PFS attributable to pazopanib was estimated using Kalbfleish-Prentice estimation to account for these patients. The independently reviewed overall response rate was 34.7% (95% CI, 28% to 41%), and the median PFS was 51.7 weeks. The PFS in the randomized comparison (n = 55) was 11.9 months for pazopanib versus 6.2 months for placebo (P = 0.0128).
Further analysis of this study was recently reported at the 2010 American Society of Clinical Oncology meeting. Suttle et al performed a retrospective analysis of the pharmacokinetics of pazopanib as related to patient outcome.35 Of the 225 patients enrolled on the trial, Cmin data was available for 205 patients at four weeks and 185 patients at 12 weeks. When separated into quartiles and stratified by PFS, it was observed that patients with a week 4 Cmin > 20.6 μg/mL had a median PFS of 49.4 weeks versus 20.3 weeks for patients unable to achieve this plasma level of drug. In addition, response rate was improved in this group (45% versus 18%), as was mean percent tumor shrinkage (37.8% vs. 8.8%). Importantly, nearly 70% of patients assessed at this time point (143/205) were able to reach or exceed this drug level. No data related to toxicity stratified by plasma level was available. While still a retrospective analysis, these data support both the initial dosage chosen for this drug as well as a potential role for maintaining dose intensity during treatment whenever possible.
Pazopanib Phase III results in metastatic renal cell carcinoma
The results of a single phase III trial with pazopanib in metastatic renal cell carcinoma were recently published by Sternberg et al.36 This trial was a randomized, double blind, placebo controlled trial in patients with clear cell, or predominantly clear cell, histology who either received no prior therapy or who had progressed on one prior cytokine-based systemic therapy. Patients were randomized in a 2:1 fashion to receive pazopanib at 800 mg once daily or placebo. Randomization was stratified according to performance status, prior nephrectomy, and treatment history. Patients who progressed on the placebo arm were allowed to enroll on an open label study of pazopanib, with seventy patients (48%) choosing this option. The primary endpoint of this trial was progression free survival with secondary endpoints of overall survival, confirmed objective response rate, duration of response, and safety.
Between April 2006 and April 2007, 435 patients were enrolled from 80 centers worldwide, with 290 randomized to pazopanib and 145 to placebo. The experimental and control arms were well balanced with regards to MSKCC risk category (94% vs. 92% with favorable/intermediate group, respectively) and prior nephrectomy (89% vs. 88%, respectively). The study was originally designed to include only cytokine-pretreated patients, but was rapidly amended to include treatment-naïve patients as well. Therefore, of the patients enrolled, 233 (54%) were treatment-naïve and 202 (46%) had been previously treated with IFN-α or IL-2. The experimental and control arms were well balanced in this regard, with the percentage of treatment naïve patients at 53% vs. 54%, respectively.
Pazopanib was found to significantly improve PFS compared to placebo (median 9.2 months vs. 4.2 months; HR, 0.46; 95% CI, 0.34 to 0.62; P < 0.0001) in the overall study population. The improvement in PFS was more pronounced in the treatment naïve subpopulation (median 11.1 vs. 2.8 months; HR, 0.40; 95% CI, 0.27 to 0.60; P < 0.0001), though the pretreated subpopulation also showed a significant improvement (median 7.4 vs. 4.2 months, HR, 0.54; 95% CI, 0.35 to 0.84; P < 0.001) as well. The objective response rate in this study was 30% (95% CI, 25.1 to 35.6), with a median duration of response of 58.7 weeks. At the time of reporting, the percentage of patients on study greater than 12 months reached 32% in the pazopanib arm and 15% in the placebo arm. Interim overall survival results did not meet significance and final results will be reported when data mature. Importantly, predefined subgroup analyses of PFS supported the pazopanib arm in all categories (MSKCC risk category, treatment history, gender, age, performance status).
