Abstract
We report a novel approach to the synthesis of GlcNAcstatins—members of an emerging family of potent and selective inhibitors of peptidyl O-GlcNAc hydrolase build upon tetrahydroimidazo[1,2-a]pyridine scaffold. Making use of a streamlined synthetic sequence featuring de novo synthesis of imidazoles from glyoxal, ammonia and aldehydes, a properly functionalised linear GlcNAcstatin precursor has been efficiently prepared starting from methyl 3,4-O-(2′,3′-dimethoxybutane-2′,3′-diyl)-α-d-mannopyranoside. Subsequent ring closure of the linear precursor in an intramolecular SN2 process furnished the key fused d-mannose-imidazole GlcNAcstatin precursor in excellent yield. Finally, a sequence of transformations of this key intermediate granted expeditious access to a variety of the target compounds bearing a C(2)-phenethyl group and a range of N(8) acyl substituents. The versatility of the new approach stems from an appropriate choice of a set of acid labile permanent protecting groups on the monosaccharide starting material. Application was demonstrated by the synthesis of GlcNAcstatins containing polyunsaturated and thiol-containing amido substituents.
Graphical abstract
1. Introduction
Reversible posttranslational modification of cytoplasmic and nuclear proteins in eukaryotic cells by glycosylation of (surface exposed) serines and threonines with 2-acetamido-2-deoxy-β-d-glucopyranosyl residues (O-GlcNAc) is believed to play important roles in diverse cellular processes, such as transcription, translation, signal transduction and protein trafficking and degradation.1–3 There is evidence to suggest that the faulty interplay between O-GlcNAcylation and competitive or synergistic phosphorylation of specific proteins is involved in progression and pathology of several metabolic and neurodegenerative diseases. Thus an increased level of O-GlcNAcylation on proteins along the insulin signalling pathway has been proposed to be involved in insulin sensitivity,4,5 and hyper-phosphorylation of the microtubule-associated protein tau that marks the development of the Alzheimer disease was shown to be reciprocal with the abnormally low O-GlcNAcylation.6
The molecular mechanisms and biological implications of O-GlcNAc attachment/hydrolysis are currently the subject of intense multidisciplinary investigation. In a striking contrast to the conceptually related reversible protein phosphorylation, regulated by a plethora of highly specific kinases and phosphatases, the dynamic cycling of O-GlcNAc in eukaryotes is achieved by a single pair of enzymes: peptidyl O-GlcNAc transferase (OGT, CAZY family GT417) and peptidyl O-GlcNAc hydrolase (OGA, GH84).
It has so far not been possible to study the cell biological effect of hypo-O-GlcNAcylation due to a lack of OGT inhibitors. However, OGA, with a number of stable functional substrate analogues available, provides an excellent opportunity to study the cellular effects of inhibitor-induced hyper-O-GlcNAcylation.
A number of potent OGA inhibitors have been developed in the recent decades, including PUGNAc (Ki=50 nM, hOGA)8–10 and NAG-thiazoline (Ki=70 nM, hOGA)11,12 (Fig. 1). Although both compounds are prone to hydrolysis in aqueous solutions and also potently inhibit lysosomal β-hexosaminidases (HexA/HexB), they became the standard tools for studying protein over O-GlcNAcylation. Recently, systematic variation of the size and electronic properties of the thiazoline substituent resulted in the discovery of the highly potent, selective and hydrolytically stable inhibitor Thiamet-G (Ki=21±3 nM, hOGA).13
Figure 1.
In parallel, a search for novel, potent and selective OGA inhibitors focussed our attention on the naturally occurring hexosaminidase inhibitor nagstatin14,15 (Fig. 1). Its unique tetrahydroimidazo[1,2-a]pyridine (fused sugar-imidazole) bicyclic scaffold and outstanding potency fuelled the research of Tatsuta et al. on the total synthesis of the parent compound and analogues.16,17 Ensuing work of Vasella et al. has established sugar-imidazoles among the most powerful β-glycosidase inhibitors.18 The origin of this potency was attributed to the half-chair conformation of the bicyclic core mimicking the flattened geometry of the putative pyranosyl oxocarbenium ion during the hydrolysis of glycosidic bond. The importance of the [1,2-a] fused imidazole ring was deduced to stem from in-plane protonation of the pseudo-anomeric nitrogen atom with a laterally positioned catalytic acid, that would proceed along the same trajectory as protonation of a glycosidic bond to yield the β-anomeric oxygen leaving group.19 Additionally, in contradiction with the earlier Tatsuta’s data, C(2) substituted fused sugar-imidazoles were found to show improved inhibition over unsubstituted analogues. The effect, particularly profound in the phenethyl-substituted fused hexose-imidazoles,20 has been also replicated for the series of C(2) phenethyl-substituted fused pentose-imidazoles.21 Surprisingly, the inhibition of hexosaminidases with nagstatin and analogous fused 2-acetamido-2-deoxy sugar-imidazoles, was only tested very recently, when synthesis of gluco-nagstatin was reported by Vasella et al.22,23
Based on these data and the availability of the crystal structure of a bacterial OGA (bOGA) homologue from Clostridium perfringens in complex with PUGNAc24 we designed GlcNAcstatin 6 (Fig. 1)—the founding member of a novel family of OGA inhibitors built on the fused d-glucosamine-imidazole scaffold. In a preliminary communication we reported synthesis and enzymatic profiling of this compound to reveal its outstanding potency and selectivity (Ki=4 pM, bOGA).25 Recently we reported the evaluation of a small set of GlcNAcstatins as inhibitors of human OGA to confirm that both potency and selectivity could also be achieved with the human enzyme.26
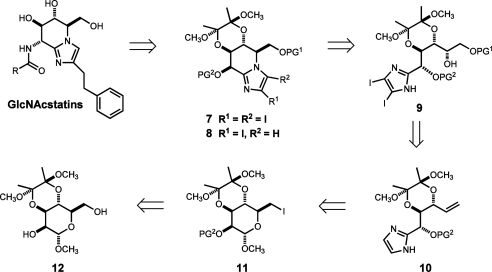
The synthesis of GlcNAcstatin was provisionally designed as a consolidation of two existing pathways to the fused sugar-imidazoles (Fig. 2).25 It exploited the highly efficient SN2 substitution of the OH(8) group in the manno-configured C(2)-branched derivative 4 (tetrahydroimidazo[1,2-a]pyridine numbering) leading to the gluco-azide 5 in accordance with Vasella’s findings.23 In turn, the key iodo-imidazole 3 was prepared using an extensively modified Tatsuta’s approach from the l-xylose 1 through the linear polyhydroxylated l-gulo-imidazole 2.16 The overall competence of the synthesis, however, was undermined by the poor efficacy of the opening step, which required separation of mixtures of diastereomers and unproductive protective group exchange. Further progress on the synthesis of GlcNAcstatins aimed at the development of more potent/selective inhibitors of hOGA was hampered by routine supply of the required intermediates. Aiming at the synthesis of derivatives bearing unsaturated or thiol-containing amide substituents, the abolishment of an array of permanent benzyl protecting groups on the sugar moiety would result in optimization of the synthetic scheme. To fulfil these objectives we developed a novel synthetic approach to GlcNAcstatins, making use of de novo synthesis of linear polyhydroxylated imidazoles21,27 from glyoxal, ammonia and chiral aldehydes available by reductive cleavage of sugar primary iodides (Bernet–Vasella reaction).28
Figure 2.
2. Results and discussion
We identified methyl 3,4-O-(2′,3′-dimethoxybutane-2′,3′-diyl)-α-d-mannopyranoside 12, easily available on a large scale,29 as the optimal monosaccharide starting material (Scheme 1). Robust 3,4-O-bis-acetal protecting group in 12, meeting the criteria for the acid labile permanent protecting group, would keep corresponding hydroxyls intact through the synthesis. The rigid bicyclic framework of 12 would also allow efficient differentiation of the remaining hydroxyl groups via selective alkylation of the OH(2) according to the Ley’s protocol30 thus securing a streamlined subsequent introduction of the 6-iodo substituent (12→11). Reductive cleavage of 11 would then afford the open-chain aldehyde, which is instantly ready for the three component construction of the imidazole ring (11→10). Asymmetric dihydroxylation of the double bond in 10 followed by selective protection of OH(6) with suitable silyl ether (acid labile) and bis-iodination of the imidazole ring would generate the required l-gulo configured cyclisation precursor 9.
Scheme 1.
We anticipated that after this point a synthetic sequence comprising of tetrahydroimidazo[1,2-a]pyridine ring closure (9→7) and selective C(3) deiodination of the imidazole (7→8) would give access to the key fused d-mannose-imidazole precursor of GlcNAcstatins 8, similar to the one successfully transformed into GlcNAcstatin in the provisional synthesis.25
In the event selective p-methoxybenzylation of the starting mannoside 12 with 1.15 equiv of p-methoxybenzyl chloride (PMBCl) in the presence of n-Bu4NI provided the expected 2-O-PMB ether 13 in about 50% yield along with 25% of 2,6-di-O-PMB derivative 14 (Scheme 2). Notably, the control experiment with benzyl bromide afforded the corresponding 2-O-Bn ether in 78% yield, in agreement with the original data.30 We found that the primary PMB group in compound 14 could be selectively removed by hydrogenolysis under controlled conditions to give a 10:1:9 mixture of 2-O- and 6-O-monoalkylated derivatives and the starting material 14 from which the 2-O-PMB ether 13 was isolated in 50% yield after flash chromatography. This observation was used advantageously to increase the overall yield of the requisite compound 13 to 60%, starting from the bis-acetal 12. Substitution of the OH(6) in 13 was then achieved using PPh3/imidazole/I2 in hot toluene31 to furnish the iodo-derivative 15 in 75% yield on 30 mmol scale. The ensuing reductive splitting of the pyranoside ring of 15 using a variant of the Bernet–Vasella reaction28 with freshly activated zinc in aqueous THF at 65 °C gave the intermediate open-chain aldehyde, which on treatment with 40% glyoxal solution in 7 M methanolic ammonia initially at 0 °C and then at 70 °C for 2 h consistently produced the key intermediate imidazole 16 in a rewarding 70% overall yield.
Scheme 2.
Reagents and conditions: (i) PMBCl, NaH, n-Bu4NI, DMF, rt, 16 h, 50%; (ii) I2, ImH, PPh3, toluene, 70 °C, 3 h, 75%; (iii) H2, Pd/C, MeOH/EtOAc, 3 h, 50%; (iv) (a) Zn, THF/H2O (10:1), 65 °C, 1 h, (b) 40% aqueous glyoxal, 7 M NH3/MeOH, 0–70 °C, 2 h, 70%.
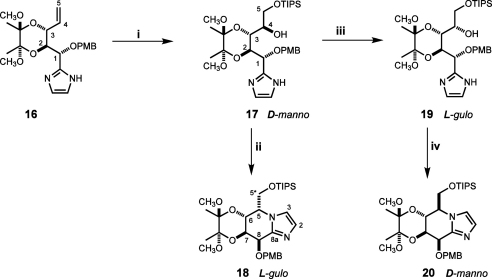
Initial catalytic dihydroxylation of the double bond in the open-chain imidazole 16 with K2(OsO4)/K3[Fe(CN)6] was found to be highly stereoselective, yet unfortunately providing the unwanted d-manno-configured derivative 17 as a sole product isolated in 78% yield after selective silylation of the primary hydroxy group with triisopropyl chlorosilane (Scheme 3). Strikingly, running the reaction in the presence of varying amounts of the β-selective (DHQD)2-PHAL ligand (0.1–5%) did not affect the stereochemical outcome or efficiency of the process, suggesting an overpowering intrinsic facial stereospecificity of the substrate.32 The absolute configuration of 17 was established after its transformation into the fused sugar-imidazole derivative 18 by treatment with Tf2O in pyridine for 1 h at rt. Formation of the bicyclic compound was confirmed by HRMS and NMR spectra. In particular, the presence of the (C8a)–(H5) and (C3)–(H5) (tetrahydroimidazo[1,2-a]pyridine numbering) cross-peaks in the HMBC spectra proved that imidazole moiety was covalently bound to C(5). Assuming clean inversion of the configuration at C(5) occurred during cyclisation, observation of the nuclear Overhauser effect (NOE) between protons H(7) and H(5∗a, b) in compound 18 was only compatible with its l-gulo configuration and, respectively, d-manno configuration of the starting compound 17.
Scheme 3.
Reagents and conditions: (i) (a) K2(OsO4)/K3[Fe(CN)6], K2CO3, CH3SO2NH2, t-BuOH/THF/water, rt, 24 h, (b) TIPSCl, Py, 50 °C, 16 h, 78%; (ii) Tf2O, pyridine, −15 °C tort, 1 h, 92%; (iii) (a) (COCl)2, DMSO, DCM, −60 °C then Et3N, (b) NaBH4, EtOH, rt, 16 h, 80%; (iv) Tf2O, Py, C2H4Cl2, −15 °C then 50 °C, 3 h, 90%.
