Abstract
Background
Epstein–Barr virus (EBV)–associated post-transplantation lymphoproliferative disease (PTLD) develops in 1 to 10 percent of transplant recipients, in whom it can be treated by a reduction in the level of immunosuppression. We postulated that the tissue expression of the small RNA transcribed by the EBER-1 gene during latent EBV infection would identify patients at risk for PTLD.
Methods
We studied EBER-1 gene expression in liver specimens obtained from 24 patients 2 days to 22 months before the development of PTLD, using in situ hybridization with an oligonucleotide probe. Control specimens were obtained from 20 recipients of allografts with signs of injury due to organ retrieval, acute graft rejection, or viral hepatitis in whom PTLD had not developed 9 to 71 months after the biopsy.
Results
Of the 24 patients with PTLD, 17 (71 percent) had specimens in which 1 to 40 percent of mononuclear cells were positive for the EBER-1 gene. In addition, 10 of these 17 patients (59 percent) had specimens with histopathological changes suggestive of EBV hepatitis. In every case, EBER-1–positive cells were found within the lymphoproliferative lesions identified at autopsy. Only 2 of the 20 controls (10 percent) had specimens with EBER-1–positive cells (P< 0.001), and such cells were rare.
Conclusions
EBER-1 gene expression in liver tissue precedes the occurrence of clinical and histologic PTLD. The possibility of identifying patients at risk by the method we describe here and preventing the occurrence of PTLD by a timely reduction of immunosuppression needs to be addressed by future prospective studies.
POST-TRANSPLANTATION Iymphoproliferative disease (PTLD), either polyclonal or monoclonal, complicates the clinical course of 1 to 10 percent of organ-transplant recipients.1-3 Immunohistochemical studies have demonstrated that the lymphoid cells within the lesions of PTLD almost invariably contain Epstein–Barr virus (EBV), primarily in a state of latent infection.4,5
The EBER-1 gene is expressed early during latent EBV infection and codes for a small messenger RNA (mRNA) expressed at up to 107 copies per cell.6 We and others have previously demonstrated the value of the detection of EBER-1 RNA for identifying EBV-infected cells in formalin-fixed paraffin-embedded tissues.7,8
In the current investigation, we used in situ hybridization to examine a series of liver-biopsy specimens from pediatric liver recipients in whom PTLD developed, as well as a control group of patients in whom this disorder did not develop, for evidence of cells expressing the EBER-1 gene. We found that such expression may permit early identification of patients at risk for PTLD.
Methods
By reviewing the autopsy and surgical pathology files at the Children’s Hospital of Pittsburgh for the years 1982 through 1989, we identified 24 liver recipients who met the two criteria for entry to our study: PTLD had been diagnosed histopathologically,3 and at least one liver-biopsy specimen obtained before the diagnosis of PTLD was available for evaluation.
Control patients were drawn from the same files as the patients with PTLD. The controls had had no documented PTLD during a follow-up period of at least nine months after the performance of the liver biopsy yielding a specimen selected to serve as a control. Pertinent clinical information about the group with PTLD and the control group was obtained from medical records. Histopathological evaluation of the liver specimens selected for study was performed on 4-μm sections of tissue routinely embedded in paraffin and stained with hematoxylin and eosin.
The in situ hybridization studies of EBV RNA were performed with a 30-base digoxigenin-labeled oligonucleotide complementary to a portion of the EBER-1 gene. The details of the technique have been published elsewhere.8 The appearance of a brown or blue-brown color in the nucleus was considered a positive reaction. This method has previously detected EBV RNA from an EBV-infected Raji-cell line, but does not detect EBV in the T-cell line Molt, which does not contain EBV. Lymphoid tissue from an EBV-seronegative patient and tissues infected with herpes simplex virus type 1 , papillomavirus type 16, and adenovirus showed no cross-reactivity. Tissue from a patient with PTLD who was known to be positive for EBV served as a positive control in each run. Any slide negative for EBV RNA was tested for preservation of RNA with the use of a polydeoxythymidine probe.9,10
The frequency of EBV -infected cells was determined in a semi-quantitative fashion by counting the total number of EBER-1 cells in relation to the total number of lymphocytes seen in parallel sections prepared from the same biopsy specimen and stained with hematoxylin and eosin. The cells in the hepatic sinusoids and portal tracts were counted. In small specimens, all available cells were counted; in larger specimens, every second or third portal tract was exa mined.