Further trials with pazopanib include: 1) the extension trial of patients enrolled to the placebo arm in the Phase III trial (discussed above); 2) an ongoing phase III open-label trial, COMPARZ (Pazopanib Versus Sunitinib in the Treatment of Subjects With Locally Advanced and/or Metastatic Renal Cell Carcinoma); and the PISCES (Patient Preference Study of Pazopanib Versus Sunitinib in Advanced or Metastatic Kidney Cancer) trial. The COMPARZ trial is designed to test pazopanib versus sunitinib (Sutent®, Pfizer) in locally advanced and/or metastatic RCC patients who have had no prior treatment. Approximately 876 patients with treatment naïve metastatic clear cell RCC will be included. The PISCES trial will address patient preferences between pazopanib and sunitinib. This trial is a randomized, double-blind, crossover study of pazopanib versus sunitinib in patients with metastatic RCC who have received no prior systemic therapy. Approximately 160 patients are planned.37
Adverse events with pazopanib
Pazopanib exhibits a similar toxicity profile to other agents in the VEGFR TKI class of agents as summarized in Table 2. Although comparisons across trials do not allow definitive conclusions, there appears to be a lower incidence of hand foot syndrome, diarrhea, asthenia and myelosuppression in the Phase III trial with pazopanib compared to the Phase III trials of sunitinib and sorafenib. There was a 40% incidence of hypertension in the phase III trial with pazopanib which appears to be somewhat higher compared to other VEGFR TKIs. Of note, the incidence of Grade 3 hypertension was less than 1% and 4% of patients on the Phase II and Phase III trials, respectively. Arterial thrombotic events occurred in 3% of pazopanib-treated patients, of which 2% were myocardial infarction/ischemia and 1% due to cerebrovascular accident/TIA. The incidence of hemorrhagic events (all grades) in the pazopanib arm was 13% compared with 5% in the placebo arm. Laboratory abnormalities observed included predominantly grade 1/2 electrolyte abnormalities, including hypo-phosphatemia, -calcemia, -natremia, and -magnesemia. In combination with clinical findings of prolonged QT intervals and torsades de pointes, this led to the recommendation that electrocardiograms and electrolytes be monitored in patients considered at risk.30
Table 2.
Adverse events reported in Phase III trials with VEGFR TKIs.
| Adverse events |
Pazopanib (%)36 |
Sunitinib (%)25 |
Sorafenib (%)24 |
|||
|---|---|---|---|---|---|---|
| All | G3–4 | All | G3–4 | All | G3–4 | |
| AST elevation | 53 | 9 | 52 | 2 | – | – |
| ALT elevation | 53 | 12 | 46 | 2 | – | – |
| Hyperglycemia | 41 | 1 | – | – | ||
| Hypertension | 40 | 4 | 24 | 8 | 17 | 4 |
| Neutropenia | 34 | 2 | 72 | 11 | – | – |
| Thrombocytopenia | 32 | 1 | 65 | 8 | – | – |
| Bleeding | 13 | NR | 12 | 1 | 15 | 3 |
| Rash | – | – | 19 | 2 | 40 | 1 |
| Fatigue/asthenia | 19 | 3 | 51 | 7 | 37 | 5 |
| Diarrhea | 11 | 4 | 53 | 5 | 43 | 2 |
| Stomatitis | <0 | <1 | 25 | 1 | NR | 1 |
| Hand-foot syndrome | <0 | <1 | 20 | 5 | 30 | 6 |
| Hypothyroidism | – | – | 6 | 1 | – | – |
| Heart failure | – | – | 10 | 2 | 3 | 3 |
| Renal impairment | – | – | 66 | 1 | – | – |
Death resulting from AEs was reported in 4% of patients in the pazopanib arm and 3% of patients in the placebo arm. Four patients (1%) in the pazopanib arm had fatal AEs that were assessed by the investigator as attributable to study treatment: ischemic stroke, abnormal hepatic function and rectal hemorrhage, peritonitis/bowel perforation, and abnormal hepatic function (one patient each). Importantly, of the two patients who died of peritonitis/bowel perforation in the phase II and phase III trials, one had RCC metastasis present at the site of perforation and the other had a history of diverticulitis.