The origin of the exceptional substrate-controlled selectivity of the dihydroxylation reaction is not immediately obvious. It is possible that it could stem from formation of a complex of osmium tetroxide with the imidazole; in this case, formation of the d-manno-configured product would result from the intramolecular delivery of the reagent onto the eclipsed rotamer of the double bond. On the other hand, the same stereochemical outcome could result from an effective shielding of one of the diastereotopic faces of the double bond by the bulky C1 substituent. In this case, however, the double bond should react in a less favourable bisecting conformation. Whatever the origin of the unique stereoselectivity of the process, the reported observations provide a rare glimpse on the barely explored stereodirecting properties of bis-acetal protected substrates in dihydroxylation reactions.33
In an attempt to invert the configuration of OH(4) in compound 17 we have found that it remained unchanged in the Mitsunobu esterification with DIAD/PPh3/p-nitrobenzoic acid. However, a simple two-step Swern oxidation34—NaBH4 reduction sequence cleanly provided a 1:10 mixture of the epimeric alcohols 17 and 19. The ample difference of the retention factors allowed the individual isomers to be isolated in 9% and 80% yields, respectively, by chromatographic separation. Operational simplicity and scalability of the devised sequence resulted in only a limited exploration of alternative, more selective, reducing agents. Addition of cerium chloride (NaBH4, CeCl3·7H2O/MeOH; −78 °C to rt) did not change the stereoselectivity, whereas DIBAL failed to reduce the intermediate ketone even at ambient temperature.
The configuration of OH(4) in compound 19 was formally established after closure of the bicyclic compound 20, which required heating of the intermediate bis-triflate at 50 °C for 3 h to go to completion (Scheme 3). The observation of the NOE between H(6) and H(5∗a, b) in compound 20 established its d-manno absolute configuration and consequently the l-gulo configuration of compound 19.
Similar to the stereochemical outcome of the dihydroxylation of 16, the formal Felkin–Anh stereochemistry of the reduction of the intermediate ketone leading to the preferential formation of the alcohol 19 is difficult to rationalise. Due to the presence of the rigid bis-acetal framework adorned with the imidazole ring and multiple alkoxy substituents, a choice of opportunities for remote chelate formation is endless making the analysis in terms of chelate or non-chelate models not conclusive.
In line with our previous findings25 we initially decided to convert the open-chain imidazole 19 into the bis-iodo-derivative 21 prior to cyclisation to avoid the drastic iodination conditions used by Vasella et al. for iodination of preformed fused sugar-imidazoles.35 As expected, iodination of 19 with 2.5 equiv of NIS in MeCN proceeded smoothly to give the requisite compound 21 (Scheme 4). However, all attempts to effect its annulation into the targeted tetrahydroimidazo[l,2-a]pyridine derivative 22 failed in marked contrast with the highly effective cyclisation of the parent non-iodinated compound 19 and previously observed smooth cyclisation of the benzylated/benzoylated analogue not possessing bis-acetal protection.25 The lack of reactivity may be explained by the spatial congestion developing in a transition state between the iodine substituent at C(3) position of the imidazole ring and the bulky primary triisopropylsilyloxy group.
Scheme 4.
Reagents and conditions: (i) NIS 2.5 equiv, MeCN, 6 h, rt, 95%; (ii) Tf2O, Py, C2H4Cl2, −15 °C then 50 °C; (iii) NIS 10 equiv, DMF, 85 °C, 36 h, 80% or NIS 3 equiv, MeCN, PPTS, 80 °C, 3 h, 65%; (iv) EtMgBr, THF, 0 °C, 10 min, 88%.
Finally we found that post-cyclisation iodination of the fused sugar-imidazole 20 using an even more vigorous variant of Vasella’s original conditions (10 equiv NIS, DMF, 85 °C, 36 h) furnished the key diiodide 22 as slightly yellow oil in 80% yield after chromatographic purification of the very dark reaction mixture (Scheme 4). Alternatively, iodination of 20 could be performed using 3 equiv of NIS in MeCN at 80 °C in the presence of 1 equiv of PPTS36 giving the requisite compound 22 in 60% yield. The detected formation of p-methoxybenzyl iodide as a by-product pointed at an inadequate stability of the PMB protective group towards the NIS/PPTS combination as a principal reason for the diminished efficacy of bis-iodination. In the concluding step, the regioselective mono-deiodination of 22 at C(3) with EtMgBr/THF at 0 °C was executed without incident to give the key mono-iodoimidazole 23 in 88% yield.
It is worth noting that explorative one-step iodination/iodocyclisation of the unsaturated open-chain imidazole 16 with 3 equiv of NIS resulted in transient formation of the bis-iodinated imidazole 24 and, finally, triply iodinated tetrahydro-5H-imidazo[1,2-a]azepine derivative 25 in 78% yield as a result of apparent anti-Baldwin 6-endo-trig cyclisation (Scheme 5). The structure of the compound 25 was initially deduced from the high field shift of C(6) (δ 26.7) (imidazo-azepine numbering) in the 13C NMR spectrum, pointing at the iodine substitution site. This was further confirmed by observation of the (C9a)–(H5a)/(5b) cross-peaks in the HMBC spectrum, revealing covalent connection of the imidazole and the methylene group of the azepine ring. The configuration of the C(6) iodo substituent was established from the presence of the H(6)–H(8) cross-peak in the NOESY spectrum showing spatial proximity of the corresponding protons.
Scheme 5.
Reagents and conditions: (i) NIS 3 equiv, MeCN, 6 h, rt, 78%.
Subsequently, a highly efficient Sonogashira coupling37 of the key iodoimidazole 23 with phenylacetylene provided the fully assembled precursor of GlcNAcstatins 26 with the C(2) phenylethynyl substituent in place in 93% yield (Scheme 6). Critical stereoselective conversion of the manno-configured 26 into the gluco-azide 28 was achieved in two steps starting with oxidative removal of the PMB protective group with DDQ (26→27; 83%) followed by azidation of the alcohol 27 with DPPA/DBU under optimized conditions (toluene, 80 °C, 2 h) to produce the requisite azide 28 in 93% yield as the single product. The gluco-configuration of the compound 28 was unambiguously deduced from the value of the corresponding coupling constant (J7,8 9.5 Hz) as well as the presence of H(6)–H(8) cross-peak in the NOESY spectrum.
Scheme 6.
Reagents and conditions: (i) PhCCH, CuI, Et3N, Pd(PPh3)4, DMF, 80 °C, 16 h, 93%; (ii) DDQ, DCM/H2O, 4 h, 83%; (iii) DPPA, DBU, toluene, rt then 80 °C, 2 h, 93%; (iv) (a) H2, Pd/C, MeOH or EtOAc, 1 h (b) (RCO)2O, Et3N, DCM for 29–31 or PyBOP, DIPEA, RCO2H, DCM, rt, 16 h, for 32–34; (v) TFA/H2O (95:5), rt, 36 h, 60–80%; (vi) DMF, MeONa/MeOH, DTT, 2 h, 56%.
Starting from the azide 28 a panel of fully protected N-acylated GlcNAcstatins precursors 29–34 encompassed derivatives of acetic, propionic, iso-butyric, valeric, 2,4-pentanedioic38 and 3-(acetylthio)propionic acids has been prepared using a uniform sequence consisted of simultaneous hydrogenation both of the triple bond and the azido group over Pd catalyst followed by reaction of the intermediate amine with suitable acylating agents.
As the concluding step of the synthesis, removal of the permanent protecting groups (TIPS and 3,4-O-dimethoxybutanediyl bis-acetal) was intended to be achieved with acid hydrolysis in one step. In the event the reaction was found to be prohibitively slow. When solutions of a substrate in DCM were exposed to the increasing (10–50%) concentrations of TFA/water (95:5) in DCM, both protecting groups remained intact for a period of several hours. Moreover, it appeared that the TIPS group could be selectively removed by treatment with 4 M HCl in THF for 16 h, leaving the butanediyl bis-acetal protection mostly unchanged. Our findings appear to be in agreement with a singular report suggesting that removal of 3,4-O-bis-acetal protection in N-acetyl glucosamine derivatives could be a slow process.39 It seems that protonation of the imidazole ring of the fused N-acetylated glucosamine-imidazoles 29–34 contributes significantly to the acid stability of the butanediyl bis-acetal protection making it even more resistant towards hydrolysis than in N-acetyl glucosamine derivatives. Finally, global deprotection of 29–34 was achieved by 36 h treatment with aqueous TFA (95:5) to furnish the requisite compounds 35–40 in acceptable yields after reversed phase chromatography purification. To obtain the 3-thiopropionic acid derivative 41, S-acetyl protection was finally removed from 40 with MeONa/MeOH in the presence of dithioerythrol.
3. Conclusions
We reported here a novel approach to the synthesis of a family of potent and selective inhibitors of peptidyl O-GlcNAcase (GlcNAcstatins) built on the fused d-glucosamine-imidazole scaffold. At the outset of the synthesis a short practical route to the densely functionalised open-chain imidazole bearing a terminal double bond has been developed using a novel combination of Bernet–Vasella reaction and de novo imidazole synthesis. Dihydroxylation of the double bond in this intermediate was found to be exclusive substrate controlled process leading to formation of the unwanted d-manno-configured product. The inversion of configuration of the OH(4) group has been achieved by a serendipitously found by-pass method using stereoselective reduction of the intermediate ketone. The obtained l-gulo substrate resisted all attempts to be cyclised into bicyclic derivative when iodine atoms have been preinstalled at the imidazole ring. In contrast, seamless cyclisation of the non-iodinated precursor into the requisite fused d-mannose-imidazole was easily achieved using the standard protocol. Ultimately, a Sonogashira reaction of C(2) iodo-derivative of the cyclised compound with phenylacetylene provided highly efficient access to the advanced intermediate in the GlcNAcstatin synthesis bearing a phenylethynyl substituent at the C(2) position. Starting from this compound, a set of GlcNAcstatins with assorted amido substituents has been prepared. Although the very last step of the synthesis, i.e., acidolytic removal of the permanent triisopropylsilyl and cyclic bis-acetal protections was found to be rather sluggish, the versatility of the new synthetic approach was clearly demonstrated by the preparation of GlcNAcstatin derivatives of 2,4-pentanedioic and 3-mercaptopropionic acids intrinsically incompatible with the hydrogenolytic deprotection routines. The new approach also gives access to rapid future exploration of alternative C(2) substituents that may yield even more potent, selective and cell-permeable GlcNAcstatin derivatives.
4. Experimental
4.1. General
All reactions were performed in oven-dried glassware under an inert atmosphere (argon) unless noted otherwise. Dichloromethane, acetonitrile, triethylamine were anhydrous grade solvents from Fluka kept over molecular sieves. Analytical thin layer chromatography (TLC) was performed on Merck silica gel 60 F254 aluminium plates (0.25 mm). Compounds were visualized by UV light, or by dipping the plate into acidic potassium permanganate aqueous solution followed by washing out the excess of the reagent, or by charring the plate at ca. 300 °C after dipping in a 5% phosphomolybdic acid solution in ethanol. Flash chromatographic separations were performed on Isco RediSep flash columns using Buchi gradient pump system. NMR spectra were recorded on Bruker AVANCE II 500 spectrometer. Splitting patterns of spectral multiplets are indicated as s, singlet; d, doublet; br s broad singlet; br d broad doublet; t triplet; quint quintet. Signals were assigned by means of DEPT, COSY, HSQC, and HMBC spectra. High-resolution mass spectra (HRMS) were obtained on a microTOF Bruker Daltonics instrument. Optical rotations were measured in chloroform on a Perkin–Elmer 343 polarimeter at 20 °C.
FT-IR spectra were recorded on Perkin–Elmer Spectrum BX instrument.
4.1.1. (2′S,3′S)-Methyl 2-O-(4-methoxybenzyl)-3,4-O-(2′,3′-dimethoxybutane-2′,3′-diyl)-α-d-mannopyranoside (13) and (2′S,3′S)-methyl 2,6-di-O-(4-methoxybenzyl)-3,4-O-(2′,3′-dimethoxybutane-2′,3′-diyl)-α-d-mannopyranoside (14)
To a cooled (ice-bath) solution of 12 (15.16 g, 49.16 mmol) and p-methoxybenzyl chloride (7.5 mL, 55.31 mmol) in DMF (250 mL) sodium hydride (60% in oil; 4 g, 100 mmol) was added in portions. The reaction was stirred for 1 h and then removed from the ice-bath; n-Bu4NI (1.8 g, 4.87 mmol) was added at this moment. The reaction was further stirred for 16 h at rt. An excess of sodium hydride was quenched by careful addition of ice chips with external cooling (ice-bath). After gas evolution ceased the reaction was partitioned between water and ethyl acetate and the layers were separated. The organic layer was washed with water. The aqueous layers were additionally extracted with ethyl acetate. The combined organic layer was dried and concentrated. The residue was purified by flash chromatography in Tol/EA 5→50% to give and 11.37 g (26.5 mmol, 54%) of the title compound 13 as foam and 6.58 g (11.99 mmol, 25%) of the title compound 14 as clear syrup.
4.1.2. Compound 13
δH (500 MHz, CDCl3) 7.38–7.32 (2H, m, CH2PhOMe), 6.88–6.82 (2H, m, CH2PhOMe), 4.87 and 4.58 (AB spectrum, J 11.4 Hz, CH2PhOMe), 4.63 (1H, d, J1,2 1.3 Hz, H-1), 4.18 (1H, t, J3,4=J4,5=10.3 Hz, H-4), 4.05 (1H, dd, J3,4 10.3, J2,3 2.8 Hz, H-3), 3.81 (1H, dd, J6a,6b 11.7, J6a,5 2.6 Hz, H-6a), 3.77 (3H, s, OMe), 3.78–3.75 (1H, m, H-6b), 3.72–3.68 (1H, m, H-5), 3.68 (1H, dd, J2,3 2.8, J1,2 1.3 Hz, H-2), 3.28 (6H, s, 2×OMe), 3.27 (3H, s, OMe), 1.34 (3H, s, Me), 1.29 (3H, s, Me).
