Abstract
Purpose
The aim of this study was to determine whether pharmacologically manipulated resting refraction, amplitude, and starting point affect accommodative and disaccommodative dynamics in anesthetized adolescent rhesus monkeys.
Methods
Pilocarpine and atropine were applied topically to manipulate resting refraction, accommodative amplitude, starting point, and end point in two monkeys with permanent electrodes in the Edinger-Westphal nucleus. Accommodation was centrally stimulated with submaximal and maximal current amplitudes. Dynamic accommodative responses were measured with infrared photorefraction before and during the course of action of the drugs. Accommodative and disaccommodative dynamics were analyzed in terms of peak velocity as a function of amplitude, starting point, and end point.
Results
Pilocarpine caused a myopic shift in resting refraction of 11.62 ± 1.17 D. Centrally stimulated accommodative amplitude was 10.08 ± 1.15 D before pilocarpine and 0.68 ± 0.29 D after pilocarpine. Changes were found in accommodative dynamics as a function of starting point and in disaccommodative dynamics as a function of amplitude and end point. Accommodative amplitude was 11.25 ± 0.18 D before atropine administration and 0.52 ± 0.11 D after atropine administration. Accommodative dynamics as a function of amplitude were not substantially altered during the course of pilocarpine-induced accommodation or atropine-induced cycloplegia.
Conclusions
Accommodative response amplitude is reduced with pilocarpine by shifting the eye to a more myopic state and with atropine by cycloplegia. Pharmacologic manipulations showed that accommodative and disaccommodative dynamics in anesthetized monkeys depend on amplitude, starting point, and end point of the response and on the contributions of neural and receptor activity.
Accommodation is a dynamic process that allows the eye to focus on objects at different distances. In humans, accommodation can occur reflexively1 and by a voluntary effort to focus on a near object.2,3 Dynamic accommodation can be produced in anesthetized rhesus monkeys by stimulating the Edinger-Westphal (EW) nucleus.4–6
Dynamics of far-to-near (accommodative) or near-to-far (disaccommodative) responses can be quantified by velocity and acceleration characteristics.3,5,7 The relationship between amplitude and peak velocity of a response is known as a main sequence.8 Dynamic analysis of EW-stimulated accommodation in anesthetized adolescent rhesus monkeys shows that the main sequence increases linearly over the full range for accommodation and disaccommodation, with disaccommodation occurring progressively faster than accommodation.5 With supramaximal current stimulation (i.e., a current amplitude greater than required to achieve maximum accommodation), peak velocity of accommodation increases further without a further increase in response amplitude. Peak velocity of disaccommodation does not increase further but depends on the disaccommodative response amplitude.9
Several studies have examined accommodative dynamics in humans. In young subjects, the time constant of accommodation and the peak velocity of disaccommodation back to baseline increase linearly with response amplitude.3,10 Peak velocity of accommodation increases at low response amplitudes, saturates at higher amplitudes,3 and is slower than for disaccommodation.2,11 The dynamics of accommodation and disaccommodation are influenced by response amplitude and starting point.12,13 Therefore, response amplitude, starting configuration of the accommodative structures, associated biomechanical characteristics, and neuromuscular activity may play a role in the dynamics of the response.
In rhesus monkeys, muscarinic agonists such as pilocarpine and carbachol cause a progressive ciliary muscle contraction, a myopic refractive shift, and, hence, a decrease in EW-stimulated accommodative response amplitude.9,14 Muscarinic antagonists such as atropine and pirenzepine cause cycloplegia because the ciliary muscle neuromuscular receptors are blocked. Pharmacologic stimulation can therefore be used in monkeys to alter refractive state, EW-stimulated accommodative response amplitude, and starting point. EW stimulation after pilocarpine allows evaluation of the dynamic accommodative response as the amplitude decreases with the pilocarpine-induced myopic shift and from more proximal starting points. EW stimulation after atropine allows evaluation of the dynamic accommodative response as the maximum amplitude decreases because of cycloplegia. Atropine and pilocarpine change maximum response amplitude through different actions at the ciliary neuromuscular junctions without affecting the EW neurons. This allows determination of the effects that receptor blockage, partial muscle stimulation, shift in starting point, and reduced response amplitude have on the accommodative response dynamics. Studies of agonists and antagonists have used similar techniques to evaluate their effects on accommodative dynamics in monkeys.9,14,15
Adolescent rhesus monkeys have high pharmacologically9,16,17 and centrally stimulated accommodative amplitudes.5,18,19 The accommodative mechanism20,21 and anterior segment anatomy22,23 are similar to those in humans. The age-related loss of accommodative amplitude, when adjusted for lifespan, is similar to the progression of presbyopia in humans24 and has a similar etiology.25,26 Rhesus monkeys are widely recognized as an ideal animal model for studies of human accommodation and presbyopia.16,17,22,24–27 In this study, pilocarpine and atropine were used to alter response amplitude and starting point in adolescent rhesus monkeys to study the effects on accommodative and disaccommodative dynamics during centrally stimulated accommodation.
Methods
Experiments were performed on four eyes of two rhesus monkeys, monkey 111 (age 6) and monkey 38 (age 7). Both monkeys had previously undergone bilateral iridectomy and had stimulating electrodes permanently implanted in the EW nucleus of the midbrain.19 The monkeys are used in multiple experimental protocols, and the iridectomies,28 justification for them, and absence of effects on centrally stimulated accommodation have been described previously.5,29 In each monkey, experiments were performed at least 1 week apart. All experiments conformed to the ARVO Statement for Use of Animals in Ophthalmic and Vision Research and were conducted under an institutionally approved animal protocol.