A major difference with pazopanib and other VEGFR TKIs includes an apparent higher probability of severe hepatotoxicity and hyperbilirubinemia with pazopanib. Elevations in the liver enzyme ALT occurred in 65% of patients, of which 12% experienced Grade 3–4 toxicity. ALT elevation recovered to ≤grade 1 after dose modification, interruption or cessation of drug in 87% of patients while the remaining 13% did not have adequate follow up data for reporting. One patient died of abnormal hepatic function that was attributed to study drug was later found to have extensive hepatic infiltration of tumor. These findings led to a black box warning for pazopanib stating “Increases in serum transaminase levels and bilirubin were observed. Severe and fatal hepatotoxicity has occurred. Measure liver chemistries before the initiation of treatment and regularly during treatment”. A genetic analysis performed by Xu et al attempted to identify genetic markers that may predict risk of ALT and/or bilirubin elevation in patients treated with pazopanib.38 Serum samples from 225 patients from the Phase II trial and 290 patients from the Phase III trial were analyzed for numerous genetic polymorphisms. Interestingly, the UGT1A1 TA repeat polymorphism was strongly associated with maximum on-treatment bilirubin concentration and bilirubin increase from baseline. None of the other markers tested was related to elevation of ALT. While screening for the UGT1A1 TA repeat polymorphism was not recommended, it is important to consider that isolated elevations of total bilirubin may not indicate pazopanib-induced hepatotoxicity.
Patient Preference
Overall patient preference amongst the evidence-based first-line treatments for favorable or intermediate risk metastatic renal cell carcinoma is unknown. These questions have been examined in retrospective studies, but may be subject to bias. Discussion of both side effects and the convenience of oral therapy, among other factors, will be critical in the decision-making process for patients with mRCC. Although the lack of head-to-head comparison studies limits definitive conclusions, we believe pazopanib may exhibit several key differences versus previously approved VEGFR TKIs. Reviewing the toxicity profiles of VEGFR TKIs in renal cell carcinoma would suggest that the lower incidence of fatigue, diarrhea and hand-foot syndrome might favor pazopanib (Table 2). However, patients with poorly controlled/difficult to treat hypertension or baseline liver dysfunction may well have greater difficulty with pazopanib. These questions regarding patient preferences and differential toxicity profiles will hopefully be answered more definitively in the prospective PISCES study discussed above.
Pazopanib: Place in Therapy
Treatment naïve patients
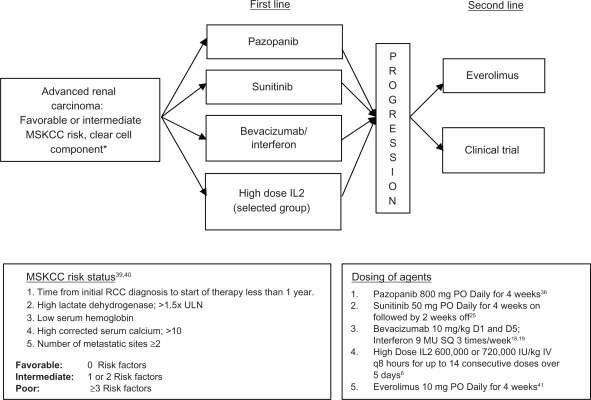
With the dramatic advances in treatment of mRCC, a question that would have been unheard of ten years ago now comes to the forefront: What is the best first line systemic therapy for mRCC? We support an evidence-based approach to upfront therapy based on the inclusion criteria in phase III trials and other patient characteristics. Important factors include MSKCC risk group,39,40 number and type of prior therapies, histologic subtype, and patient-specific factors. The strongest evidence for pazopanib is in the first line setting for patients with favorable/intermediate risk, predominantly clear cell renal carcinoma (see Figure 1). Other appropriate agents for treatment-naïve patients falling into an intermediate or favorable risk group include sunitinib, or bevacizumab plus interferon-α.2,5,39,41 For poor risk or non clear cell histology patients, there is Level 1 evidence for a survival advantage of temsirolimus, an intravenously administered inhibitor of the mammalian target of rapamycin (mTOR) pathway, when compared with interferon-α alone in the first line setting.42 Patients’ co-morbidities which could be exacerbated by agent-specific toxicities should be considered before initiating treatment. Other factors that may play a role include convenience of oral agents as well as prescription drug coverage status (ie, out-of-pocket cost).