δC (126 MHz, CDCl3) 159.1, 130.7, 129.7, 129.1, 128.2, 113.62, 113.6, 113.5, 100.6 (C-1), 99.8, 99.5, 75.2 (C-2), 72.9, 71.1 (C-5), 69 (C-3), 63.6 (C-4), 61.4 (C-6), 55.2, 54.6, 47.9, 47.8, 17.8, 17.7. νmax (KBr) 3060, 3030, 2981, 2950, 1487, 1382, 1216, 1134, 892 cm−1. [α]D +132.3 (c 1.0, CHCl3). Rf=0.28; Tol/EA 30%. HRMS-(TOF): MH+, found 429.2128. C21H33O9 requires 429.2125.
4.1.3. Compound 14
δH (500 MHz, CDCl3) 7.41–7.35 (2H, m, CH2PhOMe), 7.28–7.23 (2H, m, CH2PhOMe), 6.90–6.81 (4H, m, 2×CH2PhOMe), 4.88 and 4.63 (2H, AB spectrum, J 11.7 Hz, CH2PhOMe), 4.70 (1H, d, J1,2 1.4 Hz, H-1), 4.59 and 4.53 (2H, AB spectrum, J 11.6 Hz, CH2PhOMe), 4.17 (1H, t, J3,4=J4,5=10.2 Hz, H-4), 4.07 (1H, dd, J3,4 10.2, J3,2 2.9 Hz, H-3), 3.90–3.85 (1H, m, H-5), 3.83 (s, 3H, OMe), 3.82 (s, 3H, OMe), 3.76 (1H, dd, J6a,6b 11.1, J6a,5 2.2 Hz, H-6a), 3.72 (1H, dd, J6a,6b 11.1, J6b,5 5.5 Hz, H-6b), 3.68 (1H, dd, J3,2 2.9, J1,2 1.6 Hz, H-2), 3.35 (3H, s, OMe), 3.29 (3H, s, OMe), 3.21 (3H, s, OMe), 1.35 (3H, s, Me), 1.29 (3H, s, Me).
δC (126 MHz, CDCl3) 159.1, 159, 130.9, 130.7, 129.6, 129.1, 113.6, 100.4 (C-1), 99.8, 99.5, 75.4 (C-2), 73.1, 72.7, 71 (C-5), 69.2 (C-3), 68.6 (C-6), 63.8 (C-4), 55.1, 54.5, 47.83, 47.8, 17.85, 17.8. [α]D +140.6 (c 1.0, CHCl3). Rf=0.6; Tol/EA 30%. HRMS-(TOF): MH+, found 549.2691. C29H41O10 requires 549.2700.
4.1.4. Compound 13 from 14
A solution of 13 (6.5 g, 11.84 mmol) in MeOH/ethyl acetate 1:1 (120 mL) was hydrogenated under slight positive pressure of H2 (balloon) in the presence of Pd/C 20% catalyst (0.6 g) for 3 h at rt. The reaction mixture was filtered through a pad of Celite. The filter cake was washed additionally with MeOH; the combined filtrate was concentrated. The residue was purified by flash chromatography in Tol/EA 5→50% to give 2.56 g (5.98 mmol, 50%) of the title compound 13 and 2.72 g (4.95 mmol, 41%) of the starting material 14.
4.1.5. (2′S,3′S)-Methyl 6-deoxy-2-O-(4-methoxybenzyl)-3,4-O-(2′,3′-dimethoxybutane-2′,3′-diyl)-6-iodo-α-d-mannopyranoside (15)
To a solution of 13 (11.37 g, 26.53 mmol) and PPh3 (10.23 g, 39 mmol) in toluene (270 mL) imidazole (5.41 g, 79.5 mmol) and iodine (9.13 g, 36 mmol) were sequentially added to form a biphasic mixture with brown coloured lower layer. The reaction was placed into preheated oil bath (70 °C) and stirred for 4 h while the colour gradually faded. The reaction was cooled and concentrated. The residue was dissolved in CHCl3, absorbed on silica (80 g) and purified by flash chromatography in PE/EA 0→20% to give 11.13 g (20.67 mmol, 78%) of the title compound 15 as white solid.
δH (500 MHz, CDCl3) 7.39–7.35 (2H, m, CH2PhOMe), 6.90–6.86 (2H, m, CH2PhOMe), 4.87 and 4.62 (2H, AB spectrum, J 11.7 Hz, CH2PhOMe), 4.68 (1H, d, J1,2 1.3 Hz, H-1), 4.04 (1H, dd, J3,4 10.1, J3,2 2.8 Hz, H-3), 3.95 (1H, dd, J3,4 10.1, J4,5 9.1 Hz, H-4), 3.83 (3H, s, OMe), 3.69 (1H, m, H-5), 3.68 (1H, dd, J2,3 2.8, J1,2 1.3 Hz, H-2), 3.59 (1H, dd, J6a,6b 10.5, J6a,5 2.2 Hz, H-6a), 3.39 (3H, s, OMe), 3.31 (3H, s, OMe), 3.29 (3H, s, OMe), 3.26 (1H, dd, J6a,6b 10.5, J6b,5 8.8 Hz, H-6b), 1.36 (3H, s, Me), 1.32 (3H, s, Me).
δC (126 MHz, CDCl3) 159.1, 130.7, 129.6, 113.6, 100.5 (C-1), 99.9, 99.7, 75.2 (C-2), 72.8, 70.7 (C-5), 68.7 (C-3), 67.7 (C-4), 55.3, 54.9, 48.2, 48.1, 17.8, 5.2 (C-6). νmax (KBr) 3084, 3052, 2968, 2934, 2863, 1591, 1472, 1430, 988, 763 cm−1. Rf=0.35; PE/EA 10%, [α]D 151.5 (c 1.00, CHCl3). Mp 82.5 °C petroleum ether/chloroform. HRMS-(TOF): MNa+, found 561.0977. C21H31INaO8 requires 561.0961.
4.1.6. 2-[(R)-[(2S,3R,5R,6R)-5,6-Dimethoxy-5,6-dimethyl-3-vinyl-1,4-dioxan-2-yl]-[(4-methoxyphenyl)methoxy]methyl]-1H-imidazole (16)40
Zinc dust (13.7 g, 210 mmol) was activated by swirling with 3 M HCl (50 ml) for 3 min. The acid was decanted and the sediment was washed (3×50 ml) with water, then EtOH (50 ml) and finally with ether (50 ml). The light grey powder was dried in vacuum and added to a solution of 15 (11.13 g, 20.67 mmol) in THF/water (10:1) (100 mL). The reaction was brought to reflux (85 °C oil bath) and stirred for 40 min. The reaction was cooled to rt and filtered through a pad of Celite. The filter cake was washed with ether. The combined filtrate was concentrated, diluted with CHCl3 and washed with brine. The aqueous layer was extracted additionally with CHCl3 twice. The combined organic layer was dried, concentrated and dried briefly in vacuum to give clear oily residue (about 10 g). The residue was dissolved in 7 M methanolic ammonia (100 mL) at 4 °C (ice-bath) and mixed with 40% aqueous glyoxal solution (10 mL). The reaction was removed from the cooling bath and further stirred at rt for 1.5 h while the initially formed thick white precipitate dissolved and solution progressively turned yellow. The reaction was then heated up to 70 °C and kept at this temperature for 2 h; the solution turned brown with brown sediment. The reaction was cooled and volatiles were removed in vacuum. The residue was dissolved in CHCl3/MeOH, absorbed on silica (50 g) and purified by flash chromatography in Tol/Me2CO 5→30% to give 6.1 g (14.58 mmol, 70%) of the title compound 16 as clear syrup.
δH (500 MHz, CDCl3) 7.27–7.21 (2H, m, CH2PhOMe), 7.07 (2H, s, H-4′, H-5′), 6.87–6.82 (2H, m, CH2PhOMe), 5.69 (1H, ddd, J4,5 trans 17.3, J4,5 cis 10.3, J3,4 7.7 Hz, H-4), 5.24 (2H, m, H-5a, H-5b), 4.60 (1H, d, J1,2 2.4, H-1), 4.52–4.45 (2H, m, CH2PhOMe), 4.12 (1H, dd, J2,3 10.4, J1,2 2.4 Hz, H-2), 3.79 (3H, s, OMe), 3.66 (1H, ddt, J2,3 10.4, J3,4 7.8, J3,5 0.8 Hz, H-3), 3.38 (s, 3H, OMe), 3.12 (3H, s, OMe), 1.41 (3H, s, Me), 1.30 (3H, s, Me).
δC (126 MHz, CDCl3) 159.1, 144.7 (C-2′), 133.0 (C-4), 130.1, 129.2 (br s, C-4′/5′), 120.9 (C-5), 116.4 (br s, C-4′/5′), 113.7, 98.7, 98.5, 73 (C-2), 72.4 (C-1), 70.7, 70.3 (C-3), 55.2, 48.3, 48, 17.8, 17.5. νmax (KBr) 3067, 3033, 2869, 1877, 1812, 1599, 1535, 1497, 1455, 1364, 1343, 1113, 1028, 963 cm−1. Rf=0.25; Tol/Me2CO 30%, [α]D +41.6 (c 0.62, CHCl3). HRMS-(TOF): MH+, found 419.2185. C22H31N2O6 requires 419.2182.
4.1.7. (1R)-1-[(2R,3R,5R,6R)-3-[(R)-1H-Imidazol-2-yl-[(4-methoxyphenyl)methoxy]methyl]-5,6-dimethoxy-5,6-dimethyl-1,4-dioxan-2-yl]-2-triisopropylsilyloxy-ethanol (17)40
Freshly prepared solution of K3Fe(CN)6 (0.888 g, 2.7 mmol), K2CO3 (0.373 g, 2.7 mmol) and K2OsO4·2H2O (0.008 g, 0.022 mmol) in t-BuOH/water 1:1 (16 mL) was added to a solution of 16 (0.375 g, 0.9 mmol) and methanesulfonamide (0.095 g, 1 mmol) in t-BuOH/THF 1:1 (2.7 mL). The resulting slurry was vigorously stirred for 16 h. The reaction was quenched by addition of an excess of Na2S2O5 (1 g), stirred for 0.5 h rt, diluted with water and extracted with ethyl acetate three times. The combined organic layer was dried and concentrated to give 0.41 g (0.9 mmol) of the crude product; the TLC revealed complete consumption of the starting material and formation of a more polar single product (Tol/Me2CO 40%; Rf=0.15), (DCM/MeOH 5%; Rf=0.3).
The residue was dissolved in pyridine (5 mL) and treated with triisopropyl chlorosilane (0.213 mL, 1 mmol). The reaction was kept at 50 °C for 16 h. The reaction was cooled to rt, quenched by addition of MeOH (0.5 mL), kept for 20 min at rt and concentrated. The residue was dissolved in DCM and washed with water. The layers were separated; the aqueous layer was extracted with DCM once more. The combined organic layer was dried and concentrated. The residue was purified by flash chromatography in PE/EA 5→50% to give 0.422 g (0.693 mmol, 77%) of the title compound 17 as viscous syrup.
δH (500 MHz, CDCl3) 7.28–7.24 (2H, m, CH2PhOMe), 7.04 (2H, s, H-4′, H-5′), 6.88–6.83 (2H, m, CH2PhOMe), 5.35 (1H, d, J1,2 2.9 Hz, H-1), 4.55 (2H, s, CH2PhOMe), 4.26 (1H, dd, J2,3 10.1, J1,2 2.9 Hz, H-2), 3.89 (1H, dd, J5a,5b 9.9, J5a,4 3.6 Hz, H-5a), 3.80 (3H, s, OMe), 3.77 (1H, dd, J5a,5b 9.9, J5b,4 3.4 Hz, H-5b), 3.61 (1H, dt, J4,5 8.5, J4,5b=J4,5b 3.5 Hz, H-4), 3.38 (3H, s, OMe), 3.34 (1H, dd, J2,3 10.1, J3,4 8.6, H-3), 3.11 (3H, s, OMe), 1.38 (3H, s, Me), 1.26 (3H, s, Me), 1.11–0.97 (21H, m, Si(CH(Me)2)3).
δC (126 MHz, CDCl3) 159, 145.8 (C-2′), 130.5, 129.1, 113.7, 98.4, 98.3, 73.3 (C-1), 73.0 (C-2), 71.7 (C-4), 71.1, 67.2 (C-3), 63.1 (C-5), 55.2, 48.3, 48, 17.9, 17.7, 17.4, 11.9. νmax (KBr) 3028, 3012, 2989, 2899, 1732, 1455, 1383, 1229, 1159, 1072, 857 cm−1. Rf=0.33; PE/EA 40%, [α]D +54.2 (c 1.0, CHCl3). HRMS-(TOF): MH+, found 609.3565. C31H53N2O8Si requires 609.3571.
4.1.8. (2S,3S,4aS,5R,10S,10aR)-2,3-Dimethoxy-5-((4-methoxybenzyl)oxy)-2,3-dimethyl-10-(((triisopropylsilyl)oxy)methyl)-2,3,4a,5,10,10a-hexahydro-[1,4]dioxino[2,3-d]imidazo[1,2-a]pyridine (18)41
To a solution of 17 (0.057 g, 0.095 mmol) and Py (0.032 mL, 0.38 mmol) in DCM (1.5 mL) cooled to −15 °C trifluoromethanesulfonic anhydride (0.047 mL, 0.28 mmol) was added dropwise. The reaction was allowed to warm-up to the rt and stirred for 1 h at rt. The reaction was diluted with DCM and washed with a mixture of NaHCO3 solution and brine. The aqueous layer was extracted with DCM once more. The combined organic layer was dried and concentrated. The residue was purified by flash chromatography Tol/EA 10→40% to give 0.055 g (0.094 mmol, 100%) of the title compound 18 as glassy solid.