Monkeys were initially anesthetized with 10 mg/kg intramuscular ketamine and 0.5 mg/kg intramuscular acepromazine. Surgical depth anesthesia was maintained for the duration of the experiment with intravenous propofol (Propoflo; Abbott Laboratories, North Chicago, IL), with an initial bolus of 0.15 mg/kg followed by constant perfusion of 0.5 mg/kg per minute. Monkeys were placed prone with the head facing forward in a head holder. The eyelid was held open with a speculum. Sutures were tied beneath the lateral and medial rectus muscles to reduce EW-stimulated accommodative convergent eye movements. A custom-made polymethylmethacrylate (PMMA) contact lens (Metro Optics, Dallas, TX) was placed on the cornea to prevent dehydration and to maintain optical quality.
A static stimulus response function was first measured with a Hartinger coincidence refractometer (HCR).5,30 For each increasing stimulus current amplitude, the accommodative response was measured three times and averaged. Stimulus amplitudes from 0 μA to an amplitude that produced the maximum accommodative response available to that eye were delivered with 4-second-long stimulus trains of 600-μs pulses at 72 Hz. The stimulus/response function was unique to each eye tested and was subsequently used to calibrate the dynamic photorefraction.
Infrared photorefraction was used to measure accommodative responses dynamically and to determine the main sequence relationships.5 Before drug instillation, responses to approximately five increasing stimulus current amplitudes spanning the full accommodative range available to each eye were recorded to a digital videotape at 30 Hz. The eye was illuminated with a custom-made bank of 20 infrared diodes in front of a knife-edge aperture shielding the lower half of the lens of an infrared-sensitive charge-coupled device (CCD) video camera (Cohu, San Diego, CA). Images were analyzed off-line, frame by frame, with image analysis software (Optimas; Media Cybernetics, Silver Springs, MD). The slope of the mean vertical luminance profile over 40% of the iridectomized pupil was converted to refraction using the calibration function generated from the static stimulus response function.5,15 For each stimulus amplitude, five 4-second-long stimulus trains were delivered, and the last three responses were analyzed and averaged.
Pilocarpine Experiments
Experiments were performed on both eyes of monkey 38 and twice on the left eye of monkey 111. After the initial baseline measurements, 0.2 mL of 2% pilocarpine was instilled into the eye, with the contact lens on, allowing the drops to collect in the lower lid tear meniscus and behind the contact lens and to diffuse into the anterior chamber. Five 4-second stimuli at a current amplitude required to elicit a submaximal response (submaximal stimulus) and five stimuli at a current amplitude required to elicit a maximal response (maximal stimulus) were delivered to the EW nucleus every 3 minutes. After 15 minutes, 0.2 mL of 2% pilocarpine was again instilled. After 30 minutes, 0.2 mL of 6% pilocarpine was delivered every 15 minutes until no further noticeable EW-stimulated accommodative response occurred in the live photorefraction video image.
Atropine Experiments
Atropine experiments were performed on both eyes of both monkeys. After the pre-atropine baseline measurements, the contact lens was removed, and 40% atropine in agar was applied iontophoretically for 8 seconds each on the nasal and temporal cornea.5,29 The contact lens was replaced, and five submaximal and five maximal stimuli were delivered to the EW nucleus every 3 minutes until cycloplegia occurred at approximately 60 minutes, as determined from the live photorefraction images. Analysis of post-atropine responses to submaximal stimuli on the left eye of monkey 111 was not included because of large drifting eye movements that occurred between stimulations (as can occur under propofol anesthesia). The maximal stimulus caused the eye to return to primary gaze position; therefore, analysis of responses to the maximal stimulus was possible. Once the full effect of atropine appeared to have occurred, a static stimulus response function was again measured with the HCR to verify the extent of cycloplegia.
Dynamic Accommodation Analysis
Dynamic accommodative responses were analyzed to determine the amplitude and peak velocity of the response for accommodation and disaccommodation.5,15 For each individual accommodative response, a function was fitted to both the accommodative and the disaccommodative phases5:
where A is the response amplitude, x is the time, and τ is the time constant. The derivative of these functions with respect to time gives the velocity of the response from which peak velocity was determined.5
Immediately before drug application, a main sequence was determined for responses spanning the full range of accommodation available to each eye. After pilocarpine instillation, the change in resting refraction was determined with photorefraction every 3 minutes. Dynamics of the accommodative and disaccommodative responses to maximal and submaximal stimuli were evaluated at successive time points at which an approximately 1-D myopic shift in resting refraction had occurred. Similarly, after atropine iontophoresis, the dynamics of the accommodative and disaccommodative responses to maximal and submaximal stimuli were evaluated for each 3-minute interval, and response dynamics were evaluated at successive time points at which an approximately 1-D decrease in amplitude had occurred.
Additional analyses were performed on the post-pilocarpine dynamic responses. Peak velocity of the responses to submaximal and maximal stimuli were analyzed as a function of the change in starting point from the pre-pilocarpine resting refraction (i.e., as a function of the pilocarpine-induced myopic shift). Peak velocity of the response was also analyzed as a function of the total accommodative change induced by EW and pilocarpine (EW + pilocarpine) stimulation combined.
Results
Representative examples from two eyes (Fig. 1) and overall normalized results from all eyes (Fig. 2) of the time course of pilocarpine and atropine on dynamically measured accommodative responses to submaximal and maximal EW stimulation are shown. Because of the different response amplitudes, results in Figure 2 are normalized to the response amplitude achieved with the maximal stimulus amplitude before drug treatment.
