Abstract
Background
Chronic ethanol consumption is associated with hepatic lipid peroxidation and the deposition or retention of aldehyde-adducted proteins postulated to be involved in alcohol-induced liver injury. The purpose of this study was to characterize hepatocellular formation of aldehyde-protein adducts during early stages of alcohol-induced liver injury.
Methods
Female Sprague Dawley® rats were subjected to the intragastric administration of a low-carbohydrate/high-fat total enteral nutrition diet or a total enteral nutrition diet containing ethanol for a period of 36 days. Indexes of hepatic responses to ethanol were evaluated in terms of changes in plasma alanine aminotransferase activity, hepatic histopathologic analysis, and induction of cytochrome P-4502E1 (CYP2E1). Immunohistochemical methods were used to detect hepatic proteins modified with malondialdehyde (MDA) or 4-hydroxynonenal (4-HNE) for subsequent quantitative image analysis.
Results
After 36 days of treatment, rats receiving the alcohol-containing diet displayed hepatic histopathologies characterized by marked micro- and macrosteatosis associated with only minor inflammation and necrosis. Alcohol administration resulted in a 3-fold elevation of plasma alanine aminotransferase activity and 3-fold increases (p < 0.01) in hepatic CYP2E1 apoprotein and activity. Quantitative immunohistochemical analysis revealed significant (p < 0.01) 5-fold increases in MDA- and 4-HNE modified proteins in liver sections prepared from rats treated with alcohol. The MDA- or 4-HNE modified proteins were contained in hepatocytes displaying intact morphology and were colocalized primarily with microvesicular deposits of lipid. Aldehyde-modified proteins were not prevalent in parenchymal or nonparenchymal cells associated with foci of necrosis or inflammation.
Conclusions
These results suggest that alcohol-induced lipid peroxidation is an early event during alcohol-mediated liver injury and may be a sensitizing event resulting in the production of bioactive aldehydes that have the potential to initiate or propagate ensuing proinflammatory or profibrogenic cellular events.
Keywords: Lipid Peroxidation, Aldehyde-Modified Proteins, Liver Injury, Malondialdehyde, 4-Hydroxynonenal, Immunohistochemistry
Oxidative insult to cells is associated with the production of free radicals capable of initiating the peroxidation of membrane lipids. The autocatalytic process of lipid peroxidation results in the production of a variety of n-alkanals and alkenals derived from ω-6-polyunsaturated fatty acids such as arachidonic and linoleic acid. As noted in a recent comprehensive review (Poli and Schaur, 2000), there is significant evidence that these aldehydic products of lipid peroxidation may be involved in several chronic diseases including Parkinson’s disease, arteriosclerosis, and diabetes mellitus as well as chronic inflammatory diseases of the liver including alcoholic liver disease, hepatitis C, hepatic iron overload, and primary biliary cirrhosis.
The molecular and cellular mechanisms by which the process of lipid peroxidation initiates or propagates liver injury and fibrosis are complex. At the molecular level, the production of reactive oxygen species such as superoxide anion radical (O2-.) is an essential event in the formation of hydroxyl radical (OH-.), which can then abstract a hydrogen atom from cellular membrane lipids, resulting in the autocatalytic production of lipid radicals and the peroxidative degradation of membrane lipids (Esterbauer et al., 1990, 1991). Whereas it is predictable that the destruction of membrane lipids would have a direct effect on cellular homeostasis, various lipid-derived products resulting from lipid peroxidation have been demonstrated to have multiple biological or toxicologic effects. For instance, when present in high concentrations, two major products of lipid peroxidation, 4-hydroynonenal (4-HNE) and malondialdehyde (MDA), display cytotoxic effects in several different cell types (Schaur et al., 1990). At low micromolar or submicromolar concentrations, 4-HNE has been shown to alter cellular proliferation (Cambiaggi et al., 1997), interfere with intracellular proteolysis (Okada et al., 1999), prevent nuclear factor-κB activation (Page et al., 1999), and activate activator protein-1 (Camandola et al., 1997). An involvement of both MDA and 4-HNE in the process of liver fibrosis is supported by the observation that these aldehydic products of lipid peroxidation increase procollagen type I messenger RNA and protein in cultured human stellate cells (Parola et al., 1996).