δH (500 MHz, CDCl3) 7.47–7.41 (2H, m, CH2PhOMe), 7.07 (1H, d, J2,3 1.3 Hz, H-2), 7.01 (1H, d, J2,3 1.3 Hz, H-3), 6.90–6.83 (2H, m, CH2PhOMe), 4.95 and 4.78 (2H, AB spectrum, J 11.1 Hz, CH2PhOMe), 4.83 (1H, dd, J6,7 11.1, J6,5 7.0 Hz, H-6), 4.78 (1H, d, J8,7 3.8 Hz, H-8), 4.39 (1H, dd, J7,6 11.1, J7,8 3.8 Hz, H-7), 4.34–4.29 (1H, m, H-5), 4.12 (1H, dd, J5*a,5*b 10.5, J5*a,5 3.0 Hz, H-5∗a), 3.98 (1H, dd, J5*a,5*b 10.6, J5*b,5 5.1 Hz, H-5∗b), 3.80 (3H, s, OMe), 3.32 (3H, s, OMe), 3.25 (3H, s, OMe), 1.41 (3H, s, Me), 1.34 (3H, s, Me), 1.10–0.97 (21H, m, Si(CH(Me)2)3).
δC (126 MHz, CDCl3) 158.9, 143.5 (C-8a), 131, 129.5, 129.2 (C-2), 119.1 (C-3), 113.54, 113.5, 113.4, 99.4, 99.3, 72.0, 70.1 (C-8), 67.2 (C-7), 62.8 (C-6), 62.5 (C-5∗), 58.2 (C-5), 55.2, 48.1, 48.03, 18.01, 17.9, 17.6, 11.8. νmax (KBr) 3090, 3067, 1952, 1879, 1810, 1751, 1603, 1525, 1496, 1310, 1085, 1028, 980 cm−1. Rf=0.3; Tol/EA 30%, [α]D −27.6 (c 1.0, CHCl3). HRMS-(TOF): MH+, found 591.3460. C31H51N2O7Si requires 591.3466.
4.1.9. (1S)-1-[(2R,3R,5R,6R)-3-[(R)-1H-Imidazol-2-yl-[(4-methoxyphenyl)methoxy]methyl]-5,6-dimethoxy-5,6-dimethyl-1,4-dioxan-2-yl]-2-triisopropylsilyloxy-ethanol (19)40
To a solution of DMSO (0.97 mL, 13.7 mmol) in DCM (65 mL) trifluoroacetic anhydride (1.43 mL, 10.25 mmol) was added dropwise at −60 °C. The reaction was stirred for 20 min then a solution of 17 (5.2 g, 8.54 mmol) in DCM (20 mL) was added via capillary. The reaction was further stirred for 45 min before Et3N (4.76 mL, 34.16 mmol) was added dropwise. The reaction was stirred for 5 min at −60 °C and then was warmed to rt. The reaction was quenched by addition of 10% citric acid solution and diluted with DCM. The layers were separated; the organic layer was successively washed with water and a mixture of saturated NaHCO3 solution and brine. The aqueous layers were additionally extracted with DCM; the combined organic layer was dried and concentrated to yield a semi-solid residue (5.25 g); TLC PE/EA 50% showed disappearance of the starting material (Rf=0.45) and formation of more polar product (Rf=0.35).
The residue was dissolved in EtOH (60 mL) and treated with NaBH4 (0.37 g, 10 mmol) at rt for 16 h. The reaction was quenched by addition of acetic acid and concentrated. The residue was partitioned between 1 M HCl and DCM and the layers were separated. The organic layer was washed successively with water and a mixture of saturated NaHCO3 solution and brine. The aqueous layers were additionally extracted with DCM. The combined organic layer was dried and concentrated. The residue was purified by flash chromatography in Tol/EA 20→50% to give 0.53 g (0.88 mmol, 9%) of the starting material 17 and 4.8 g (7.9 mmol, 81%) of the title compound 19 as viscous syrup.
δH (500 MHz, CDCl3) 7.29–7.24 (2H, m, CH2PhOMe), 7.03 (2H, s, H-4′, H-5′), 6.88–6.81 (2H, m, CH2PhOMe), 4.77 (1H, d, J1,2 2.6 Hz, H-1), 4.66 and 4.58 (2H, AB spectrum, J 11.5 Hz, CH2PhOMe), 4.66 (1H, dd, J2,3 10.4, J2,1 2.6 Hz, H-2), 3.80 (3H, s, OMe), 3.74–3.64 (3H, m, H-3, H-5a, H-5b), 3.53 (1H, ddd, J 7.7, 6.3, 1.4 Hz, H-4), 3.35 (3H, s, OMe), 3.16 (3H, s, OMe), 1.38 (3H, s, Me), 1.31 (3H, s, Me), 1.07–0.94 (21H, m, Si(CH(Me)2)3).
δC (126 MHz, CDCl3) 159.1, 145.1 (C-2′/C3′), 130.3, 129.2, 113.7, 98.7, 73.2 (C-1), 71.3, 69.94 (C-4/C-2), 69.9 (C-4/C-2), 66.6 (C-3), 62.7 (C-5), 55.2, 48.2, 47.95, 17.95, 17.8, 17.4, 11.8. νmax (KBr) 3030, 3015, 2993, 2899, 1735, 1455, 1383, 1230, 1161, 1072, 855 cm−1. Rf=0.35; Tol/EA 40%, [α]D +68.6 (c 1.0, CHCl3). HRMS-(TOF): MH+, found 609.3562. C31H53N2O8Si requires 609.3571.
4.1.10. (2S,3S,4aS,5R,10R,10aR)-2,3-Dimethoxy-5-((4-methoxybenzyl)oxy)-2,3-dimethyl-10-(((triisopropylsilyl)oxy)methyl)-2,3,4a,5,10,10a-hexahydro-[1,4]dioxino[2,3-d]imidazo[1,2-a]pyridine (20)41
To a solution of 19 (4.57 g, 7.5 mmol) and pyridine (2.42 mL, 30 mmol) in C2H4Cl2 (75 mL) cooled to −15 °C trifluoromethanesulfonic anhydride (3.8 mL, 22.58 mmol) was added dropwise. The reaction was warmed to rt. After 10 min the reaction was placed into preheated oil bath (50 °C) and stirred for 3 h. The reaction was cooled down, diluted with DCM and washed with a mixture of NaHCO3 solution and brine. The aqueous layer was extracted with DCM once more. The combined organic layer was dried and concentrated. The residue was purified by flash chromatography Tol/EA 10→40% to give 4.29 g (7.26 mmol, 97%) of the title compound 20 as clear syrup.
δH (500 MHz, CDCl3) 7.31–7.26 (2H, m, CH2PhOMe), 7.24 (1H, d, J2,3 1.2 Hz, H-2), 6.99 (1H, d, J2,3 1.2 Hz, H-3), 6.79–6.73 (2H, m, CH2PhOMe), 4.77 and 4.65 (2H, AB spectrum, J 11.4 Hz, CH2PhOMe), 4.69 (1H, d, J8,7 3.2 Hz, H-8), 4.35 (1H, dd, J6,7 10.5, J6,5 9.2 Hz, H-6), 4.13 (1H, dd, J5*a,5*b 10.7, J5*a,5 1.9 Hz, H-5∗a), 3.96 (1H, ddd, J5,6 9.1, J5,5*b 7.2, J5,5*a 1.9 Hz, H-5), 3.92 (1H, dd, J7,6 10.6, J7,8 3.3 Hz, H-7), 3.84 (1H, dd, J5*b,5*a 10.7, J5*b,5 7.2 Hz, H-5∗b), 3.71 (3H, s, OMe), 3.21 (3H, s, OMe), 3.19 (3H, s, OMe), 1.32 (3H, s, Me), 1.27 (3H, s, Me), 1.07–0.87 (21H, m, Si(CH(Me)2)3).
δC (126 MHz, CDCl3) 158.9, 143.2 (C-8a), 130.8, 129.5 (C-3), 129.2, 118.8 (C-2), 113.4, 99.5, 99.3, 71.3, 69.7 (C-8), 69.4 (C-7), 64.6 (C-5∗), 62.5 (C-6), 60.6 (C-5), 55.2, 48.2, 48.1, 17.9, 17.6, 17.7, 11.8. νmax (KBr) 3088, 3060, 1946, 1880, 1801, 1751, 1603, 1525, 1499, 1310, 1083, 1034, 982 cm−1. Rf=0.38; Tol/EA 40%, [α]D +40.4 (c 1.0, CHCl3). HRMS-(TOF): MH+, found 591.3465. C31H51N2O7Si requires 591.3466.
4.1.11. (S)-1-((2R,3R,5S,6S)-3-((R)-(4,5-Diiodo-1H-imidazol-2-yl)((4-methoxybenzyl)oxy)methyl)-5,6-dimethoxy-5,6-dimethyl-1,4-dioxan-2-yl)-2-((triisopropylsilyl)oxy)ethanol (21)40
To a solution of 19 (0.121 g, 0.2 mmol) in MeCN (2 mL) NIS (0.099 g, 0.44 mmol) was added at rt. The reaction was further stirred for 40 min in the dark. The reaction was diluted with DCM and washed with 0.5 M aqueous sodium thiosulfate (Na2S2O3) solution. The aqueous layer was extracted with DCM once more. The combined organic layer was dried and concentrated. The residue was absorbed on silica (2 g) and purified by flash column chromatography in Tol/EA gradient 5→20% to give 0.133 g (0.15 mmol, 77%) of the title compound 21 as foam.
δH (500 MHz, CDCl3) 7.28–7.23 (2H, m, CH2PhOMe), 6.88–6.83 (2H, m, CH2PhOMe), 4.64 and 4.6 (2H, AB spectrum, J 11.4 Hz, CH2PhOMe), 4.62 (1H, d, J1,2 2.6 Hz, H-1), 4.58 (1H, dd, J2,3 10.8, J2,12.5 Hz, H-2), 3.80 (3H, s, OMe), 3.70 (1H, dd, J5a,5b 9.2, J5a,4 6.2 Hz, H-5a), 3.65 (1H, t, J5a,5b=J5b,5=9.3 Hz, H-5b), 3.60 (1H, dd, J3,2 10.8, J3,41.2 Hz, H-3), 3.56–3.47 (1H, m, H-4), 3.36 (3H, s, OMe), 3.23 (3H, s, OMe), 2.46 (1H, br d, OH-4), 1.41 (3H, s, Me), 1.33 (3H, s, Me), 1.09–0.86 (21H, m, Si(CH(Me)2)3).
δC (126 MHz, CDCl3) 159.1, 150.7 (C-2′), 129.9, 129.2, 113.7, 98.7, 98.5, 94.6 (br s, C-4′/5′), 75.9 (br s, C-4′/5′), 72.2 (C-1), 71.3, 70.1 (C-4), 69.4 (C-2), 65.7 (C-3), 62.4 (C-5), 55.3, 48.5, 48.3, 18.0, 17.9, 17.8, 17.2, 11.8. Rf=0.18; Tol/EA 10%. [α]D −13.3 (c 1.00, CHCl3). HRMS-(TOF): MH+, found 861.1510. C31H51I2N2O8Si requires 861.1504.
4.1.12. (2S,3S,4aS,5R,10R,10aR)-7,8-Diiodo-2,3-dimethoxy-5-((4-methoxybenzyl)oxy)-2,3-dimethyl-10-(((triisopropylsilyl)oxy)methyl)-2,3,4a,5,10,10a-hexahydro-[1,4]dioxino[2,3-d]imidazo[1,2-a]pyridine (22)41
A solution of 20 (3.2 g, 5.4 mmol) in DMF (60 mL) was treated with NIS (12.1 g, 53.78 mmol) at 80 °C for 36 h. The reaction was cooled, diluted with ethyl acetate and washed with 0.5 M sodium thiosulfate solution. The layers were separated. The aqueous layer was extracted with ethyl acetate once more. The combined organic layer was dried and concentrated. The residue was absorbed on silica (60 g) and purified by flash chromatography PE/EE 5→30% to give 3.3 g (3.9 mmol, 73%) of the title compound 22 as yellowish foam.
4.1.13. With NIS/PPTS
A solution of 20 (0.296 g, 0.5 mmol), NIS (0.338 g, 0.35 mmol) and pyridinium p-toluenesulfonate (0.125 g, 0.11 mmol) in MeCN (5 mL) was stirred at 80 °C for 1 h. The reaction was cooled, diluted with ethyl acetate and washed with 0.5 M sodium thiosulfate solution. The aqueous layer was extracted with ethyl acetate once more. The combined organic layer was dried and concentrated. The residue was purified by flash chromatography PE/EE 5→30% to give 0.259 g (0.31 mmol, 62%) of the title compound 22.
δH (500 MHz, CDCl3) 7.28–7.23 (2H, m, CH2PhOMe), 6.78–6.74 (2H, m, CH2PhOMe), 4.94 (1H, dd, J6,7 10.7, J6,5 6.5 Hz, H-6), 4.69 and 4.59 (2H, AB spectrum, J 11.4 Hz, CH2PhOMe), 4.61 (1H, d, J8,7 2.8 Hz, H-8), 4.26 (1H, dd, J5*a,5*b 10.7, J5*a,5 4.8 Hz, H-5∗a), 4.03 (1H, ddd, J5,6 6.7, J5,5*a 4.8, J5,5*b 2.5 Hz, H-5), 3.87 (1H, dd, J5*a,5*b 10.7, J5*b,5 2.5 Hz, H-5∗b), 3.80 (1H, dd, J7,6 10.6, J7,8 2.8 Hz, H-7), 3.73 (3H, s, OMe), 3.26 (3H, s, OMe), 3.15 (3H, s, OMe), 1.31 (3H, s, Me), 1.26 (3H, s, Me), 0.88–0.77 (21H, m, Si(CH(Me)2)3).