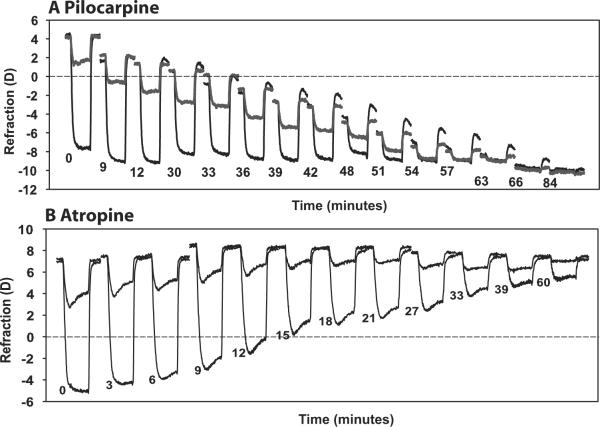
Figure 1.
Representative examples of the time course of the refractive accommodative changes to a sub-maximal and maximal stimulus current amplitude as pilocarpine (A; monkey 38, OD) and atropine (B; monkey 38, OS) takes effect. Each response is an average of three 4-second-long responses, expanded horizontally for visibility. Responses were selected at certain time points: for pilocarpine, resting refraction became approximately 1 D more myopic; for atropine, accommodative amplitude decreased by approximately 1 D. The start time of each response after topical drug application is shown.
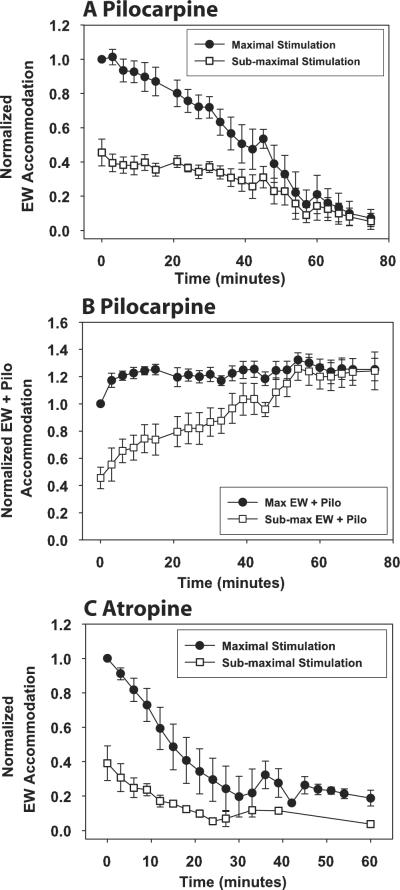
Figure 2.
For all eyes, topical pilocarpine (A) and atropine iontophoresis (C) decreased the EW-stimulated accommodative response amplitudes to submaximal (□) and maximal (●) stimuli. For EW + pilocarpine stimulation (B), responses to maximal EW stimulation increased after the first time point and then remained constant, whereas responses to submaximal EW stimulation increased and approached the maximal responses. Responses are normalized to the pre-pilocarpine maximal EW-stimulated response for each eye. Error bars represent SEM.
Pilocarpine Experiments
The pre-pilocarpine maximal EW-stimulated accommodative amplitudes (± SD) measured during the HCR stimulus response functions were: monkey 38, OS 9.81 ± 0.13 D; monkey 38, OD 12.50 ± 0.44 D; monkey 111, OSa 12.67 ± 0.08 D; and monkey 111, OSb 12.69 ± 0.14 D. Average resting refraction for all four eyes (with the contact lenses) measured with photorefraction before pilocarpine was +2.84 ± 0.60 D (mean ± SEM). Post-pilocarpine measurements were stopped when no EW-stimulated accommodative response appeared to occur at 81.25 ± 3.54 minutes after pilocarpine instillation. At this time, the mean pilocarpine-stimulated refraction was −8.78 ± 0.93 D, resulting in a significant myopic shift of 11.62 ± 1.17 D. Average maximum EW-stimulated accommodative amplitude before pilocarpine was 10.08 ± 1.15 D; at 81.25 ± 3.54 minutes after pilocarpine, it was 0.68 ± 0.29 D, resulting in a significant decrease in maximum EW-stimulated accommodation of 9.40 ± 1.13 D (Fig. 2A). EW + pilocarpine accommodation to the maximal EW stimulus amplitude increased initially and thereafter remained constant, whereas EW + pilocarpine accommodation to the submaximal EW stimulus amplitude increased toward the maximum available amplitude after pilocarpine instillation (Fig. 2B). On average, pilocarpine-stimulated accommodation at 81.25 minutes was 1.54 ± 0.93 D greater than the pretreatment maximum EW-stimulated accommodative response.
Post-Pilocarpine Submaximal Stimulation Dynamics
Peak velocity of accommodation and disaccommodation decreased linearly with EW-stimulated accommodative amplitude as pilocarpine was taking effect (Figs. 3A, 3B). When analyzed as a function of the pilocarpine-induced change in starting point, peak velocity of EW-stimulated accommodative responses to submaximal EW stimulation decreased as the starting point became more proximal (Fig. 4C), whereas peak velocity of EW-stimulated disaccommodative responses was not correlated with the starting point (Fig. 5C). For the post-pilocarpine submaximal stimulus, EW-stimulated accommodative response amplitude remained relatively constant at 3.26 ± 0.23 D (mean ± SE) for approximately 45 minutes (Fig. 2A). For Figures 3C and 4C, only responses within 45 minutes of pilocarpine instillation were included in this analysis to examine responses of similar amplitude. For accommodation and disaccommodation, peak velocity as a function of EW + pilocarpine-stimulated response amplitude decreased with total response amplitude for submaximal stimuli (Figs. 4E, 5E).