Immunohistochemical in situ hybridization studies with double labeling were performed on liver blocks obtained at autopsy from four patients with fatal PTLD. Initially, the immunohistochemical reaction was performed according to a previously published method.11 The antibodies used were directed against LCA (Dako, Carpinteria, Calif.), CD20 (L26, Dako), CD43 (Leu 22, Becton Dickinson, Mountain View, Calif.), Cam 5.2 (Becton Dickinson), and a mixture of anti-keratin antibodies composed of AE1 (Hybritech, San Diego, Calif.), Cam 5.2, VCD3 (Triton Biosciences, Alameda, Calif.), and GN-2 (Enzo Biochemicals, New York). Thereafter, in situ hybridization studies were performed as outlined above.
Results
The group with PTLD comprised 10 male and 14 female patients. Their ages at the time of transplantation ranged from 6 months to 25 years (median, 3.7 years). Their diagnoses included a variety of liver disorders, such as biliary atresia, cryptogenic cirrhosis, alpha1-antitrypsin deficiency, hereditary cholestasis, and hyperalimentation injury. One patient had received a heart transplant for idiopathic cardiomyopathy. The onset of PTLD occurred a median of 12.5 weeks after transplantation (range, 5 to 640). The anatomical sites involved by PTLD when it was first diagnosed were the liver (seven patients), the tonsils or adenoids (five patients), the gastrointestinal tract (five patients), the trachea (one patient), the bile duct (one patient), the meninges (one patient), and the lymph nodes (one patient); in three patients the disease was d isseminated.
The control group consisted of 12 male and 8 female patients 10 months to 16 years old (median, 7 years). The spe trum of primary diseases requiring transplantation in this group was similar to that in the group with PTLD. The biopsy specimens selected as controls were obtained 5 to 1790 days after transplantation (median, 27.5). Histopathological examination generally showed varying degrees of cellular rejection with or without injury due to organ retrieval, as assessed by standard criteria.12 Two controls had non-A, non-B hepatitis, and three had cytomegalovirus hepatitis (Table 1). These controls had no evidence of PTLD during clinical follow-up lasting 9 to 71 months after liver biopsy (median, 30).
Table 1.
Clinicopathological Features and EBER-1 Gene Expression in Liver Recipients
| Feature | Control Group (N = 20) |
Group with PTLD (N = 24) |
|---|---|---|
| Age range (median) — yr | 0.8–16 (7.0) | 0.5–25.0 (3.7) |
| Sex — M/F | 12/18 | 10/14 |
| no. with feature/no. Studied (%) | ||
| EBER-1 positivity | 2/20 (10) | 17/24 (71)* |
| EBV seropositivity† | 7/15 (47) | 10/16 (62) |
| Liver histopathology Cellular rejection or ischemic injury‡ |
15/20 (75) | 5/24 (21) |
| Hepatitis§ | 5/20 (25) | 17/24 (71) |
P<0.001 for comparison of the control group with the group with PTLD, by Fisher’s exact test.
For details of serologic testing for EBV, see the text.
Changes reflecting graft rejection and ischemic injury were frequently concurrent.
Hepatitis was attributed to cytomegalovirus or non-A, non-B hepatitis virus in the control group, and generally 10 EBY in the group wilh PTLD. The latter group included five patients with concurrent hepatitis and rejection (see the text for detailed histopathological findings).