The potential of these aldehydic products to modulate numerous, diverse cellular functions can be attributed to their ability to diffuse from their site of production as well as their electrophilic chemical properties that facilitate covalent interactions with nucleophilic groups of cellular proteins or nucleic acids. The potential of MDA or 4-HNE to form adducts with proteins has been documented by using purified proteins treated with 4-HNE (Uchida and Stadtman 1993), isolated cells exposed to various pro-oxidants (Hartley et al., 1997), or immunohistochemically in tissue obtained from animals treated with ethanol, iron combined with ethanol (Kono et al., 2001; Tsukamoto et al., 1995) or carbon tetrachloride (Hartley et al., 1999). Likewise, MDA- and 4HNE -protein adducts have been detected in liver samples obtained from patients with chronic liver disease such as hepatitis C (Paradis et al., 1997a) as well as in patients with hemochromatosis, Wilson’s disease, alcoholic liver disease, primary biliary cirrhosis, or cholestasis (Paradis et al., 1997b). Collectively, these studies indicate that MDA- and 4HNE-protein adducts are reliable biomarkers of hepatic lipid peroxidation associated with chronic liver injury. The involvement of lipid peroxidation in chronic liver disease is predictable given the sustained inflammatory processes associated with the progressive stages of hepatitis C, cholestasis, or alcoholic liver disease. However, the occurrence of lipid peroxidation during the early, pre-inflammatory, or sensitization stages of liver injury has not been clearly delineated.
In the present study we characterized the appearance of MDA- and 4HNE-adducted proteins in livers of rats subjected to an intragastric ethanol administration regimen specifically designed to initiate early alcohol-induced changes in the liver without extensive inflammation and hepatocellular necrosis. The results of this study indicate that MDA- and 4-HNE-adducted proteins appear in hepatocytes concurrent with the development of microsteatosis in the absence of extensive cellular injury, which suggests that the process of lipid peroxidation is an early event in alcohol-induced liver injury that occurs before extensive inflammation and necrosis are manifested.
MATERIALS AND METHODS
Chemicals
Unless specified, all reagents were purchased from Sigma Aldrich (St. Louis, MO) and were analytical grade or better. Biotinylated goat antirabbit immunoglobulin G was purchased from Gibco-BRL (Life Technologies, Inc., Gaithersburg, MD). Vectastain Elite ABC kit and diaminobenzidine tetrahydrochloride (DAB) were purchased from Pierce (Rockford, IL).
Animals
Virus-free, adult female Sprague Dawley rats (approximately 240 g) were purchased from Harlan Industries (Indianapolis, IN). Rats were kept in a facility approved by the American Association for Advancement of Laboratory Animal Care under a 12 hr light cycle and constant humidity. Each rat was conditioned by gentle, daily handling (≥7 days) before surgical implantation of a single intragastric cannula under nembutal anesthesia (Badger et al., 1993). During a 14-day surgical recovery period, rats were infused with water intragastrically at the rate of 1 ml/hr and were allowed access to food and water ad libitum. The rats were assigned randomly to one of two treatment groups described later (n = 5–6). All experimental procedures involving animals were approved by the IACUC.
Ethanol Administration
A low-carbohydrate/high-fat total enteral nutrition (TEN) diet and a TEN diet that contained ethanol isocalorically substituted for carbohydrate- and lipid-derived calories were formulated and infused as described previously (Korourian et al., 1999; Rowlands et al. 2000). The control diet was formulated so that the caloric composition was 16% protein derived, 37% carbohydrate derived, 47% lipid derived, and 0% ethanol derived. The ethanol diet was modified to contain a final composition of 16% protein-derived calories, 3% carbohydrate-derived calories, 34% ethanol-derived calories, and 47% lipid-derived calories. At initiation of the study, ethanol-treated rats were infused with ethanol-containing diet at a rate of 10 g/kg/day (28% ethanol-derived calories). The rate of ethanol infusion was progressively increased 0.5 g/kg/day to 12.5 g/kg/day (34% ethanol-derived calories) in the final 7 days of the study by substituting ethanol for carbohydrate calories. Animals infused with the control diet received isocaloric carbohydrate calories equivalent to the ethanol-derived calories. Both groups were administered control or ethanol-containing diet for a total of 36 days, and daily weights were assessed for each animal. At the conclusion of the study, a final body weight was recorded and blood was obtained for alanine aminotransferase (ALT) measurements. The rats were killed, the livers were weighed, and samples were obtained for histopathological evaluation and preparation of microsomes (Rowlands et al., 2000).