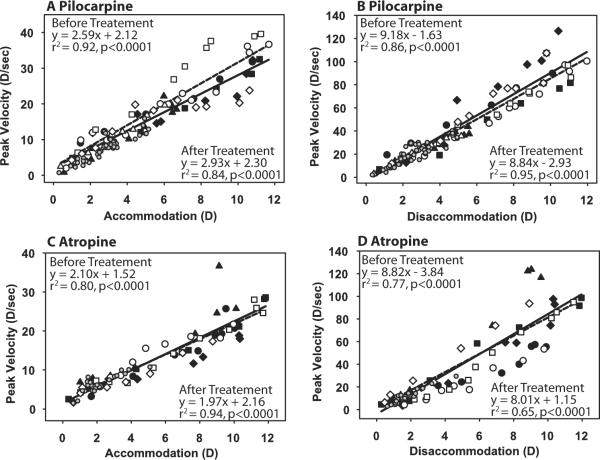
Figure 3.
Main sequence analysis for accommodation (A, C) and disaccommodation (B, D) before (closed symbols, solid lines) and after (open symbols, dashed lines) pilocarpine (A, B) and atropine (C, D). The pre-drug main sequences were determined from responses to stimuli of increasing amplitude. The post-drug main sequences were determined from progressively decreasing responses to the maximal and submaximal stimulus amplitudes. Different symbols represent different monkey eyes. Small gray circles represent the posttreatment response to submaximal stimulus amplitudes for all eyes and are not included in the linear regressions. A significant difference was observed in intercept for the pre-pilocarpine and post-pilocarpine conditions (A; P < 0.05).
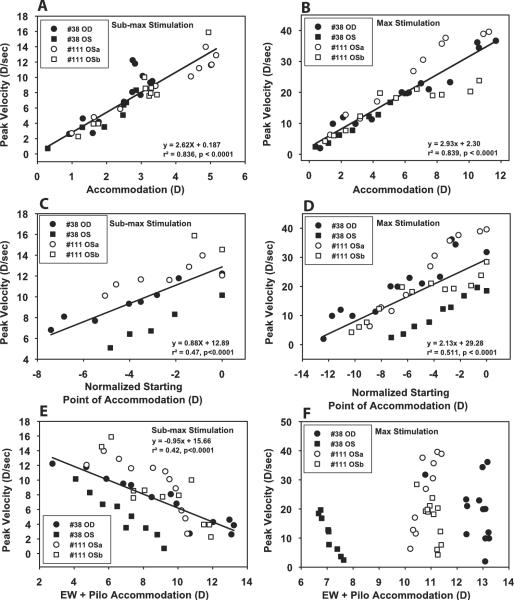
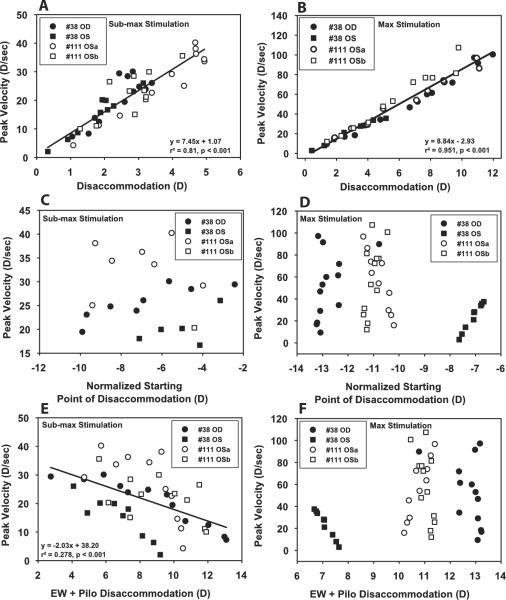
Figure 4.
There was a linear relationship between EW-stimulated response amplitude of accommodation and peak velocity for submaximal (A) and maximal (B) stimulus current amplitudes after pilocarpine instillation. When similar amplitude responses to submaximal current stimulations were considered as a function of normalized starting point, a decrease in peak velocity was observed as pilocarpine shifted the starting point to a more myopic refraction (C). When all responses to maximal current stimulations were considered, there was also a decrease in peak velocity with a myopic shift in starting point (D). Peak velocity to submaximal current stimulation correlated with total accommodation (i.e., EW + pilocarpine-stimulated responses; E) but not with maximal current stimulation (F).
Figure 5.
A linear relationship was observed between EW-stimulated response amplitude of disaccommodation and peak velocity for submaximal (A) and maximal (B) stimulus amplitudes after pilocarpine instillation. When similar amplitude responses to submaximal stimulations were considered as a function of the normalized starting point (C) or all responses to maximal stimulations (D) were considered, no correlation occurred between peak velocity and a myopic shift in starting point. Peak velocity to submaximal current stimulation was correlated with total accommodation (i.e., EW + pilocarpine-stimulated responses; E) but not for maximal current stimulation (F).