Figure 1.
Evidence-based treatment algorithm for treatment-naïve metastatic renal cell carcinoma (category 1).
Note: *Temsirolimus is FDA approved for the first line treatment of poor risk or non clear cell histology metastatic renal cell carcinoma (category 1).42
Treatment options in the second line setting, after progression on a first line VEGF signaling pathway agent, are even less clear. Inhibitors of the mammalian target of rapamycin (mTOR) pathway, including everolimus, have been reported to show efficacy in the second line setting.41 The strongest evidence supports everolimus after progression on TKIs. The role of VEGFR TKI therapies as second line treatment options are currently being evaluated in clinical trials, with the only published data in smaller phase II trials testing sorafenib either before or after treatment with sunitinib. However, the critical questions are: By what mechanisms does angiogenesis inhibition fail and what rationale would support their continued use? Reports of increasing serum VEGF levels during treatment with VEGFR TKIs would support continued use of these agents with alternative dosing strategies.43 This hypothesis is supported by a Phase II trial with escalated dose sorafenib showing a responses in 42% of patients who had progressed on standard dose.44 Other Phase II trials have shown response rates with sunitinib in bevacizumab refractory disease12 and axitinib in sorafenib refractory disease.22 Whether these results are related to more potent VEGF inhibition or inhibition of additional targets is unclear. However, these results do suggest that VEGF signaling pathway inhibitors could play an important role as second line therapies and beyond. A phase III trial comparing sorafenib and sunitinib sequencing (NCT00732914) is designed to address a related VEGFR TKI sequencing question. Whether the results of this trial will be applicable to other VEGFR TKIs such as pazopanib and axitinib is unclear.
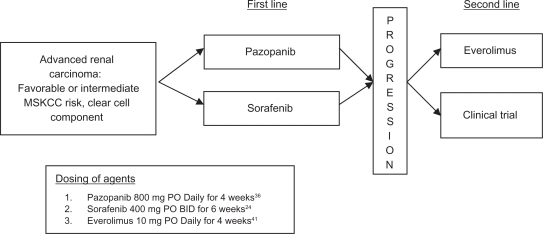
Cytokine-refractory patients
With the approval of six targeted therapy regimens for advanced renal cell carcinoma, the population of cytokine-refractory patients is dramatically shrinking. Therefore, the question of how to use these novel agents in cytokine-refractory disease is currently less pressing. However, randomized controlled studies of pazopanib36 and sorafenib24 provide category 1 (ie, Phase III RCT data) evidence supporting their use in this population (See Figure 2). As the use of VEGFR signaling pathway inhibitors for first line treatment is now standard of care, we expect to see fewer and fewer cytokine-refractory patients in clinical practice in the upcoming years.
Figure 2.
Evidence-based treatment algorithm for cytokine refractory metastatic renal cell carcinoma (category 1).
Conclusion
Pazopanib is a novel VEGFR TKI that has now demonstrated efficacy in a randomized Phase III trial in metastatic renal cell carcinoma in both the front line and cytokine-refractory settings. The unique toxicity profile of this agent, as well as the convenience of an oral therapy, makes it an attractive option in treatment of mRCC. Further trials defining the comparative efficacy, toxicity, and patient preference of pazopanib versus other VEGFR TKIs are currently underway. Further research to test the efficacy of this agent in the setting of patients who have failed prior VEGF blockade (bevacizumab, sunitinib, etc.) is needed.