In situ hybridization studies for EBER-1 expression revealed that in 17 of the 24 patients with PTLD (71 percent), one or more liver specimens obtained before the development of PTLD contained from 1 percent to 40 percent EBER-1 mRNA–positive mononuclear cells in the portal tracts and sinusoids (Fig. 1 and 2). In contrast, only 2 of 20 controls (10 percent) had mononuclear cells containing EBER-1 mRNA (P<0.001 by Fisher’s exact test); in both these controls, less than 1 percent of the cells were positive. Generally, the positive cells were small lymphocytes, although some were as large as 20 μm. Hepatocytes, bile-duct epithelial cells, and endothelial cells did not hybridize with the EBER-1 probe. In the group with PTLD, the interval between the detection of EBER-1 mRNA in mononuclear cells and the development of PTLD ranged from 0 to 50 days in 10 patients and from 51 to 100 days in 3 patients; it was more than 100 days in 4 patients. In 1 of these 17 patients, rare EBER-1 mRNA-positive cells were detected 660 days before the onset of PTLD. In 10 of the 17 patients in whom EBV-positive cells were identified, the subsequent PTLD involved the liver, whereas in the other 7 patients it involved other organs. Expression of the EBER-1 gene could be demonstrated in each of 11 specimens of tissue involved by PTLD that were studied by in situ hybridization (Fig. 3). These tissues contained abundant EBER-1–positive mononuclear cells, which accounted for more than 80 percent of the total lymphoid-cell population in some patients. Small round lymphocytes as well as cells with blastic transformation contained EBV mRNA. As in three previously reported cases of PTLD,13 rare hepatocytes also showed staining for EBER-1 mRNA (Fig. 4). Bile-duct epithelial cells and endothelial cells were negative in all patients. Double-labeling studies showed that the majority of the lymphoid cells positive for EBER-1 mRNA had membranes positive for CD20, a finding consistent with B-cel1 lineage (Fig. 5). However, cells coexpressing CD43 and EBER-1 mRNA, presumably representing T cells, were also present in small numbers.
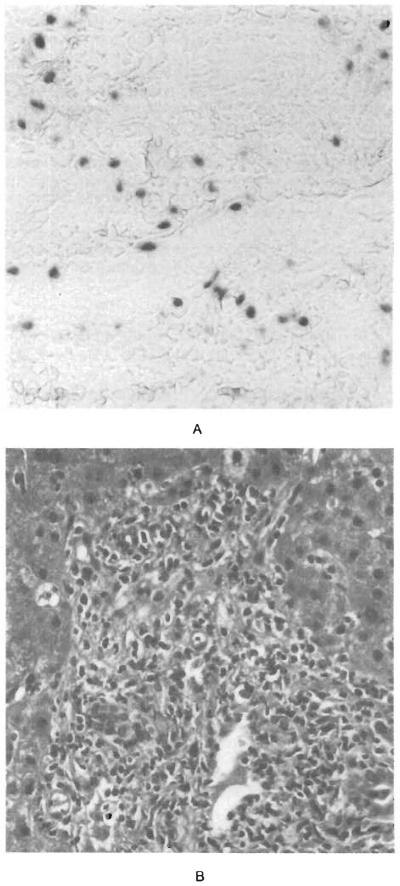
Figure 1.
Lymphocytic Infiltration of the Hepatic Portal Tracts by Mononuclear cells Positive for EBER-1 mRNA in a Patient with EBV-Associated Hepatitis.
Panel A (×210) shows scattered EBER-1–positive nuclei in approximately 20 percent of the lymphocytes infiltrating the portal tract. Panel B (hematoxylin and eosin, ×210) shows an infiltrate of activated lymphocytes involving the portal vein and simulating graft rejection.
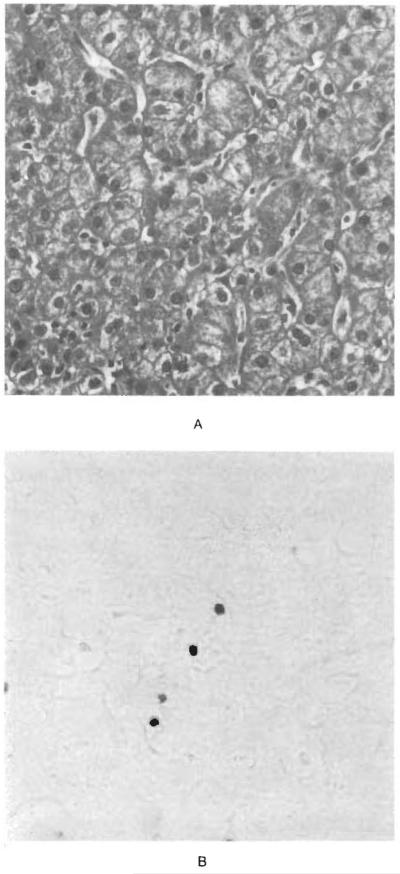
Figure 2.
Lymphocytic Infiltration of the Hepatic Sinusoids by EBER-1–Positive Cells in a Patient with EBV-Associated Hepatitis.