δC (126 MHz, CDCl3) 158.9, 150 (C-8a), 130.3, 129.6, 113.4, 99.8, 99.6, 97.4 (C-3), 80.1 (C-2), 71.4, 70.1 (C-8), 68.9 (C-7), 63.6 (C-6), 62.9 (C-5∗), 62.1 (C-5), 55.3, 48.2, 48.1, 17.8, 17.7, 17.6, 11.8. νmax (KBr) 3063, 2865, 1880, 1812, 1613, 1501, 1454, 1352, 1173, 1100, 1031, 911 cm−1. Rf=0.3; PE/EE 30%, [α]D −28.5 (c 1.0, CHCl3). HRMS-(TOF): MH+, found 843.1421. C31H49I2N2O7Si requires 843.1399.
4.1.14. (2S,3S,4aS,5R,10R,10aR)-7-Iodo-2,3-dimethoxy-5-((4-methoxybenzyl)oxy)-2,3-dimethyl-10-(((triisopropylsilyl)oxy)methyl)-2,3,4a,5,10,10a-hexahydro-[1,4]dioxino[2,3-d]imidazo[1,2-a]pyridine (23)41
To a solution of 22 (2.02 g, 2.39 mmol) in THF (25 mL) a stock 1 M solution of EtMgBr in THF (3 mL, 3 mmol) was added at 4 °C (ice-bath). After 10 min the reaction was quenched with saturated NH4Cl solution. The reaction mixture was diluted with ethyl acetate and brine and the layers were separated. The aqueous layer was extracted with ethyl acetate once more. The combined organic layer was dried and concentrated. The residue was absorbed on silica (15 g) and purified by flash chromatography PE/EA 5→15% to give 1.438 g (2.06 mmol, 84%) of the title compound 23 as foam.
δH (500 MHz, CDCl3) 7.39 (1H, s, H-3), 7.37–7.32 (2H, m, CH2PhOMe), 6.87–6.81 (2H, m, CH2PhOMe), 4.86 and 4.71 (2H, AB spectrum, J 11.2 Hz, CH2PhOMe), 4.75 (1H, d, J8,7 3.2 Hz, H-8), 4.42 (1H, dd, J6,7 10.6, J6,5 9.2 Hz, H-6), 4.16 (1H, dd, J5*a,5*b 10.8, J5*a,5 2.0 Hz, H-5∗a), 4.04 (1H, ddd, J5,6 9.1, J5,5*b 7.2, J5,5*a 2.0 Hz, H-5), 3.98 (1H, dd, J7,6 10.6, J7,8 3.2 Hz, H-7), 3.87 (1H, dd, J5*a,5*b 10.7, J5*b,5 7.1 Hz, H-5∗b), 3.79 (3H, s, OMe), 3.29 (3H, s, OMe), 3.27 (3H, s, OMe), 1.40 (3H, s, Me), 1.34 (3H, s, Me), 1.13–1.01 (21H, m, Si(CH(Me)2)3).
δC (126 MHz, CDCl3) 158.9, 145.4 (C-8a), 130.6, 129.5, 124.6 (C-3), 113.4, 99.6, 99.3, 82.2 (C-2), 71.8, 69.6 (C-8), 69.2 (C-7), 64.5 (C-5∗), 62.2 (C-6), 60.8 (C-5), 55.2, 48.2, 48.1, 17.9, 17.8, 11.8. νmax (KBr) 3063, 2874, 1875, 1734, 1495, 1453, 1368, 1257, 1112, 1023, 943 cm−1. Rf=0.38; PE/EA 15%, [α]D +53.2 (c 1.0, CHCl3). HRMS-(TOF): MH+, found 717.2435. C31H50IN2O7Si requires 717.2432.
4.1.15. (2S,3S,4aR,5R,11S,11aS)-7,8,11-Triiodo-2,3-dimethoxy-5-((4-methoxybenzyl)oxy)-2,3-dimethyl-3,4a,5,10,11,11a-hexahydro-2H-[1,4]dioxino[2,3-d]imidazo[1,2-a]azepine (25)42
To a stirred solution of 16 (0.063 g, 0.15 mmol) in MeCN (3 mL) NIS (0.111 g, 0.5 mmol) was added at rt. The reaction was further stirred for 24 h in the dark. The reaction was diluted with DCM and washed with 0.5 M sodium thiosulfate solution. The aqueous layer was extracted with DCM once more. The combined organic layer was dried and concentrated. The residue was purified by flash column chromatography in PE/EA 5→15% to give 0.093 g (0.12 mmol, 78%) of the title compound 25 as viscous syrup.
δH (500 MHz, CDCl3) 7.15–7.10 (2H, m, CH2PhOMe), 6.83–6.78 (2H, m, CH2PhOMe), 4.80 (1H, d, J9,8 1.4 Hz, H-9), 4.77 and 4.43 (2H, AB spectrum, J 11.9 Hz, CH2PhOMe), 4.50 (1H, dd, J5a,5b 14.6, J5a,6 2.8 Hz, H-5a), 4.43 (1H, dd, J5a,5b 14.6, J5b,6 11.4 Hz, H-5b), 4.31 (1H, dd, J7,6 10.8, J7,8 9.2 Hz, H-7), 3.86 (1H, br dt, J6,5b 11.4, J6,5a 2.8 Hz, H-6), 3.81 (3H, s, OMe), 3.66 (1H, dd, J8,7 9.2, J8,9 1.4 Hz, H-8), 3.43 (3H, s, OMe), 3.18 (3H, s, OMe), 1.36 (3H, s, Me), 1.31 (3H, s, Me).
δC (126 MHz, CDCl3) 159.2, 149.3 (C-9a), 129.7, 129.2, 113.6, 113.5, 100.29, 100.0, 99.9, 94.3 (C-2), 85.5 (C-3), 76.5 (C-9), 72.6, 72.1 (C-7 and C-8), 72.0, 55.3 (C-5), 54.2, 49.1, 48.3, 26.7 (C-6), 17.4, 17.2. Rf=0.32; PE/EA 15%, [α]D +3.4 (c 1.0, CHCl3). HRMS-(TOF): MH+, found 796.9078. C22H28I3N2O6 requires 796.9082.
4.1.16. (2S,3S,4aS,5R,10R,10aR)-2,3-Dimethoxy-5-((4-methoxybenzyl)oxy)-2,3-dimethyl-7-(phenylethynyl)-10-(((triisopropylsilyl)oxy)methyl)-2,3,4a,5,10,10a-hexahydro-[1,4]dioxino[2,3-d]imidazo[1,2-a]pyridine (26)41
A solution of 23 (1.43 g, 2.0 mmol), phenylacetylene (1.1 mL, 10 mmol) and Et3N (1.4 mL, 10 mmol) in DMF (20 mL) was degassed by freezing, evacuating and thawing three times. Cuprous iodide (0.038 g, 0.2 mmol) and Pd(PPh3)4 (0.23 g, 0.2 mmol) were then added to the reaction flask. The reaction was placed in the preheated (80 °C) oil bath stirred for 16 h. The reaction was cooled and concentrated. The residue was dissolved in ethyl acetate and washed with water and brine. The aqueous layer was extracted with ethyl acetate once more. The combined organic layer was dried and concentrated. The brown residue was purified by flash column chromatography in PE/EA 10→20% to give 1.28 g (1.86 mmol, 93%) of the title compound 26 as amber amorphous solid.
δH (500 MHz, CDCl3) 7.56 (1H, s, H-3), 7.55–7.52 (2H, m, CH2PhOMe), 7.41–7.36 (2H, m, Ph), 7.35–7.30 (3H, m, Ph), 6.88–6.82 (2H, m, CH2PhOMe), 4.90 and 4.80 (2H, AB spectrum, J 11.2 Hz, CH2PhOMe), 4.87–4.83 (1H, br d, H-8), 4.43 (1H, dd, J6,7 10.5, J6,5 9.2, Hz, H-6), 4.20 (1H, dd, J5*a,5*b 10.8, J5*a,5 1.9 Hz, H-5∗a), 4.06 (1H, ddd, J5,6 9.3, J5,5*b 7.3, J5,5*a 1.9 Hz, H-5), 4.03 (1H, dd, J7,6 10.6, J7,8 3.2 Hz, H-7), 3.90 (1H, dd, J5*a,5*b 10.8, J5*b,5 7.4 Hz, H-5∗b), 3.81 (3H, s, OMe), 3.30 (3H, s, OMe), 3.30 (3H, s, OMe), 1.42 (3H, s, Me), 1.36 (3H, s, OMe), 1.17–1.04 (21H, m, Si(CH(Me)2)3).
δC (126 MHz, CDCl3) 158.9, 143.7 (C-8a), 131.6, 130.7, 129.6, 128.2, 128.0, 124.4 (C-2), 123.3, 122.8 (C-3), 113.4, 99.6, 99.3, 89.3 (C-2″), 82.9 (C-2′), 71.8, 69.9 (C-8), 69.2 (C-7), 64.7 (C-5∗), 62.2 (C-6), 61.0 (C-5), 55.2, 48.2, 48.1, 18.0, 17.9, 17.72, 17.7, 11.8. νmax (KBr) 3089, 2941, 2100, 1599, 1496, 1454, 1364, 1301, 1174, 1112, 1050, 1028, 913 cm−1. Rf=0.25; PE/EA 15%, [α]D +91.4 (c 1.0, CHCl3). HRMS-(TOF): MH+, found 691.3774. C39H55N2O7Si requires 691.3779.
4.1.17. (2S,3S,4aR,5R,10R,10aR)-2,3-Dimethoxy-2,3-dimethyl-7-(phenylethynyl)-10-(((triisopropylsilyl)oxy)methyl)-2,3,4a,5,10,10a-hexahydro-[1,4]dioxino[2,3-d]imidazo[1,2-a]pyridin-5-ol (27)41
A solution of 26 (0.266 g, 0.38 mmol) in DCM/water (20:1; 5 mL) DDQ (0.15 g, 0.67 mmol) was added in one portion. The reaction was stirred for 4 h at rt. The reaction was quenched by addition of sodium metabisulfite (Na2S2O5) solution, diluted with DCM and washed successively with sodium metabisulfite (Na2S2O5) solution and a mixture of concentrated NaHCO3 solution and brine. The aqueous layer was extracted with DCM once more. The combined organic layer was dried and evaporated. The residue was purified by flash column chromatography in Tol/EA 5→50% to give 0.173 g (0.3 mmol, 80%) of the title compound 27.
δH (500 MHz, CDCl3) 7.55 (1H, s, H-3), 7.54–7.50 (2H, m, Ph), 7.34–7.27 (3H, m, Ph), 5.08 (1H, d, J8,7 3.4 Hz, H-8), 4.46 (1H, br s, OH-8), 4.38 (1H, dd, J6,7 10.5, J6,5 9.5 Hz, H-6), 4.24 (1H, dd, J5*a,5*b 10.8, J5*a,5 1.6 Hz, H-5∗a), 4.06 (1H, ddd, J5,6 9.2, J5,5*b 7.4, J5,5*a 1.6 Hz, H-5), 4.01–3.94 (2H, m, H-5∗b, H-7), 3.30 (3H, s, OMe), 3.27 (3H, s, OMe), 1.40 (3H, s, Me), 1.34 (3H, s, Me), 1.16–1.03 (21H, m, Si(CH(Me)2)3).
δC (126 MHz, CDCl3) 144.7 (C-8a), 131.5, 128.2, 128, 124.3, 123.4, 122.4 (C-3), 100.0, 99.9, 99.4, 89.2 (C-2″), 83.0 (C-2′), 68.5 (C-7), 64.5 (C-5∗), 63.2 (C-8), 61.8 (C-6), 60.8 (C-5), 48.2, 48.1, 17.9, 17.9, 17.7, 17.6, 11.8. νmax (KBr) 3089, 2945, 1951, 1731, 1686, 1602, 1534, 1496, 1384, 1263, 1092, 1028, 909 cm−1. Rf=0.2; Tol/EA 40%, [α]D +80.3 (c 1.0, CHCl3). HRMS-(TOF): MH+, found 571.3209. C31H47N2O6Si requires 571.3203.
4.1.18. (2S,3S,4aR,5S,10R,10aR)-5-Azido-2,3-dimethoxy-2,3-dimethyl-7-(phenylethynyl)-10-(((triisopropylsilyl)oxy)methyl)-2,3,4a,5,10,10a-hexahydro-[1,4]dioxino[2,3-d]imidazo[1,2-a]pyridine (28)41
To a stirred solution of 27 (0.648 g, 1.14 mmol) in toluene (20 mL) diphenylphosphoryl azide (1.23 mL, 5.7 mmol) followed by DBU (0.852 mL, 5.7 mmol) was added at rt. The reaction was placed in a preheated oil bath (80 °C) and kept for 2 h. The reaction was cooled and concentrated. The brown residue was absorbed on silica and purified by flash chromatography PE/EE 5→30% to give 0.574 g (0.96 mmol, 85%) of the title compound 28 as foam.
δH (500 MHz, CDCl3) 7.55–7.51 (2H, m, Ph), 7.47 (1H, s, H-3), 7.37–7.30 (3H, m, Ph), 4.73 (1H, d, J8,7 9.5 Hz, H-8), 4.29 (1H, d, J 9.7 Hz, H-5∗a), 4.09–3.93 (4H, m, H-5, H-5∗b, H-6, H-7), 3.38 (3H, s, OMe), 3.30 (3H, s, OMe), 1.42 (3H, s, Me), 1.37 (3H, s, Me), 1.17–1.04 (21H, m, Si(CH(Me)2)3).