Post-Pilocarpine Maximal Stimulation Dynamics
For the maximal EW stimulus, response amplitude began to decrease by approximately 6 minutes after pilocarpine instillation. There was a slight increase in the intercept of the main sequence of EW-stimulated accommodation to the maximal stimulus amplitude after pilocarpine compared with the prepilocarpine main sequence relationship (F(2,68) = 3.73, P < 0.05; slope, P = 0.22; intercept, P < 0.02). No change was observed in the main sequence of disaccommodation after pilocarpine (F(2,68) = 1.14, P = 0.33; slope, P = 0.63; intercept, 0.15; Fig. 3B).
For EW-stimulated responses to maximal stimuli, peak velocity decreased as the starting point became more proximal for accommodation (Fig. 4D) but not for disaccommodation (Fig. 5D). Peak velocity as a function of EW + pilocarpine stimulation showed no correlation with amplitude for responses to maximum stimuli for either accommodation or disaccommodation (Figs. 4F, 5F).
After pilocarpine, the end point of the disaccommodative response became more proximal. For the maximal EW stimulus, disaccommodative response amplitude decreased and peak velocity decreased linearly with disaccommodative end point (Fig. 6). For the submaximal stimuli, initially during the first 45 minutes as the end point became more proximal and the disaccommodative response amplitude was maintained, peak velocity remained constant with the disaccommodative end point (P = 0.08; r2 = 0.15). Subsequently, as the disaccommodative response amplitude for the submaximal stimuli decreased with end point, peak velocity decreased. The slope of peak velocity versus end point relationship for the submaximal stimuli was significantly flatter than for the maximal stimuli (P < 0.0001). For disaccommodation, the dynamics were clearly dependent on end point and differed for the maximal and submaximal stimuli, depending on response amplitude.
Figure 6.
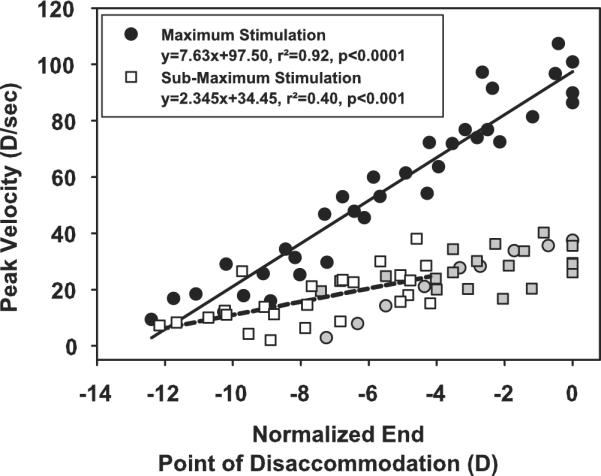
Peak velocity as a function of disaccommodative end point for maximal EW stimulation (black and grey circles, monkey 38 OS; gray circles are excluded from the linear regression analysis because of the low amplitude responses in this eye) and submaximal EW stimulation (white and grey squares; the initial responses of similar amplitude, gray squares, are excluded from the linear regression analysis). No relationship exists for the responses of similar amplitude (gray squares).
Atropine Experiments
Before atropine iontophoresis, resting refraction measured with the HCR was +3.33 ± 0.72 D. The pre-drug maximal EW-stimulated accommodative amplitudes (± SD) measured during the HCR stimulus response functions were: monkey 38, OS 11.42 ± 0.44 D; monkey 38, OD 10.92 ± 0.38 D; monkey 111, OS 10.86 ± 0.05 D; monkey 111, OD 10.92 ± 0.22. Maximum effect of atropine was determined to have occurred 48 ± 9.72 minutes after atropine administration, at which time the resting refraction was +4.52 ± 0.72 D. This resulted in a nonsignificant hyperopic shift of 1.19 ± 0.41 D (paired t test; P = 0.06). Average EW-stimulated maximum accommodative amplitude before atropine was 11.25 ± 0.18 D, and after atropine it was 0.52 ± 0.11 D, resulting in a significant decrease in accommodation of 10.73 ± 0.16 D (Fig. 2C; paired t test; P < 0.001). EW-stimulated accommodative responses to submaximal and maximal stimuli began to decrease immediately after atropine iontophoresis. The post-atropine main sequence relationship for submaximal responses was not different from the pre-atropine main sequence for the same response amplitudes. As accommodation to the maximal stimulus decreased, response dynamics were not significantly altered compared with the pre-atropine main sequence for accommodation (F(2,64) = 0.19, P = 0.83; slope, P = 0.56; intercept, P = 0.88) or for disaccommodation (F(2,64) = 0.19, P = 0.83; slope, P = 0.55; intercept, P = 0.87; Figs. 3C, 3D).
Discussion
The use of atropine and pilocarpine has allowed amplitude, starting point, and end point to be manipulated to evaluate their influence on accommodative and disaccommodative dynamics. In this study, pilocarpine was used to change the tonic status and configuration of the accommodative structures, and atropine was used to reduce response amplitude to study EW-stimulated accommodative dynamics on the assumption that the drugs per se do not influence the dynamics.
After pilocarpine, baseline refraction became more myopic, effectively shifting the starting point of the EW-stimulated accommodative response more proximally. Response amplitude to the submaximal EW stimulus remained nearly constant during the initial 45-minute myopic progression, suggesting that a fixed stimulus amplitude to the EW nucleus released a constant quantity of acetylcholine and that this was added at the neuromuscular junction to the pilocarpine already present. Although the EW-stimulated response amplitude remained constant to the submaximal stimulus, peak velocity of the accommodative response decreased and became slower in the more proximal range. This is a clear demonstration that, in anesthetized monkeys, response dynamics are dependent not only on response amplitude5 but also on starting point, as has been demonstrated in humans.12,13 It may be that as the starting point becomes more proximal, resting tension on the zonular fibers is partially released as the lens becomes more accommodated, the potential energy level of the accommodative plant is reduced, and the initial response velocity decreases.