In Panel A (×210), the sinusoids contain aggregates of lymphocytes, and the hepatocytes show focal hydropic swelling, steatosis, and nuclear pyknosis. In Panel B (×210), the sinusoids also contain EBER-1–positive lymphocytes.
Figure 3.
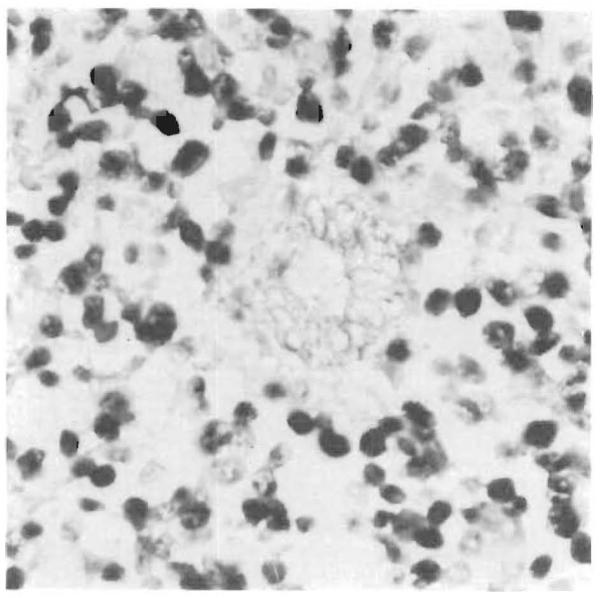
PTLD Involving a Portal Tract (× 420).
Approximately 70 percent of the cells are EBER-1–positive, with both the small round lymphocytes and the large immature lymphocytes affected. The bile-duct epithelium in the center of the field is stained with anti–Cam 5.2 antibody and does not show evidence of EBV infection.
Figure 4.
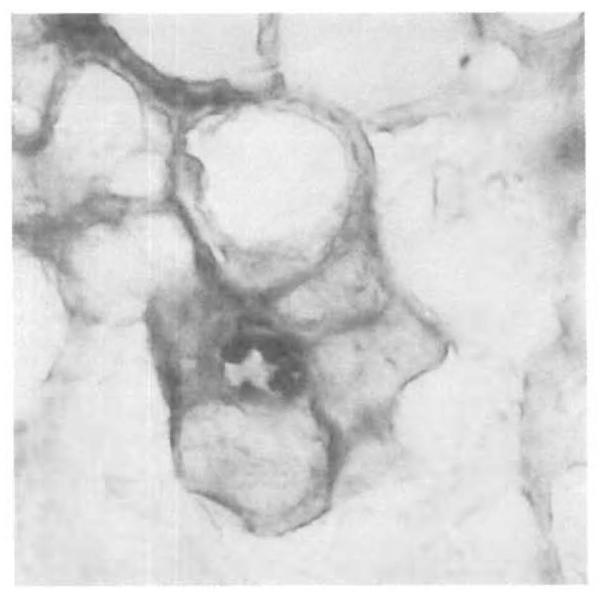
Fatal PTLD Involving Hepatocytes (×630).
Rare EBER-1–positive nuclei were found In cells conclusively identified as hepatocytes, by simultaneous immunohistochemical evaluation with the anti-keratin antibody Cam 5. 2.
Figure 5.
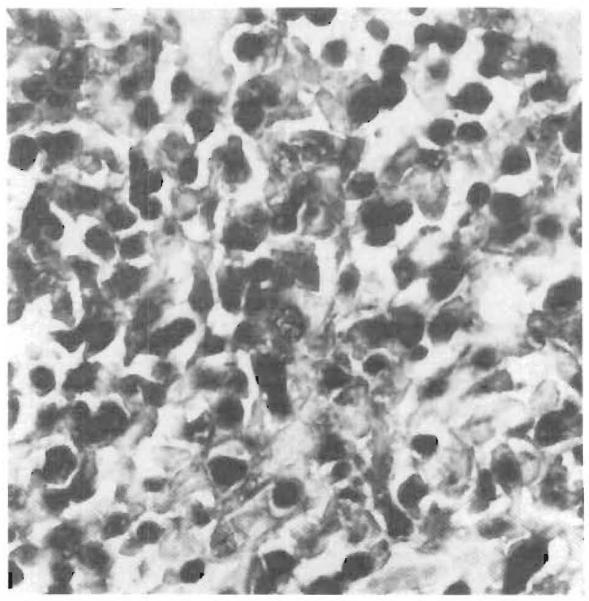
EBV-Infected Lymphocytes in the Lesions of PTLD (×420).