δC (126 MHz, CDCl3) 141.7 (C-8a), 131.5, 128.2, 128.1, 125.3 (C-2), 123.2, 121.4 (C-3), 99.65, 99.6, 89.4 (C-2″), 82.7 (C-2′), 70.6 (C-7), 64.3, 62.4 (C-5∗), 59.7, 57.5 (C-8), 48.4, 48.3, 17.9, 17.8, 17.6, 17.4, 11.9. νmax (KBr) 3089, 3032, 2950, 2835, 2109, 1454, 1407, 1362, 1332, 1269, 1145, 1098, 1018, 917 cm−1. Rf=0.26; PE/EE 20%, [α]D +98.7 (c 0.51, CHCl3). HRMS-(TOF): MH+, found 596.3275. C31H46N5O5Si requires 596.3268.
4.1.19. N-((2S,3S,4aR,5S,10R,10aR)-2,3-Dimethoxy-2,3-dimethyl-7-phenethyl-10-(((triisopropylsilyl)oxy)methyl)-2,3,4a,5,10,10a-hexahydro-[1,4]dioxino[2,3-d]imidazo[1,2-a]pyridin-5-yl)acetamide (29)41
A solution of 28 (0.045 g, 0.076 mmol) in ethyl acetate (2 mL) was stirred under slight positive pressure of H2 (balloon) in the presence of 20% Pd2(OH)2 on carbon (0.025 g) for 40 min. The reaction was filtered through a pad of Celite and concentrated. The residue was dissolved in DCM (2 mL) and treated with an excess of acetic anhydride (0.05 mL), and Et3N (0.2 mL) for 2 h at rt. The reaction was quenched with MeOH (0.1 mL), stirred for 20 min, diluted with DCM and washed successively with 1 M HCl, water and a mixture of saturated NaHCO3 solution and brine. The aqueous layers were additionally extracted with DCM. The combined organic layer was dried and concentrated. The residue was purified by flash chromatography Tol/Me2CO 5→40% to give 0.047 g (0.071 mmol, 93%) of the title compound 29 as foam.
δH (500 MHz, CDCl3) 7.21–7.16 (2H, m, Ph), 7.14–7.07 (3H, m, Ph), 6.89 (1H, s, H-3), 4.50 (1H, t, J7,6=J7,8=10.0 Hz, H-7), 4.43–4.34 (1H, br t, H-8), 4.21 (1H, dd, J5*a,5*b 11.0, J5*a,5 1.7 Hz, H-5∗a), 4.03 (1H, ddd, J5,6 9.8, J5*b,5 5.8, J5*a,5 1.7 Hz, H-5), 3.91 (1H, dd, J5*a,5*b 11.0, J5*b,5 5.8 Hz, H-5∗b), 3.72 (1H, t, J6,7=J6,5=10 Hz, H-6), 3.16 (3H, s, OMe), 3.07 (3H, s, OMe), 2.99–2.91 (1H, m, H-2′a), 2.83–2.70 (3H, m, H-2′b, H-2″a, H-2″b), 1.99 (3H, s, COCH3), 1.21 (3H, s, Me), 1.12 (3H, s, Me), 1.08–0.90 (21H, m, Si(CH(Me)2)3).
δC (126 MHz, CDCl3) 170.4 (COCH3), 143.6 (C-8a), 142.2, 141.6, 128.4, 128.3, 125.8, 113.6 (C-3), 99.4, 99.2, 67.9 (C-7), 65.8 (C-6), 62.7 (C-5∗), 59.14 (C-5), 49.5 (C-8), 48.1, 47.7, 35.8 (C-2′), 30.6 (C-2″), 23.4 (COCH3), 18.0, 17.9, 17.6, 17.5, 12.0. Rf=0.22; Tol/Me2CO 30%, [α]D +105.3 (c 1.0, CHCl3). HRMS-(TOF): MH+, found 616.3775. C33H54N3O6Si requires 616.3782.
4.1.20. N-((2S,3S,4aR,5S,10R,10aR)-2,3-Dimethoxy-2,3-dimethyl-7-phenethyl-10-(((triisopropylsilyl)oxy)methyl)-2,3,4a,5,10,10a-hexahydro-[1,4]dioxino[2,3-d]imidazo[1,2-a]pyridin-5-yl)propionamide (30)41
Prepared from 28 in 83% yield as described for preparation of 29 with replacement of acetic anhydride for propionic anhydride.
δH (500 MHz, CDCl3) 7.22–7.15 (2H, m, Ph), 7.14–7.06 (3H, m, Ph), 6.88 (1H, s, H-3), 4.53–4.38 (2H, m, H-7, H-8), 4.21 (1H, d, J5*a,5*b 10.9 Hz, H-5∗a), 4.04 (1H, dd, J5,6 9.6, J5*b,5 5.8 Hz, H-5), 3.90 (1H, dd, J5*a,5*b 11.0, J5*b,5 5.9 Hz, H-5∗b), 3.73 (1H, t, J6,5=J6,7=9.9 Hz, H-6), 3.16 (3H, s, OMe), 3.08 (3H, s, OMe), 2.92 (1H, dt, J2′a, 2′b 13.8, J 7.1 Hz, H-2′a), 2.84–2.70 (3H, m, H-2′b, H-2″a, H-2″b), 2.23 (2H, m, COCH2CH3), 1.22 (4H, s, Me), 1.15 (3H, s, Me), 1.06 (4H, t, J 7.5 Hz, COCH2CH3), 1.04–0.93 (21H, m, Si(CH(Me)2)3).
δC (126 MHz, CDCl3) 174.1 (COCH2CH3), 143.5 (C-8a), 142.1, 141.4, 128.4, 128.3, 125.8, 113.6 (C-3), 99.3, 99.2, 68.0 (C-7), 65.7 (C-6), 62.7 (C-5∗), 59.2 (C-5), 49.3 (C-8), 48.1, 47.7, 35.7 (C-2′), 30.4 (C-2″), 29.6 (COCH2CH3), 18.0, 17.95, 17.6, 17.4, 12.0, 9.8 (COCH2CH3). Rf=0.35; Tol/Me2CO 30%, [α]D +103.5 (c 1.0, CHCl3). HRMS-(TOF): MH+, found 630.3931. C34H56N3O6Si requires 630.3938.
4.1.21. N-((2S,3S,4aR,5S,10R,10aR)-2,3-Dimethoxy-2,3-dimethyl-7-phenethyl-10-(((triisopropylsilyl)oxy)methyl)-2,3,4a,5,10,10a-hexahydro-[1,4]dioxino[2,3-d]imidazo[1,2-a]pyridin-5-yl)isobutyramide (31)41
Prepared from 28 in 78% yield as described for preparation of 29 with replacement of acetic anhydride for iso-butyric anhydride.
δH (500 MHz, CDCl3) 7.19–7.15 (2H, m, Ph), 7.12–7.05 (3H, m, Ph), 6.86 (1H, s, H-3), 4.52 (1H, t, J 9.9 Hz, H-7), 4.44–4.36 (1H, br t, H-8), 4.22 (1H, dd, J5*a,5*b 11.0, J5*a,5 1.7 Hz, H-5∗a), 4.05 (1H, ddd, J5,6 9.9, J5*b,5 6.2, J5*a,5 1.3 Hz, H-5), 3.88 (1H, dd, J5*a,5*b 11.0, J5*b,5 6.1 Hz, H-5∗b), 3.71 (1H, t, J6,5=J6,7 10.1 Hz, H-6), 3.16 (3H, s, OMe), 3.09 (3H, s, OMe), 2.88 (1H, ddd, J 14.0, 11.2, 6.3 Hz, H-2′a), 2.83–2.67 (3H, m, H-2′b, H-2″a, H-2″b), 2.45–2.36 (1H, m, COCH(CH3)2), 1.21 (3H, s, Me), 1.15 (3H, s, Me), 1.09 (3H, d, J 6.9 Hz, COCH(CH3)2), 1.02 (3H, d, J 6.8 Hz, COCH(CH3)2), 1.01–0.94 (21H, m, Si(CH(Me)2)3).
δC (126 MHz, CDCl3) 177.3 (COCH(CH3)2), 143.7 (C-8a), 142.1, 141.1, 128.3, 128.2, 125.7, 113.5 (C-3), 99.3, 99.2, 67.9 (C-7), 65.7 (C-6), 62.6 (C-5∗), 59.1 (C-5), 49.3 (C-8), 48.1, 47.6, 35.6 (C-2′), 35.2 (COCH(CH3)2), 30.2 (C-2″), 20.0 (COCH(CH3)2), 19.2 (COCH(CH3)2), 18.0, 17.9, 17.6, 17.4, 11.9. Rf=0.35; [DCM/PE 1:1]-Me2CO 30%, [α]D +101.4 (c 1.0, CHCl3). HRMS-(TOF): MH+, found 644.4105. C35H58N3O6Si requires 644.4095.
4.1.22. N-((2S,3S,4aR,5S,10R,10aR)-2,3-Dimethoxy-2,3-dimethyl-7-phenethyl-10-(((triisopropylsilyl)oxy)methyl)-2,3,4a,5,10,10a-hexahydro-[1,4]dioxino[2,3-d]imidazo[1,2-a]pyridin-5-yl)pentanamide (32)41
A solution of 28 (0.05 g, 0.084 mmol) in MeOH (1.5 mL) was stirred under slight positive pressure of H2 (balloon) of H2 in the presence of 20% Pd2(OH)2 on carbon (0.05 g) for 2 h. The reaction was filtered through a pad of Celite and concentrated in vacuum. The residue was dissolved in DCM (2 mL) and treated with valeric acid (0.027 mL, 0.25 mmol), PyBOP (0.13 g, 0.25 mmol) and DIPEA (0.5 mL) for 2 h at rt. The reaction was diluted with DCM and washed successively with 1 M HCl, water and a mixture of saturated NaHCO3 solution and brine. The aqueous layers were additionally extracted with DCM. The combined organic layer was dried and concentrated. The residue was purified by flash chromatography Tol/Me2CO 5→25% to give 0.046 g (0.076 mmol, 90%) of the title compound 32 as foam.
δH (500 MHz, CDCl3) 7.20–7.16 (2H, m, Ph), 7.13–7.07 (3H, m, Ph), 6.88 (1H, s, H-3), 4.50 (1H, t, J7,6=J7,8=10.0 Hz, H-7), 4.41–4.32 (1H, br t, H-8), 4.21 (1H, dd, J5*a,5*b 11.0, J5*a,5 1.8 Hz, H-5∗a), 4.04 (1H, ddd, J5,6 9.7, J5*b,5 5.8, J5*a,5 1.5 Hz, H-5), 3.90 (1H, dd, J5*a,5*b 11.0, J5*b,5 5.9 Hz, H-5∗b), 3.72 (1H, t, J6,7=J6,5=10.0 Hz, H-6), 3.16 (3H, s, OMe), 3.07 (3H, s OMe), 2.98–2.87 (1H, m, H-2′a), 2.83–2.68 (3H, m, H-2′b, H-2″a, H-2″b), 2.26 (1H, dt, J 15.1, 7.7 Hz, COCH2C2H4CH3), 2.21–2.11 (1H, m, COCH2CH2CH2CH3), 1.58–1.48 (2H, m, COCH2CH2CH2CH3), 1.28 (2H, dq, J 14.7, 7.4 Hz, COCH2CH2CH2CH3), 1.21 (3H, s, Me), 1.13 (3H, s, Me), 1.07–0.93 (21H, m, Si(CH(Me)2)3), 0.79 (3H, t, J 7.4 Hz, COCH2CH2CH2CH3).
δC (126 MHz, CDCl3) 173.4 (COCH2CH2CH2CH3), 143.6 (C-8a), 142.1, 141.4, 128.3, 128.2, 125.7, 113.4 (C-3), 99.3, 99.1, 67.9 (C-7), 65.7 (C-6), 62.6 (C-5∗), 59.0 (C-5), 49.4 (C-8), 48.1, 47.6, 36.4 (COCH2CH2CH2CH3), 35.7 (C-2′), 30.5 (C-2″), 27.8 (COCH2CH2CH2CH3), 22.4 (COCH2CH2CH2CH3), 18.0, 17.9, 17.5, 17.4, 13.8 (COCH2CH2CH2CH3), 11.9. Rf=0.32; Tol/Me2CO 20%, [α]D +101.3 (c 0.35, CHCl3). HRMS-(TOF): MH+, found 658.4239. C36H60N3O6Si requires 658.4251.
4.1.23. (E)-N-((2S,3S,4aR,5S,10R,10aR)-2,3-Dimethoxy-2,3-dimethyl-7-phenethyl-10-(((triisopropylsilyl)oxy)methyl)-2,3,4a,5,10,10a-hexahydro-[1,4]dioxino[2,3-d]imidazo[1,2-a]pyridin-5-yl)penta-2,4-dienamide (33)41
Prepared from 28 in 93% yield as described for preparation of 32 with replacement of valeric acid for 2,4-pentanedienic acid.