In humans, voluntary accommodation has been reported to be slower in the near range (5–8 D31), faster in the near range (4.5–6 D12 and 4.6–8.6 D32), or shows no change with starting point (3–4.5 D33). In our study, in anesthetized monkeys, response velocity was found to be slower in the near range. Pilocarpine stimulation served to shift the baseline to a more proximal starting point. In humans, starting point is shifted proximally by a neuronally driven accommodative effort. This involves visual and neural feedback for the proximal shift in starting point and the additional accommodative response. Perhaps these differences or the fact that the monkeys were anesthetized in these experiments resulted in response dynamics being different from those of conscious humans. It has been suggested that, in conscious humans, the accommodative neural controller may compensate for reduced speed by increasing its neural output,33 thus maintaining or increasing the peak velocity. It has also been suggested that the saturation of peak velocity in human subjects at higher accommodative amplitudes3 is caused by mid-brain neurons firing with an initial pulse followed by a step (pulse-step).33 Behavioral studies in monkeys also show that the EW neuron firing frequency increases with an increase in accommodative response.34,35 In the experiments described here, EW-stimulated accommodation was achieved with a step stimulus with constant pulse frequency. EW stimuli of various forms and frequencies could have been constructed and tested for their effects on accommodative dynamics, but that was beyond the scope of the present study.
The main sequence relationship for low-amplitude, EW-stimulated responses before atropine instillation was not significantly different from the main sequence relationship for low-amplitude responses to maximal stimulations after atropine reduced the accommodative response amplitude. Previous studies show that delivering a supramaximal stimulus to a nonatropinized eye results in greater peak velocity than occurs with maximal stimulation.9 Atropine bound to and blocked a proportion of the ciliary muscle receptors and decreased the response amplitude to the maximal stimulus, yet the peak velocity for this fixed stimulus amplitude decreased as the response amplitude decreased. The same maximal stimulus amplitude was delivered to the EW nucleus as before atropine instillation, so the same number of EW neurons were recruited and the same amount of acetylcholine was released at the neuromuscular junction, with diminished effect on the blocked receptors of the ciliary muscle. This suggests that, in anesthetized monkeys, peak velocity is influenced by the number of ciliary neuromuscular receptors activated. It also suggests that the increase in peak velocity that occurs with a supramaximal stimulus in the non–drug-treated eye occurs because maximal stimulus amplitude does not activate all available ciliary muscle receptors to achieve the maximum accommodative response amplitude. It is interesting that the post-atropine response amplitudes to the submaximal stimulus decreased even though the eye clearly had greater amplitude during this early period, suggesting that the constant amount of acetylcholine released with submaximal EW stimulation could not bind to free receptors and stimulate the ciliary muscle in the presence of low concentrations of atropine.
In contrast to atropine, after pilocarpine, the accommodative dynamics for the maximum stimulus showed a small but significant increase in the intercept of the main sequence relationship (Fig. 3A), apparently largely caused by individual variability in the response dynamics of one eye. As the baseline starting point became more proximal after pilocarpine administration, less EW-stimulated accommodative range was available, yet the same maximal stimulus amplitude was delivered. Therefore, as with atropine, the maximal stimulus effectively became a supramaximal stimulus by the release of excess acetylcholine over what was required to achieve the maximum available response amplitude. However, peak velocity was decreased rather than maintained. In this case, some of the neuromuscular receptors were already bound by pilocarpine when the EW stimulus was delivered. As pilocarpine bound to the receptors, fewer unbound receptors were available for the acetylcholine released by EW stimulation. Thus, in the presence of the maximal EW stimulus, peak velocity decreased as the number of receptors activated by the EW stimulation decreased, the EW-stimulated response amplitude decreased, and the starting point of the response became more proximal. In the case of the submaximal EW stimulation in the presence of pilocarpine, the first two points did not apply, but peak velocity still decreased as the starting point became more proximal. Therefore, it appears that in the absence of a change in amplitude, it was the proximal shift in starting point that was the predominant influence on the response dynamics.
The slope and intercept of the post-atropine and post-pilocarpine disaccommodation main sequence remained unchanged compared with the pre-drug condition, suggesting that the dynamics of disaccommodation are dependent on the response amplitude and the restoration of forces of the accommodative structures rather than stimulus amplitude in the absence of consciousness.5 In our study of anesthetized monkeys, peak velocity of disaccommodation was independent of starting point for a constant amplitude response. This is in contrast to studies in humans, which show that the speed of step responses for disaccommodation depends on the starting point. In anesthetized monkeys, visual feedback was absent, and disaccommodation was totally passive and included no neural firing. However, it has been suggested that disaccommodation is influenced by neural firing and is not a passive process in humans.13 Neural firing when focusing from near to far has also been demonstrated in alert rhesus monkeys with single-unit recording techniques.36 The results shown here also demonstrate that the dynamics of disaccommodation are dependent on the amplitude of the response and on the disaccommodative end point. Peak velocity for disaccommodative responses with the same end point depends on the response amplitude.