Acknowledgments
The authors would like to thank Dr. Douglas McNeel for his thoughtful critique of this manuscript. This work was supported by NIH Grants T32 CA009614 and K12 CA087718.
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Linehan WM WM, Zbar B. The genetic basis of cancer of the kidney. J Urol. 2003 Dec;170(6 Pt 1):2163–72. doi: 10.1097/01.ju.0000096060.92397.ed. [DOI] [PubMed] [Google Scholar]
- 3.Kim WY, Kaelin WG. Role of VHL Gene Mutation in Human Cancer. J Clin Oncol. 2004;22(24):4991–5004. doi: 10.1200/JCO.2004.05.061. [DOI] [PubMed] [Google Scholar]
- 4.Linehan WM, Zbar B, Bates BE, Zelefsky MJ, Yang JC. Cancer of the Kidney and Ureter. In: deVita VT HS, Rosenberg SA, editors. Cancer Principles and Practice of Oncology. 6th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2001. pp. 1362–96. [Google Scholar]
- 5.Cohen HT, McGovern FJ. Renal-Cell Carcinoma. N Engl J Med. 2005;353(23):2477–90. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- 6.Fisher RI RS, Fyfe G. Long-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinoma. Cancer J Sci Am. 2000;6(Suppl 1):S55–7. [PubMed] [Google Scholar]
- 7.Rini BI. Vascular endothelial growth factor-targeted therapy in metastatic renal cell carcinoma. Cancer. 2009;115(S10):2306–12. doi: 10.1002/cncr.24227. [DOI] [PubMed] [Google Scholar]
- 8.Gnarra JR, Tory K, Weng Y, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. 1994;7(1):85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- 9.Schraml P, Struckmann K, Hatz F, et al. VHL mutations and their correlation with tumour cell proliferation, microvessel density, and patient prognosis in clear cell renal cell carcinoma. The Journal of Pathology. 2002;196(2):186–93. doi: 10.1002/path.1034. [DOI] [PubMed] [Google Scholar]
- 10.Gallou C, Joly D, Méjean A, et al. Mutations of the VHL gene in sporadic renal cell carcinoma: Definition of a risk factor for VHL patients to develop an RCC. Human Mutation. 1999;13(6):464–75. doi: 10.1002/(SICI)1098-1004(1999)13:6<464::AID-HUMU6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 11.Ainsworth NL, Lee JS, Eisen T. Impact of anti-angiogenic treatments on metastatic renal cell carcinoma. Expert Rev Anticancer Ther. 2009;9(12):1793–805. doi: 10.1586/era.09.144. [DOI] [PubMed] [Google Scholar]
- 12.Rini BI, Michaelson MD, Rosenberg JE, et al. Antitumor Activity and Biomarker Analysis of Sunitinib in Patients With Bevacizumab-Refractory Metastatic Renal Cell Carcinoma. J Clin Oncol. 2008;26(22):3743–8. doi: 10.1200/JCO.2007.15.5416. [DOI] [PubMed] [Google Scholar]
- 13.Rasmuson T, Grankvist K, Jacobsen J, Ljungberg B. Impact of serum basic fibroblast growth factor on prognosis in human renal cell carcinoma. European Journal of Cancer. 2001;37(17):2199–203. doi: 10.1016/s0959-8049(01)00290-8. [DOI] [PubMed] [Google Scholar]
- 14.Wechsel HWBK, Feil G, Loeser W, Lahme S, Petri E. Renal cell carcinoma: relevance of angiogenetic factors. Anticancer Res. 1999 Mar–Apr;19(2C):1537–40. [PubMed] [Google Scholar]