δH (500 MHz, CDCl3) 7.16 (2H, m, Ph), 7.11–7.05 (3H, m, Ph), 7.00 (1H, dd, J 15.1, 11.0 Hz, COCHCHCHCH2), 6.96 (1H, s, H-3), 6.26 (1H, dt, J 17.0, 10.5, 10.5 Hz, COCHCHCHCH2), 6.00 (1H, d, J 15.2 Hz, COCHCHCHCH2), 5.34 (1H, d, Jtrans 16.9 Hz, COCHCHCHCH2), 5.23 (1H, d, Jcis 10.1 Hz, COCHCHCHCH2), 4.63–4.43 (2H, m, H-7, H-8), 4.24 (1H, d, J5*a,5*b 11 Hz, H-5∗a), 4.09 (1H, dd, J5,6 9.9, J5*b,5 6.5 Hz, H-5), 3.92 (1H, dd, J5*a,5*b 11.1, J5*b,5 6.1 Hz, H-5∗b), 3.71 (1H, t, J6,7=J6,5=10.0 Hz, H-6), 3.15 (3H, s, OMe), 2.98 (3H, s, OMe), 2.92 (1H, m, H-2′a), 2.84–2.70 (3H, m, H-2′b, H-2″a, H-2″b), 1.20 (3H, s, Me), 1.06 (3H, s, Me), 1.05–0.91 (21H, m, Si(CH(Me)2)3).
δC (126 MHz, CDCl3) δ 164.2 (COCHCHCHCH2), 152.3, 143.2 (C-8a), 142.1 (COCHCHCHCH2), 135.2 (COCHCHCHCH2), 132.9, 128.6, 127.7, 125.9 (COHCHCHCH2), 121.7 (COCHCHCHCH2), 118.5, 116.1 (C-3), 99.3, 99.1, 68.1 (C-7), 65.3 (C-6), 63.0 (C-5∗), 58.7 (C-5), 49.7 (C-8), 48.6, 47.9, 35.4 (C-2′), 35.0 (C-2″), 18.0, 17.7, 17.5, 17.4, 12.0. Rf=0.3; Tol/Me2CO 20%. [α]D +97.4 (c 0.85, CHCl3). HRMS-(TOF): MH+, found 654.3943. C36H60N3O6Si requires 654.3938.
4.1.24. S-(3-(((2S,3S,4aR,5S,10R,10aR)-2,3-Dimethoxy-2,3-dimethyl-7-phenethyl-10-(((triisopropylsilyl)oxy)methyl)-2,3,4a,5,10,10a-hexahydro-[1,4]dioxino[2,3-d]imidazo[1,2-a]pyridin-5-yl)amino)-3-oxopropyl) ethanethioate (34)41
Prepared from 28 in 74% yield as described for preparation of 32 with replacement of valeric acid for S-acetyl-3-mercaptopropionic acid.
δH (500 MHz, CDCl3) 7.21–7.15 (2H, m, Ph), 7.12–7.07 (3H, m, Ph), 6.87 (1H, s, H-3), 4.46 (1H, t, J 9.9 Hz, H-7), 4.4 (1H, br t, H-8), 4.21 (1H, d, J 10.8 Hz, H-5∗a), 4.03 (1H, dd, J 9.8, 5.8 Hz, H-5), 3.89 (1H, dd, J 10.9, 5.9 Hz, H-5∗b), 3.72 (1H, t, J 9.9 Hz, H-6), 3.15 (3H, s, OMe), 3.09 (3H, s, OMe), 3.02 (2H, m, H-2′a, H-2′b), 2.92 (1H, m, H-2″a), 2.75 (3H, m, H-2″b, COCH2CH2SAc), 2.51 (2H, m, COCH2CH2SAc), 2.17 (3H, s, SCOCH3), 1.22 (3H, s, Me), 1.15 (3H, s, Me), 1.02–0.95 (21H, m, Si(CH(Me)2)3).
δC (126 MHz, CDCl3) 195.7 (SCOCH3), 170.8 (COC2H4SAc), 143.2 (C-8a), 142.1, 141.7, 128.4, 128.2, 125.8, 113.6 (C-3), 99.3, 99.2, 68.0 (C-7), 65.7 (C-6), 62.7 (C-5∗), 59.1 (C-5), 49.6 (C-8), 48.1, 47.8, 36.1 (COCH2CH2SAc), 35.7 (C-2′), 30.4 (C-2″), 24.7 (SCOCH3), 18.0, 17.9, 17.6, 17.4, 11.9. Rf=0.35; Tol/EA 20%, [α]D +101.4 (c 1.0, CHCl3). HRMS-(TOF): MH+, found 704.3763. C36H58N3O7SSi requires 704.3765.
4.1.25. N-((5R,6R,7R,8S)-6,7-Dihydroxy-5-(hydroxymethyl)-2-phenethyl-5,6,7,8-tetrahydroimidazo[1,2-a]pyridin-8-yl)acetamide (35)41
A solution of 29 (0.046 g, 0.075 mmol) in 95% trifluoroacetic acid (2 mL) was kept for 36 h at rt. The reaction was diluted with toluene, concentrated in vacuum and co-evaporated with toluene once more. The residue was purified on Phenomenex Luna 21×100 5 μm C18(2) column, gradient 5–95% MeCN in water (0.1% NH3) at flow rate 25 mL/min. Appropriate fractions were pooled, concentrated to approximately 1/3 of the initial volume in vacuum and freeze dried to give 0.02 g (0.058 mmol, 77%) of the title compound 35 as amorphous solid.
δH (500 MHz, CDCl3/CD3OD) 7.07–7.01 (2H, m, Ph), 6.98–6.92 (3H, m, Ph), 6.65 (1H, s, H-3), 4.68 (1H, d, J8,7 8.3 Hz, H-8), 3.86 (1H, dd, J5*a,5*b 12.2, J5*a,5 2.8 Hz, H-5∗a), 3.71 (1H, dd, J5*a,5*b 12.2, J5*b,5 4 Hz, H-5∗b), 3.63 (2H, m, H-5, H-6), 3.53 (1H, dd, J7,8 8.5, J7,6 8.3 Hz, H-7), 2.66 (2H, m, H-2′a, H-2′b), 2.57 (2H, m, H-2″a, H-2″b), 1.86 (3H, s, COCH3).
δC (126 MHz, CDCl3/CD3OD) δ 177.2 (COCH3), 146.5, 144.4 (C-8a), 130.8, 128.5 (C-2), 117.2 (C-3), 78.0 (C-7), 72.8 (C-6), 64.4 (C-5), 64.2(C-5∗), 54.5 (C-8), 37.9 (C-2′), 34.1 (C-2″), 22.4 (COCH3). HRMS-(TOF): MH+, found 346.1761. C18H24N3O4 requires 346.1767.
4.1.26. N-((5R,6R,7R,8S)-6,7-Dihydroxy-5-(hydroxymethyl)-2-phenethyl-5,6,7,8-tetrahydroimidazo[1,2-a]pyridin-8-yl)propionamide (36)41
Prepared starting from compound 30 as described for 35 in 73% yield; amorphous solid.
δH (500 MHz, pyridine-d5) 8.97 (1H, d, JNH,8 8.2 Hz, NHCOC2H5), 7.40 (1H, s, H-3), 7.30 (3H, m, Ph), 7.21 (2H, m, Ph), 5.85 (1H, t, J8,7=JNH,8=8.5 Hz, H-8), 4.67 (1H, dd, J5*a,5*b 11.5, J5*a,5 1.8 Hz, H-5∗a), 4.59 (1H, t, J6,7=J6,5=8.6 Hz, H-6), 4.43 (2H, m, H-5∗b, H-7), 4.32 (1H, ddd, J5,6 8.6, J5*b,55.4, J5*a,5 2.4 Hz, H-5), 3.07 (4H, m, H-2′a, H-2′b, H-2″a, H-2″b), 2.45 (2H, m, COCH2CH3), 1.21 (3H, t, J=7.5 Hz, COCH2CH3).
δC (126 MHz, pyridine-d5) 176.8 (COCH2CH3), 146.5, 144.9, 144.4 (C-8a), 130.8, 130.7, 128.1 (C-2), 116.4 (C-3), 77.5 (C-7), 72.3 (C-6), 64.7 (C-5), 64.0 (C-5∗), 53.7 (C-8), 38.3 (C-2′), 33.3 (C-2″), 31.7 (COCH2CH3), 12 (COCH2CH3). HRMS-(TOF): MH+, found 360.1922. C19H26N3O4 requires 360.1923.
4.1.27. N-((5R,6R,7R,8S)-6,7-Dihydroxy-5-(hydroxymethyl)-2-phenethyl-5,6,7,8-tetrahydroimidazo[1,2-a]pyridin-8-yl)isobutyramide (37)41
Prepared starting from compound 31 as described for 35 in 70% yield; amorphous solid.
δH (500 MHz, pyridine-d5) 8.81 (1H, br d, JNH,8 NHCO), 7.28 (1H, br s, H-3), 7.17–7.2 (4H, m, Ph), 7.07–7.11 (1H, m, Ph), 5.73 (1H, t, J8,7=JNH,8=8.4 Hz, H-8), 4.55 (1H, dd, J5*a,5*b 11.7, 2.6 Hz, H-5∗a), 4.47 (1H, t, J7,6=J7,8=8.6 Hz, H-7), 4.3 (2H, m, H-5∗b, H-6), 4.2 (1H, m, H-5), 2.95 (4H, m, H-2′a, H-2′b, H-2″a, H-2″b), 2.66 (1H, quint, COCH(CH3)2), 1.19 (3H, d, J 6.8 Hz, COCH(CH3)2), 1.18 (3H, d, J 6.8 Hz, COCH(CH3)2).
δC (126 MHz, pyridine-d5) 180.1 (COCH(CH3)2), 146.4, 144.9, 144.4 (C-8a), 130.8, 130.7, 128.1 (C-2), 116.5 (C-3), 77.6 (C-7), 72.3 (C-6), 64.8 (C-5), 64.1 (C-5∗), 53.7 (C-8), 38.3 (C-2′), 37.7 (C-2″), 33.3 (COCH(CH3)2), 22.1 (COCH(CH3)2), 21.9 (COCH(CH3)2). HRMS-(TOF): MH+, found 374.2075. C20H28N3O4 requires 374.2080.
4.1.28. N-((5R,6R,7R,8S)-6,7-Dihydroxy-5-(hydroxymethyl)-2-phenethyl-5,6,7,8-tetrahydroimidazo[1,2-a]pyridin-8-yl)pentanamide (38)41
Prepared starting from compound 32 as described for 35 in 68% yield; amorphous solid.
δH (500 MHz, DMSO) 7.98 (1H, d, JNH,8 9.0 Hz, NHCO), 7.32–7.22 (4H, m, Ph), 7.21–7.15 (1H, m, Ph), 7.01 (1H, s, H-3), 5.42 (1H, d, J 4.8 Hz, OH-6), 5.24 (1H, d, J 4.8 Hz, OH-7), 4.98 (1H, t, J 7.6 Hz, OH-5∗), 4.75 (1H, t, J8,7=J8,NH=8.8 Hz, H-8), 4.00 (1H, ddd, J5*a,5*b 11.3, 4.4, 1.4 Hz, H-5∗a), 3.75–3.68 (1H, m, H-5∗b), 3.69–3.61 (2H, m, H-6, H-5), 3.62–3.53 (1H, m, H-7), 2.91–2.78 (2H, m, H-2′a, H-2′b), 2.68 (2H, t, J 8.3 Hz, H-2″a, H-2″b), 2.15 (2H, t, J 7.4 Hz, COCH2CH2CH2CH3), 1.59–1.47 (2H, m, COCH2CH2CH2CH3), 1.44–1.30 (2H, m, COCH2CH2CH2CH3), 0.89 (3H, t, J 7.3 Hz, COC3H6CH3).
δC (126 MHz, DMSO d6) 173.4 (NHCO), 144.5, 142.1 (C-8a), 141.6, 129.0, 126.7, 113.9 (C-3), 73.3 (C-6), 69.4 (C-5/C-7), 61.9 (C-5/C-7), 61.1 (C-5∗), 50.2 (C-8), 36.0 (COCH2CH2CH2CH3, C-2′), 31.2 (C-2″), 28.3 (COCH2CH2CH2CH3), 22.6 (COCH2CH2CH2CH3), 14.3 (COCH2CH2CH2CH3). HRMS-(TOF): MH+, found 388.2232. C21H30N3O4 requires 388.2236.
4.1.29. (E)-N-((5R,6R,7R,8S)-6,7-Dihydroxy-5-(hydroxymethyl)-2-phenethyl-5,6,7,8-tetrahydroimidazo[1,2-a]pyridin-8-yl)penta-2,4-dienamide (39)41
Prepared staring from compound 33 as described for 35 in 48% yield; amorphous solid.
δH (500 MHz, DMSO) 8.39 (1H, d, JNH,8 8.7 Hz, NHCO), 7.31–7.23 (4H, m, Ph), 7.22–7.14 (1H, m, Ph), 7.09 (1H, dd, J 15.2, 11.1 Hz, COCHCHCHCH2), 7.06 (1H, s, H-3), 6.54 (1H, dt, J 16.9, 10.5 Hz, COCHCHCHCH2), 6.15 (1H, d, J 15.2 Hz, COCHCHCHCH2), 5.63 (1H, d, Jtrans 16.7 Hz, COCHCHCHCH2), 5.44 (1H, d, Jcis 11.4 Hz, COCHCHCHCH2), 5.5 (1H, br s, OH-6), 5.38 (1H, br s, OH-7), 5.03 (1H, br s, OH-5∗), 4.86 (1H, t, J8,7=J8,NH=8.9 Hz, H-8), 4.01 (1H, dd, J5*a,5*b 10.9, 2.8 Hz, H-5∗a), 3.79–3.64 (3H, m, H-5, H-5∗b, H-6), 3.63–3.56 (1H, m, H-7), 2.93–2.73 (2H, m, H-2′a, H-2′b), 2.68 (2H, t, J 8.1 Hz, H-2″a, H-2″b).