Given the use of pharmacologic agents to alter the refractive state, starting point, and accommodative amplitude, it might not have been entirely clear to what extent the altered accommodative dynamics were caused by receptor blockage, altered amplitude, or altered starting point. However, experiments in which the starting point and therefore the amplitude are manipulated by EW stimulation, without pharmacology, may shed more light on the extent to which receptor activation or blockage can affect accommodative dynamics. It is possible to shift the starting point proximally under neuronal control in rhesus monkeys using a digital stimulator. This would eliminate the pharmacology and resultant receptor blockage and may provide more insight into the cause of the altered response dynamics.
In vitro studies have suggested that accommodative dynamics are dictated by the accommodative plant, mainly the lens and ciliary body,37 and that the starting configuration of the lens substance and capsule dictate the elastic forces of the lens capsule.38,39 Although results from a study in humans support this suggestion,12 results from the present study suggest that accommodative dynamics to EW-stimulated accommodation in anesthetized rhesus monkeys depends on neural and biomechanical contributions because amplitude of accommodation (from the same starting point) and starting point (with the same amplitude) influence accommodative dynamics.
In conclusion, topical application of pharmacologic agents has allowed manipulation of resting refraction, accommodative amplitude, starting point of the response, and neural and receptor activation to gain a better understanding of the various contributions to accommodative and disaccommodative dynamics. This ultimately may enable us to understand how aging affects the biomechanics of the accommodative plant and, perhaps, influences accommodative dynamics.
Acknowledgments
Supported by National Eye Institute (NEI) Grant 1 RO1 EY014651, NEI Grant P30 EY07751 to the University of Houston College of Optometry, NEI Grant 5 T32 EY07024 to the University of Texas Health Science Center at Houston (AG, LAO), and an Ezell Fellowship from the American Optometric Foundation (LAO).
Footnotes
Disclosure: L.A. Ostrin, None; A. Glasser, None
References
- 1.Fincham EF. The accommodation reflex and its stimulus. Br J Ophthalmol. 1951;35:381–393. doi: 10.1136/bjo.35.7.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heron G, Charman WN, Schor C. Dynamics of the accommodation response to abrupt changes in target vergence as a function of age. Vision Res. 2001;41:507–519. doi: 10.1016/s0042-6989(00)00282-0. [DOI] [PubMed] [Google Scholar]
- 3.Kasthurirangan S, Vilupuru AS, Glasser A. Amplitude dependent accommodative dynamics in humans. Vision Res. 2003;43:2945–2956. doi: 10.1016/j.visres.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Croft MA, Kaufman PL, Crawford KS, et al. Accommodation dynamics in aging rhesus monkeys. Am J Physiol. 1998;275:R1885–R1897. doi: 10.1152/ajpregu.1998.275.6.R1885. [DOI] [PubMed] [Google Scholar]
- 5.Vilupuru AS, Glasser A. Dynamic accommodation in rhesus monkeys. Vision Res. 2002;42:125–141. doi: 10.1016/s0042-6989(01)00260-7. [DOI] [PubMed] [Google Scholar]
- 6.Vilupuru AS, Glasser A. The relationship between refractive and biometric changes during Edinger-Westphal stimulated accommodation in rhesus monkeys. Exp Eye Res. 2005;80:349–360. doi: 10.1016/j.exer.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bharadwaj SR, Schor CM. Acceleration characteristics of human ocular accommodation. Vision Res. 2005;45:17–28. doi: 10.1016/j.visres.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 8.Bahill AT, Clark MR, Stark L. The main sequence, a tool for studying human eye movements. Math Biosci. 1975;24:191–204. [Google Scholar]
- 9.Ostrin LA, Glasser A. Comparisons between pharmacologically and Edinger-Westphal-stimulated accommodation in rhesus monkeys. Invest Ophthalmol Vis Sci. 2005;46:609–617. doi: 10.1167/iovs.04-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciuffreda KJ, Kruger PB. Dynamics of human voluntary accommodation. Am J Optom Physiol Opt. 1988;65:365–370. doi: 10.1097/00006324-198805000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Schaeffel F, Wilhelm H, Zrenner E. Inter-individual variability in the dynamics of natural accommodation in humans: relation to age and refractive errors. J Physiol. 1993;461:301–320. doi: 10.1113/jphysiol.1993.sp019515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasthurirangan S, Glasser A. Influence of amplitude and starting point on accommodative dynamics in humans. Invest Ophthalmol Vis Sci. 2005;46:3463–3472. doi: 10.1167/iovs.04-1408. [DOI] [PubMed] [Google Scholar]