Immunohistochemical evaluation with anti-CD20 antibody, combined with in situ hybridization, showed that in PTLD the majority of EBV-infected lymphocytes are B cells. Cells positive for CD20 are stained brown, and those positive for EBER-1 mRNA are stained blue-black.
Serologic data temporally related to the biopsy were obtained for 16 of the 24 patients with PTLD (through the courtesy of Dr. M .C. Breinig, University of Pittsburgh). Six patients were judged either to be seronegative for EBV or to have titers transiently positive for EBV because of multiple perioperative blood transfusions; all were negative for EBER-1 mRNA. The other 10 patients were seropositive and found to be positive for EBER-1 mRNA as well. Serologic data were available for 15 of the 20 controls. Both EBER-1–positive controls were seropositive for EB; one was undergoing primary seroconversion at the time of biopsy. Five controls were seropositive for EBV but EBER-1–negative. The eight other controls were seronegative as well as EBER-1–negative.
Histopathological examination of liver specimens from the 17 patients with PTLD whose mononuclear cells were positive for EBER-1 mRNA showed that 15 patients had changes indicating EBV hepatitis, consisting of activated mononuclear infiltrates in the portal tracts associated with lobular disarray and sinusoidal lymphocytes arranged in linear beads and small aggregates. One patient had mononuclear inflammatory infiltrates that spared the portal tracts and resembled lobular hepatitis. Five patients, in addition to the changes of EBV hepatitis, had a mixed lymphocytic, polymorphonuclear, and eosinophilic portal-tract infiltrate associated with bile-duct injury, indicating a mild acute cellular rejection. One patient had a necrotizing granulomatous hepatitis with adenovirus inclusions. When PTLD supervened in the patients with EBV hepatitis, the mononuclear-cell infiltrates expanded and acquired atypical nuclear characteristics.
Liver specimens obtained from 7 of the 24 patients before PTLD developed were negative for EBER-1 mRNA. The histologic correlates of these tissues were mild acute cellular rejection with or without coexisting injury due to organ retrieval (five patients) or injury due to hyperalimentation (one patient); non-specific changes consisting of mild hepatocellular swelling with scattered polymorphonuclear leukocytes and lymphocytes in the sinusoids were observed in one patient (Table 1).
Discussion
The oropharyngeal mucosa, parotid and lacrimal glands, lymph nodes, and circulating lymphocytes are known to be anatomical sites where latent EBV may persist after acute infectious mononucleosis.8, 14-17 The present study indicates that lymphocytes of the hepatic portal tract and sinusoids can also harbor EBV, and that an increase in the number of EBER-1–positive cells precedes clinically and histologically evident PTLD. The detection of such EBV-positive cells in the absence of evidence of graft rejection should prompt the physician to consider decreasing the level of immunosuppression, since EBV infections, even when PTLD is advanced, are responsive to immunomodulation.2 Prospective studies will be necessary to assess the positive predictive value of EBER-1–positive cells in regard to the possible development of PTLD in a particular patient.
The presence of EBV-positive lymphoid cells before the occurrence of lymphoproliferative disease has also been observed in lymph nodes from patients with the acquired immunodeficiency syndrome; this finding is consistent with current notions of a multistep process of carcinogenesis induced by EBV.18-21 The possible role of EBER-1 mRNA in the evolution of PTLD is unknown, but it has been suggested that EBER-1 genes direct the synthesis of critical viral proteins in vivo22.
The fact that EBV-positive cells in PTLD lesions were generally positive for CD20 is consistent with the notion that the EBV receptors required for the virus to enter the cells are normally found on B cells.23 The less frequent occurrence of EBER-1 mRNA in CD43-positive cells adds to growing evidence that benign as well as neopla stic T cells can occasionally be infected with EBV.8,24,25
Several authors have described the histopathological features of clinically presumed EBV hepatitis in patients who were not graft recipients26-28 and in recipients of liver allografts.29 Our study documents the presence of EBV-infected cells in the liver tissue of transplant recipients. EBV hepatitis in liver allografts may be overlooked if the pathologist does not pay close attention to lobular disarray and sinusoidal lymphocytosis. The demonstration of the presence of a virus by in situ hybridization is very helpful in reaching a diagnosis when mononuclear infiltrates are subtle, but it is unnecessary when numerous transformed lymphocytes can be clearly seen within the sinusoids, arranged in discrete aggregates and linear chains. However, serologic data should be obtained in the latter case, since non-A, non-B hepatitis can cause similar changes.30
Acknowledgments
Supported by funds from the Pathology Education and Research Foundation and Children’S Hospital of Pittsburgh and by grants (HL-13108 and CA-50341) from the National Institutes of Health.