- 15.Rennel ES, Harper SJ, Bates DO. Therapeutic potential of manipulating VEGF splice isoforms in oncology. Future Oncology. 2009;5(5):703–12. doi: 10.2217/fon.09.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cebe-Suarez S, Zehnder-Fjallman A, Ballmer-Hofer K. The role of VEGF receptors in angiogenesis; complex partnerships. Cellular and Molecular Life Sciences. 2006;63(5):601–15. doi: 10.1007/s00018-005-5426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang JC, Haworth L, Sherry RM, et al. A Randomized Trial of Bevacizumab, an Anti-Vascular Endothelial Growth Factor Antibody, for Metastatic Renal Cancer. N Engl J Med. 2003;349(5):427–34. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Escudier B, Bellmunt J, Negrier S, et al. Phase III Trial of Bevacizumab Plus Interferon Alfa-2a in Patients With Metastatic Renal Cell Carcinoma (AVOREN): Final Analysis of Overall Survival. J Clin Oncol. 2010;28(13):2144–50. doi: 10.1200/JCO.2009.26.7849. [DOI] [PubMed] [Google Scholar]
- 19.Rini BI, Halabi S, Rosenberg JE, et al. Phase III Trial of Bevacizumab Plus Interferon Alfa Versus Interferon Alfa Monotherapy in Patients With Metastatic Renal Cell Carcinoma: Final Results of CALGB 90206. J Clin Oncol. 2010;28(13):2137–43. doi: 10.1200/JCO.2009.26.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raymond E, Faivre S, Vera K, et al. Final results of a phase I and pharmacokinetic study of SU11248, a novel multi-target tyrosine kinase inhibitor, in patients with advanced cancers. Proc Am Soc Clin Oncol. 2003;22:192. [Google Scholar]
- 21.Strumberg D, Richly H, Hilger RA, et al. Phase I Clinical and Pharmacokinetic Study of the Novel Raf Kinase and Vascular Endothelial Growth Factor Receptor Inhibitor BAY 43-9006 in Patients With Advanced Refractory Solid Tumors. J Clin Oncol. 2005;23(5):965–72. doi: 10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 22.Rini BI WG, Hudes G, Stadler WM, et al. Axitinib (AG-013736; AG) in patients (pts) with metastatic renal cell cancer (RCC) refractory to sorafenib. J Clin Oncol. 2007. 2007 ASCO Annual Meeting Proceedings Part I. Vol 25, No. 18S (Jun 20 Supplement):5032.
- 23.Hurwitz HI, Dowlati A, Saini S, et al. Phase I Trial of Pazopanib in Patients with Advanced Cancer. Clinical Cancer Research. 2009;15(12):4220–7. doi: 10.1158/1078-0432.CCR-08-2740. [DOI] [PubMed] [Google Scholar]
- 24.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in Advanced Clear-Cell Renal-Cell Carcinoma. N Engl J Med. 2007;356(2):125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 25.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus Interferon Alfa in Metastatic Renal-Cell Carcinoma. N Engl J Med. 2007;356(2):115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 26.Motzer RJ, Hutson TE, Tomczak P, et al. Overall Survival and Updated Results for Sunitinib Compared With Interferon Alfa in Patients With Metastatic Renal Cell Carcinoma. J Clin Oncol. 2009;27(22):3584–90. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Limvorasak S, Posadas EM. Pazopanib: therapeutic developments. Expert Opin Pharmacother. 2009;10(18):3091–02. doi: 10.1517/14656560903436493. [DOI] [PubMed] [Google Scholar]
- 28.Sonpavde G, Hutson TE, Sternberg CN. Pazopanib for the treatment of renal cell carcinoma and other malignancies. Drugs Today (Barc) 2009;45(9):651–61. doi: 10.1358/dot.2009.45.9.1414786. [DOI] [PubMed] [Google Scholar]
- 29.Kumar R, Knick VB, Rudolph SK, et al. Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Molecular Cancer Therapeutics. 2007;6(7):2012–21. doi: 10.1158/1535-7163.MCT-07-0193. [DOI] [PubMed] [Google Scholar]