- 13.Bharadwaj SR, Schor CM. Dynamic control of ocular disaccommodation: first- and second-order dynamics. Vision Res. 2006;46:1019–1037. doi: 10.1016/j.visres.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ostrin LA, Frishman LJ, Glasser A. Effects of pirenzepine on pupil size and accommodation in rhesus monkeys. Invest Ophthalmol Vis Sci. 2004;45:3620–3628. doi: 10.1167/iovs.04-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ostrin LA, Glasser A. The effects of phenylephrine on pupil diameter and accommodation in rhesus monkeys. Invest Ophthalmol Vis Sci. 2004;45:215–221. doi: 10.1167/iovs.03-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koretz JF, Bertasso AM, Neider MW, et al. Slit-lamp studies of the rhesus monkey eye, II: changes in crystalline lens shape, thickness and position during accommodation and aging. Exp Eye Res. 1987;45:317–326. doi: 10.1016/s0014-4835(87)80153-7. [DOI] [PubMed] [Google Scholar]
- 17.Bito LZ, DeRousseau CJ, Kaufman PL, Bito JW. Age-dependent loss of accommodative amplitude in rhesus monkeys: an animal model for presbyopia. Invest Ophthalmol Vis Sci. 1982;23:23–31. [PubMed] [Google Scholar]
- 18.Chin NB, Ishikawa S, Lappin H, et al. Accommodation in monkeys induced by midbrain stimulation. Invest Ophthalmol. 1968;7:386–396. [PubMed] [Google Scholar]
- 19.Crawford K, Terasawa E, Kaufman PL. Reproducible stimulation of ciliary muscle contraction in the cynomolgus monkey via a permanent indwelling midbrain electrode. Brain Res. 1989;503:265–272. doi: 10.1016/0006-8993(89)91673-9. [DOI] [PubMed] [Google Scholar]
- 20.Glasser A, Kaufman PL. The mechanism of accommodation in primates. Ophthalmology. 1999;106:863–872. doi: 10.1016/S0161-6420(99)00502-3. [DOI] [PubMed] [Google Scholar]
- 21.Glasser A, Wendt M, Ostrin L. Accommodative changes in lens diameter in rhesus monkeys. Invest Ophthalmol Vis Sci. 2006;47:278–286. doi: 10.1167/iovs.05-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koretz JF, Neider MW, Kaufman PL, et al. Slit-lamp studies of the rhesus monkey eye, I: survey of the anterior segment. Exp Eye Res. 1987;44:307–318. doi: 10.1016/s0014-4835(87)80014-3. [DOI] [PubMed] [Google Scholar]
- 23.van Alphen GW. The adrenergic receptors of the intraocular muscles of the human eye. Invest Ophthalmol. 1976;15:502–505. [PubMed] [Google Scholar]
- 24.Kaufman PL, Bito LZ, DeRousseau CJ. The development of presbyopia in primates. Trans Ophthalmol Soc UK. 1982;102:323–326. [PubMed] [Google Scholar]
- 25.Croft MA, Glasser A, Heatley G, et al. The zonula, lens, and circumlental space in the normal iridectomized rhesus monkey eye. Invest Ophthalmol Vis Sci. 2006;47:1087–1095. doi: 10.1167/iovs.04-1524. [DOI] [PubMed] [Google Scholar]
- 26.Croft MA, Glasser A, Heatley G, et al. Accommodative ciliary body and lens function in rhesus monkeys, I: normal lens, zonule and ciliary process configuration in the iridectomized eye. Invest Ophthalmol Vis Sci. 2006;47:1076–1086. doi: 10.1167/iovs.04-1523. [DOI] [PubMed] [Google Scholar]
- 27.Bito LZ, Kaufman PL, DeRousseau CJ, Koretz J. Presbyopia: an animal model and experimental approaches for the study of the mechanism of accommodation and ocular aging. Eye. 1987;1(pt 2):222–230. doi: 10.1038/eye.1987.41. [DOI] [PubMed] [Google Scholar]
- 28.Kaufman PL, Lütjen-Drecoll E. Total iridectomy in the primate in vivo: surgical technique and postoperative anatomy. Invest Ophthalmol. 1975;14:766–771. [PubMed] [Google Scholar]
- 29.Crawford KS, Kaufman PL, Bito LZ. The role of the iris in accommodation of rhesus monkeys. Invest Ophthalmol Vis Sci. 1990;31:2185–2190. [PubMed] [Google Scholar]
- 30.Fincham EF. The coincidence optometer. Proc Phys Soc (London) 1937;49:456–468. [Google Scholar]
- 31.Shirachi D, Liu J, Lee M, et al. Accommodation dynamics, I: range nonlinearity. Am J Optom Physiol Opt. 1978;55:631–641. doi: 10.1097/00006324-197809000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Beers APA, Van Der Heijde GL. In vivo determination of the biomechanical properties of the component elements of the accommodative mechanism. Vision Res. 1994;34:2897–2905. doi: 10.1016/0042-6989(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 33.Schor CM, Bharadwaj SR. Pulse-step models of control strategies for dynamic ocular accommodation and disaccommodation. Vision Res. 2006;46:242–258. doi: 10.1016/j.visres.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 34.Gamlin PD, Zhang Y, Clendaniel RA, Mays LE. Behavior of identified Edinger-Westphal neurons during ocular accommodation. J Neurophysiol. 1994;72:2368–2382. doi: 10.1152/jn.1994.72.5.2368. [DOI] [PubMed] [Google Scholar]
- 35.Judge SJ, Cumming BG. Neurons in the monkey midbrain with activity related to vergence eye movement and accommodation. J Neurophysiol. 1986;55:915–930. doi: 10.1152/jn.1986.55.5.915. [DOI] [PubMed] [Google Scholar]
- 36.Gamlin PD, Clarke RJ. Single-unit activity in the primate nucleus reticularis tegmenti pontis related to vergence and ocular accommodation. J Neurophysiol. 1995;73:2115–2119. doi: 10.1152/jn.1995.73.5.2115. [DOI] [PubMed] [Google Scholar]
- 37.Ejiri M, Thompson HE, O'Neill WD. Dynamic visco-elastic properties of the lens. Vision Res. 1969;9:233–244. doi: 10.1016/0042-6989(69)90003-0. [DOI] [PubMed] [Google Scholar]
- 38.Fisher RF. The significance of the shape of the lens and capsular energy changes in accommodation. J Physiol. 1969;201:21–47. doi: 10.1113/jphysiol.1969.sp008740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fisher RF. The force of contraction of the human ciliary muscle during accommodation. J Physiol. 1977;270:51–74. doi: 10.1113/jphysiol.1977.sp011938. [DOI] [PMC free article] [PubMed] [Google Scholar]