References
- 1.Hanto DW, Gajl-Peczalska KJ, Frizzera G, et al. Epstein-Barr virus (EBV) induced polyclonal and monoclonal B-cell lymphoproliferative diseases occurring after renal transplantation: clinical, pathologic, and virologic findings and implications for therapy. Ann Surg. 1983;198:356–69. doi: 10.1097/00000658-198309000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Nalesnik MA, Porter KA, et al. Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporine-steroid therapy. Lancet. 1984;1:583–7. doi: 10.1016/s0140-6736(84)90994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nalesnik MA, Jaffe R, Starzl TE, et al. The pathology of posttransplant Iymphoproliferative disorders occurring in the setting of cyclosporine A-prednisone immunosuppression. Am J Pathol. 1988;133:173–92. [PMC free article] [PubMed] [Google Scholar]
- 4.Young L, Alfieri C, Hennessy K, et al. Expression of Epstein–Barr virus transformation–associated genes in tissues of patients with EBV lymphoproliferative disease. N Engl J Med. 1989;321:1080–5. doi: 10.1056/NEJM198910193211604. [DOI] [PubMed] [Google Scholar]
- 5.Thomas JA, Hotchin NA, Allday MJ, et al. Immunohistology of Epstein-Barr virus-associated antigens in B cell disorders from immunocompromised individuals. Transplantation. 1990;49:944–53. doi: 10.1097/00007890-199005000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Rooney C, Howe JG, Speck SH, Miller G. Influences of Burkitt’s lymphoma and primary B cells on latent gene expression by the nonimmortalizing P3J-HR-1 strain of Epstein-Barr virus. J Virol. 1989;63:1531–9. doi: 10.1128/jvi.63.4.1531-1539.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howe JG, Steitz JA. Localization of Epstein-Barr virus-encoded small RNAs by in situ hybridization. Proc Natl Acad Sci USA. 1986;83:9006–10. doi: 10.1073/pnas.83.23.9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss LM, Chen YY, Liu XF, Shibata D. Epstein-Barr virus and Hodgkin’s disease: a correlative in situ hybridization and polymerase chain reaction study. Am J Pathol. 1991;139:1259–65. [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss LM, Movahed LA, Chen YY, et al. Detection of immunoglobulin light-chain mRNA in lymphoid tissues using a practical in situ hybridization method. Am J Pathol. 1990;137:979–88. [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss LM, Chen YY. Effect of different fixatives on the detection of nucleic acids from paraffin-embedded tissues by in situ hybridization using oligonucleotide probes. J Histochem Cytochem. 1991;39:1237–42. doi: 10.1177/39.9.1918942. [DOI] [PubMed] [Google Scholar]
- 11.Sheibani K, Tubbs RR. Enzyme immunohistochemistry: technical aspects. Semin Diagn Pathol. 1984;1:235–50. [PubMed] [Google Scholar]
- 12.Demetris AJ, Jaffe R, Starzl TE. A review of adult and pediatric Post-transplant liver pathology. Pathol Annu. 1987;22(2):347–86. [PubMed] [Google Scholar]
- 13.Randhawa PS, Jaffe RJ, Demetris AJ, et al. The systemic distribution of Epstein-Barr virus genomes in fatal post-transplantation Iymphoproliferative disorders: an in situ hybridization study. Am J Pathol. 1991;138:1027–33. [PMC free article] [PubMed] [Google Scholar]
- 14.Strauch B, Andrews L-L, Siegel N, Miller G. Oropharyngeal excretion of Epstein-Barr virus by renal transplant recipients and other patients treated with immunosuppressive drugs. Lancet. 1974;1:234–7. doi: 10.1016/s0140-6736(74)92546-x. [DOI] [PubMed] [Google Scholar]