δC (126 MHz, DMSO d6) 164.7 (COCHCHCHCH2), 158.8, 152.7, 143.1 (C-8a), 141.9 (COCHCHCHCH2), 139.3, 135.2 (COCHCHCHCH2), 128.2, 128.0, 126.6, 125.7 (COCHCHCHCH2), 123.8 (COCHCHCHCH2), 113.6(C-3), 72.8 (C-7), 68.5 (C-6), 61.0 (C-5∗), 60.2 (C-5), 49.5 (C-8), 38.8 (C-2′), 35.2 (C-2″). HRMS-(TOF): MH+, found 384.1919. C21H26N3O4 requires 384.1923.
4.1.30. S-(3-(((5R,6R,7R,8S)-6,7-Dihydroxy-5-(hydroxymethyl)-2-phenethyl-5,6,7,8-tetrahydroimidazo[1,2-a]pyridin-8-yl)amino)-3-oxopropyl) ethanethioate (40)41
Prepared starting from compound 34 as described for 35 in 75% yield; amorphous solid.
δH (500 MHz, pyridine-d5) 9.31 (1H, d, JNH,8 8.4 Hz, NHCO), 7.40 (1H, s, H-3), 7.33–7.27 (2H, m, Ph), 7.24–7.17 (3H, m, Ph), 5.87 (1H, t, J8,7=J8,NH=8.6 Hz, H-8), 4.67 (1H, dd, J5*a,5*b 11.4, J5*a,5 1.6 Hz, H-5∗a), 4.60 (1H, t, J6,7=J6,5=8.6 Hz, H-6), 4.44 (2H, m, H-5∗b, H-7), 4.32 (1H, ddd, J5,6 8.6, J5*b,5 4.7, J5*a,5 1.6 Hz, H-5), 3.52–3.36 (2H, m), 3.17–2.97 (4H, m), 2.96–2.82 (2H, m), 2.16 (3H, s, SCOCH3).
δC (126 MHz, pyridine-d5) 197.4 (SCOCH3), 173.8 (CONH), 146.3, 144.9, 144.4 (C-8a), 130.8, 130.7, 128.1, 116.5 (C-3), 77.2 (C-7), 72.3 (C-6), 64.8 (C-5), 64.0 (C-5∗), 53.8 (C-8), 38.4, 38.2, 33.3, 32.3 (SCOCH3), 27.3. HRMS-(TOF): MH+, found 434.1756. C21H28N3O5S requires 434.1750.
4.1.31. N-((5R,6R,7R,8S)-6,7-Dihydroxy-5-(hydroxymethyl)-2-phenethyl-5,6,7,8-tetrahydroimidazo[1,2-a]pyridin-8-yl)-3-mercaptopropanamide (41)41
To a solution of 40 (0.042 g, 0.1 mmol) and dithiothreitol (0.015 g) in DMF (1.5 mL) stock 25% solution of MeONa in MeOH (0.03 mL) was added at rt. The reaction was stirred for 2 h and quenched with AcOH (0.05 mL). The reaction was concentrated in vacuum and the residue was purified on the C18(2) column as described above to give 0.023 g (0.056 mmol, 56%) of the title compound 41 as amorphous solid.
δH (500 MHz, MeOD) 7.29 (2H, m, Ph), 7.24 (1H, s, H-3), 7.23–7.15 (3H, m, Ph), 4.90 (1H, d, J8,7 7.6 Hz, H-8), 4.15 (1H, dd, J5*a,5*b 12.1, 2.6 Hz, H-5∗a), 4.10–4.05 (1H, m, H-5), 4.02 (1H, dd, J7,6 8.5, J7,8 7.8 Hz, H-7), 3.99 (1H, dd, J5*a,5*b 12.5, 5.2 Hz, H-5∗b), 3.92 (1H, dd, J6,7 8.6, J6,5 7.7 Hz, H-6), 2.98–2.88 (4H, m), 2.84–2.74 (2H, m), 2.70–2.60 (2H, m).
δC (126 MHz, MeOD) 174.5 (NHCO), 145.3, 144.9, 144.4 (C-8a), 130.8, 128.7, 128.5, 126.7, 116.5 (C-3), 71.0 (C-7), 68.1 (C-6), 62.9 (C-5), 60.1 (C-5∗), 49.2 (C-8), 39.8, 34.8, 27.6, 20.0. HRMS-(TOF): MH+, found 392.1641. C19H26N3O4S requires 392.1644.
Acknowledgements
This work was supported by a Wellcome Trust Senior Research Fellowship to DvA.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.tet.2010.07.037.
Contributor Information
Vladimir S. Borodkin, Email: vsborodkin@dundee.ac.uk.
Daan M.F. van Aalten, Email: dmfvanaalten@dundee.ac.uk.
Mol files
The following ZIP file contains the MOL files of the most important compounds referred to in this article.
ZIP file containing the MOL files of the most important compounds in this article.
References and notes
- 1.Torres C.R., Hart G.W. J. Biol. Chem. 1984;259:3308–3317. [PubMed] [Google Scholar]
- 2.Zachara N.E., Hart G.W. Biochim. Biophys. Acta. 2004;1673:13–28. doi: 10.1016/j.bbagen.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 3.Love D.C., Hanover J.A. Sci. STKE. 2005;312:1–14. doi: 10.1126/stke.3122005re13. [DOI] [PubMed] [Google Scholar]
- 4.Wells L., Gao Y., Mahoney J.A., Vosseller K., Chen C., Rosen A., Hart G.W. J. Biol. Chem. 2002;277:1755–1761. doi: 10.1074/jbc.m109656200. [DOI] [PubMed] [Google Scholar]
- 5.Lehman D.M., Fu D.J., Freeman A.B., Hunt K.J., Leach R.J., Johnson-Pais T., HamLington J., Dyer T.D., Arya R., Abboud H., Goring H.H., Duggirala R., Blangero J., Konrad R.J., Stern M.P. Diabetes. 2005;54:1214–1221. doi: 10.2337/diabetes.54.4.1214. [DOI] [PubMed] [Google Scholar]
- 6.Dias W.B., Hart G.W. Mol. Biosyst. 2007;3:766–772. doi: 10.1039/b704905f. [DOI] [PubMed] [Google Scholar]
- 7.Cantarel B.L., Coutinho P.M., Rancurel C., Bernard T., Lombard V., Henrissat B. Nucleic Acids Res. 2009;37:D233–D238. doi: 10.1093/nar/gkn663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haltiwanger R.S., Grove K., Philipsberg G.A. J. Biol. Chem. 1998;273:3611–3617. doi: 10.1074/jbc.273.6.3611. [DOI] [PubMed] [Google Scholar]
- 9.Horsch M., Hoesch L., Vasella A., Rast D.M. Eur. J. Biochem. 1991;197:815–818. doi: 10.1111/j.1432-1033.1991.tb15976.x. [DOI] [PubMed] [Google Scholar]
- 10.Stubbs K.A., Zhang N., Vocadlo D.J. Org. Biomol. Chem. 2006;4:839–845. doi: 10.1039/b516273d. [DOI] [PubMed] [Google Scholar]
- 11.Knapp S., Vocadlo D., Gao Z.N., Kirk B., Lou J.P., Withers S.G. J. Am. Chem. Soc. 1996;118:6804–6805. [Google Scholar]
- 12.Macauley M.S., Whitworth G.E., Debowski A.W., Chin D., Vocadlo D.J. J. Biol. Chem. 2005;280:25313–25322. doi: 10.1074/jbc.M413819200. [DOI] [PubMed] [Google Scholar]
- 13.Yuzwa S.A., Macauley M.S., Heinonen J.E., Shan X., Dennis R.J., He Y., Whitworth G.E., Stubbs K.A., McEachern E.J., Davies G.J., Vocadlo D.J. Nat. Chem. Biol. 2008;4:483–490. doi: 10.1038/nchembio.96. [DOI] [PubMed] [Google Scholar]
- 14.Aoyagi T., Suda H., Uotani K., Kojima F., Aoyama T., Horiguchi K., Hamada M., Takeuchi T. J. Antibiot. (Tokyo) 1992;45:1404–1408. doi: 10.7164/antibiotics.45.1404. [DOI] [PubMed] [Google Scholar]
- 15.Aoyama T., Naganawa H., Suda H., Uotani K., Aoyagi T., Takeuchi T. J. Antibiot. (Tokyo) 1992;45:1557–1558. doi: 10.7164/antibiotics.45.1557. [DOI] [PubMed] [Google Scholar]
- 16.Tatsuta K., Miura S., Gunji H. Bull. Chem. Soc. Jpn. 1997;70:427–434. [Google Scholar]
- 17.Tatsuta K., Miura S., Ohta S., Gunji H. J. Antibiot. (Tokyo) 1995;48:286–288. doi: 10.7164/antibiotics.48.286. [DOI] [PubMed] [Google Scholar]
- 18.Lillelund V.H., Jensen H.H., Liang X., Bols M. Chem. Rev. 2002;102:515–553. doi: 10.1021/cr000433k. [DOI] [PubMed] [Google Scholar]
- 19.Heightman T.D., Vasella A.T. Angew. Chem., Int. Ed. 1999;38:750–770. doi: 10.1002/(SICI)1521-3773(19990315)38:6<750::AID-ANIE750>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Panday N., Canac Y., Vasella A. Helv. Chim. Acta. 2000;83:58–79. [Google Scholar]
- 21.Dubost E., Le Nouen D., Streith J., Tarnus U., Tschamber T. Eur. J. Org. Chem. 2006:610–626. [Google Scholar]
- 22.Shanmugasundaram B., Debowski A.W., Dennis R.J., Davies G.J., Vocadlo D.J., Vasella A. Chem. Commun. (Cambridge, U.K.) 2006:4372–4374. doi: 10.1039/b612154c. [DOI] [PubMed] [Google Scholar]
- 23.Terinek M., Vasella A. Helv. Chim. Acta. 2005;88:10–22. [Google Scholar]
- 24.Rao F.V., Dorfmueller H.C., Villa F., Allwood M., Eggleston I.M., van Aalten D.M.F. EMBO J. 2006;25:1569–1578. doi: 10.1038/sj.emboj.7601026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorfmueller H.C., Borodkin V.S., Schimpl M., Shepherd S.M., Shpiro N.A., van Aalten D.M. J. Am. Chem. Soc. 2006;128:16484–16485. doi: 10.1021/ja066743n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dorfmueller H.C., Borodkin V.S., Schimpl M., van Aalten D.M.F. Biochem. J. 2009;420:221–227. doi: 10.1042/BJ20090110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothenberg A.S., Dauplaise D.L., Panzer H.P. Angew. Chem., Int. Ed. Engl. 1983;22:560–561. [Google Scholar]
- 28.Bernet B., Vasella A. Helv. Chim. Acta. 1979;62:1990–2016. [Google Scholar]
- 29.Hense A., Ley S.V., Osborn H.M.I., Owen D.R., Poisson J.F., Warriner S.L., Wesson K.E. J. Chem. Soc., Perkin Trans. 1. 1997:2023–2031. [Google Scholar]
- 30.Grice P., Ley S.V., Pietruszka J., Priepke H.W.M., Warriner S.L. J. Chem. Soc., Perkin Trans. 1. 1997:351–363. [Google Scholar]
- 31.Garegg P.J., Samuelsson B. J. Chem. Soc., Perkin Trans. 1. 1980:2866–2869. [Google Scholar]
- 32.Kolb H.C., Vannieuwenhze M.S., Sharpless K.B. Chem. Rev. 1994;94:2483–2547. [Google Scholar]
- 33.Ley S.V., Polara A. J. Org. Chem. 2007;72:5943–5959. doi: 10.1021/jo0703451. [DOI] [PubMed] [Google Scholar]
- 34.Mancuso A.J., Huang S.L., Swern D. J. Org. Chem. 1978;43:2480–2482. [Google Scholar]
- 35.Terinek M., Vasella A. Helv. Chim. Acta. 2003;86:3482–3509. [Google Scholar]
- 36.Wu J.P., Emeigh J., Gao D.A., Goldberg D.R., Kuzmich D., Miao C., Potocki I., Qian K.C., Sorcek R.J., Jeanfavre D.D., Kishimoto K., Mainolfi E.A., Nabozny G., Jr., Peng C., Reilly P., Rothlein R., Sellati R.H., Woska J.R., Jr., Chen S., Gunn J.A., O’Brien D., Norris S.H., Kelly T.A. J. Med. Chem. 2004;47:5356–5366. doi: 10.1021/jm049657b. [DOI] [PubMed] [Google Scholar]
- 37.Sonogashira K., Tohda Y., Hagihara N. Tetrahedron Lett. 1975:4467–4470. [Google Scholar]
- 38.Hansen S.U., Bols M. J. Chem. Soc., Perkin Trans. 1. 1999:3323–3325. [Google Scholar]
- 39.Saotome C., Kanie Y., Kanie O., Wong C.H. Bioorg. Med. Chem. 2000;8:2249–2261. doi: 10.1016/s0968-0896(00)00170-x. [DOI] [PubMed] [Google Scholar]
- 40.Numbering used for the spectra description is based on 1-(1H-imidazol-2-yl)pent-4-ene-1,2,3-triol backbone as shown for compound 16 (Scheme 2).
- 41.Numbering used for the spectra description is based on the 5-(hydroxymethyl)-5,6,7,8-tetrahydroimidazo[1,2-a]pyridine-6,7,8-triol backbone as shown for compound 18 (Scheme 3).
- 42.Numbering used for the spectra description is based on 6,7,8,9-tetrahydro-5H-imidazo[1,2-a]azepine-7,8,9-triol backbone as shown for compound 25 (Scheme 5).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ZIP file containing the MOL files of the most important compounds in this article.