- 30.Votrient (pazopanib) [package insert] Research Triangle Park NG [Google Scholar]
- 31.Shibata SLJ, Chung VM, Lenz H, et al. A phase I and pharmacokinetic single agent study of pazopanib (P) in patients (Pts) with advanced malignancies and varying degrees of liver dysfunction (LD) J Clin Oncol. 2010;28:15s. (Suppl; abstr 2571) [Google Scholar]
- 32.Dejonge MSS, Verweij J, Collins TS, et al. A phase I, open-label study of the safety and pharmacokinetics (PK) of pazopanib (P) and lapatinib (L) administered concurrently. J Clin Onco. 2006;24:142s. (Suppl; abstr 3088) [Google Scholar]
- 33.Tan ARJS, Dowlati A, Levinson K, et al. Phase I study of the safety, tolerability, and pharmacokinetics (PK) of weekly paclitaxel administered in combination with pazopanib (GW786034) J Clin Oncol. 2008;26 (May 20 Suppl; abstr 3552) [Google Scholar]
- 34.Hutson TE, Davis ID, Machiels J-PH, et al. Efficacy and Safety of Pazopanib in Patients With Metastatic Renal Cell Carcinoma. J Clin Oncol. 2010;28(3):475–80. doi: 10.1200/JCO.2008.21.6994. [DOI] [PubMed] [Google Scholar]
- 35.Suttle B, Ball HA, Molimard M, et al. Relationship between exposure to pazopanib (P) and efficacy in patients (pts) with advanced renal cell carcinoma (mRCC) J Clin Oncol. 2010;28:15s. (Suppl; abstr 3048) [Google Scholar]
- 36.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in Locally Advanced or Metastatic Renal Cell Carcinoma: Results of a Randomized Phase III Trial. J Clin Oncol. 2010;28(6):1061–8. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 37.Sternberg CN. Pazopanib in renal cell carcinoma. Clin Adv Hematol Oncol. 2010 Apr;8(4):232–3. [PubMed] [Google Scholar]
- 38.Xu C-F, Reck BH, Xue Z, et al. Pazopanib-induced hyperbilirubinemia is associated with Gilbert’s syndrome UGT1A1 polymorphism. 2010;102(9):1371–7. doi: 10.1038/sj.bjc.6605653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and Prognostic Stratification of 670 Patients with Advanced Renal Cell Carcinoma. J Clin Oncol. 1999;17(8):2530. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 40.Mekhail TM, Abou-Jawde RM, BouMerhi G, et al. Validation and Extension of the Memorial Sloan-Kettering Prognostic Factors Model for Survival in Patients with Previously Untreated Metastatic Renal Cell Carcinoma. J Clin Oncol. 2005;23(4):832–41. doi: 10.1200/JCO.2005.05.179. [DOI] [PubMed] [Google Scholar]
- 41.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. The Lancet. 2008;372(9637):449–56. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 42.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, Interferon Alfa, or Both for Advanced Renal-Cell Carcinoma. New England Journal of Medicine. 2007;356(22):2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 43.DePrimo S, Bello C, Smeraglia J, et al. Soluble protein biomarkers of pharmacodynamic activity of the multi-targeted kinase inhibitor SU11248 in patients with metastatic renal cell cancer. Proc Am Assoc Cancer Res. 2005;46:108. [Google Scholar]
- 44.Escudier B, Szczylik C, Hutson TE, et al. Randomized Phase II Trial of First-Line Treatment with Sorafenib Versus Interferon Alfa-2a in Patients with Metastatic Renal Cell Carcinoma. J Clin Oncol. 2009;27(8):1280–9. doi: 10.1200/JCO.2008.19.3342. [DOI] [PubMed] [Google Scholar]