- 15.Schuurman HJ, Schemmann MHG, de Weger RA, Aanstoot H, Hene R. Epstein-Barr virus in the sublabial salivary gland in Sjogren’s syndrome. Am J Clin Pathol. 1989;91:461–3. doi: 10.1093/ajcp/91.4.461. [DOI] [PubMed] [Google Scholar]
- 16.Crouse CA, Pflugfelder SC, Cleary T, Demick SM, Atherton SS. Detection of Epstein-Barr virus genomes in normal human lacrimal glands. J Clin Microbiol. 1990;28:1026–32. doi: 10.1128/jcm.28.5.1026-1032.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nilsson K, Klein G, Henle W, Henle G. The establishment of Iymphoblastoid lines from adult and fetal human lymphoid tissue and its dependence on EBV. Int J Cancer. 1971;8:443–50. doi: 10.1002/ijc.2910080312. [DOI] [PubMed] [Google Scholar]
- 18.Shibata D, Weiss LM, Nathwani BN, Brynes RK, Levine AM. Epstein-Barr virus in benign lymph node biopsies from individuals infected with the human immunodeficiency virus is associated with concurrent or subsequent development of non-Hodgkin’s lymphoma. Blood. 1991;77:1527–33. [PubMed] [Google Scholar]
- 19.Hanto DW, Sakamoto K, Purtilo DT, Simmons RL, Najarian RS. The Epstein-Barr virus in the pathogenesis of posttransplant Iymphoproliferative disorders: clinical, pathologic, and virologic correlation. Surgery. 1981;90:204–13. [PubMed] [Google Scholar]
- 20.Locker J, Nalesnik M. Molecular genetic analysis of lymphoid tumors arising after organ transplantation. Am J Pathol. 1989;135:977–87. [PMC free article] [PubMed] [Google Scholar]
- 21.Katz BZ, Raab-Traub N, Miller G. Latent and replicating forms of Epstein-Barr virus DNA in lymphomas and Iymphoproliferative diseases. J Infect Dis. 1989;160:589–98. doi: 10.1093/infdis/160.4.589. [DOI] [PubMed] [Google Scholar]
- 22.Swaminathan S, Tomkinson B, Kieff E. Recombinant Epstein-Barr virus with small RNA (EBER) genes deleted transforms lymphocytes and replicates in vitro. Proc Natl Acad Sci U S A. 1991;88:1546–50. doi: 10.1073/pnas.88.4.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bush JL, Radich PC. The Epstein-Barr virus receptor. Lab Med. 1989;20:318–23. [Google Scholar]
- 24.Kikuta H, Taguchi Y, Tomizawa K, et al. Epstein-Barr virus genome-positive T lymphocytes in a boy with chronic active EBV infection associated with Kawasaki-like disease. Nature. 1988;333:455–7. doi: 10.1038/333455a0. [DOI] [PubMed] [Google Scholar]
- 25.Su IJ, Hsieh MC, Lin KH, et al. Aggressive peripheral T-cell lymphomas containing Epstein-Barr viral DNA: a clinicopathologic and molecular analysis. Blood. 1991;77:799–808. [PubMed] [Google Scholar]
- 26.Markin RS, Linder J, Zuerlein K, et al. Hepatitis in fatal infectious mononucleosis. Gastroenterology. 1987;93:1210–7. doi: 10.1016/0016-5085(87)90246-0. [DOI] [PubMed] [Google Scholar]
- 27.Gowing NFC. Infectious mononucleosis: histopathologic aspects. Pathol Annu. 1975;10:1–20. [PubMed] [Google Scholar]
- 28.Lukes RJ, Cox FH. Clinical and morphologic findings in 30 fatal cases of infectious mononucleosis. Am J Pathol. 1958;34:586. abstract. [Google Scholar]
- 29.Randhawa PS, Markin RS, Starzl TE, Demetris AJ. Epstein-Barr virus-associated syndromes in immunosuppressed liver transplant recipients: clinical profile and recognition on routine allograft biopsy. Am J Surg Pathol. 1990;14:538–47. doi: 10.1097/00000478-199006000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bamber M, Murray A, Arborgh BAM, et al. Short incubation non-A, non-B hepatitis transmitted by factor VIII concentrates in patients with congenital coagulation disorders. Gut. 1981;22:854–9. doi: 10.1136/gut.22.10.854. [DOI] [PMC free article] [PubMed] [Google Scholar]