Biochemical Assays
Blood ethanol concentrations were determined before rats were killed, as previously described (Korourian et al., 1999). Serum ALT activity was assessed by a commercial kit (ALT: 2.6.1.2; Sigma Aldrich, St. Louis, MO). Cytochrome P-4502E1 (CYP2E1) activity was determined indirectly by measuring carbon tetrachloride (CCl4)-dependent lipid peroxidation of microsomes isolated from each animal as previously described (Fang et al., 1998). Briefly, isolated hepatic microsomes, corresponding to 0.5 to 1 mg of protein, were suspended in 1 ml of 50 mM potassium phosphate buffer (pH 7.4), which contained 2.15 mM CCl4 in acetone and 0.25 mM nicotinamide adenine dinucleotide phosphate. Blank incubations contained everything except that no CCl4 was present in the acetone vehicle. The reactions were terminated by the addition of 1.0 N perchloric acid, and the samples were heated in boiling water for 10 min and centrifuged. Thiobarbituric acid-reactive material (TBA or TBAR) in the supernatant was quantified spectrophotometrically at 532 nm by using a molar extinction coefficient of 156 mM−1·cm−1. The CCl4-dependent formation of TBARS was calculated by subtracting values for blank incubations from those containing CCl4. Isolated hepatic microsomes were evaluated by Western blot analysis to determine the levels of CYP2E1 apoprotein content, and immunoquantitation by densitometric scanning was as performed as described elsewhere (Fang et al., 1998).
Histopathology, Immunostaining, and Imaging
Liver samples were processed, and paraffin-embedded sections were stained with hematoxylin and eosin (H&E). H&E-stained liver sections were scored for macro- and microvesicular steatosis, inflammation (macrophage infiltration), and necrosis by a pathologist with no prior knowledge of the treatment groups. Scoring was based on a scale encompassing 1 (baseline) to 5 (most extensive) as described elsewhere (Korourian et al., 1999). Steatosis was scored as the percentage of parenchymal cells containing fat (micro- or macrosteatosis) as <25% = 1, 25 to 50% = 2, 50 to 75% = 3, and <75% = 4. The presence of inflammation, based on infiltration by polymorphonuclear leukocytes and mononuclear cells, was evaluated using a scale where no polymorphonuclear leukocytes and mononuclear cells present = 1; occasional foci of inflammatory cells = 2; widely dispersed, organized foci of inflammatory cells = 3; and frequently occurring, large foci of inflammatory cells = 4. Necrosis was assessed using a scale of 1 to 4 as follows: occasional (<1%) necrotic hepatocytes = 1; frequent (5–10%) necrotic hepatocytes = 2; small foci of necrosis (clusters >10 necrotic hepatocytes) = 3; and extensive areas of necrosis (>25% of the lobular unit) = 4. A total pathology score was determined by summing the scores for steatosis, macrophage infiltration, and necrosis.
Immunostaining was carried out as previously described (Hartley et al., 1999). Briefly, paraffin-embedded serial sections of liver, corresponding to H&E-stained sections from each animal, were deparaffinized, rehydrated, blocked in Tris-buffered saline/Tween 20 (TBST) with 5% nonfat dried milk, and incubated overnight at 4°C with immunoglobulin G-purified polyclonal rabbit antibodies specific for 4-HNE- or MDA-adducted protein epitopes. The production and characterization of these antibodies are described in detail elsewhere (Hartley et al., 1997). Briefly, the antibodies were produced in rabbits immunized with keyhole limpet hemocyanin adducted with either 4-HNE or MDA. The resulting antisera were thoroughly characterized by enzyme-linked immunosorbent assay (ELISA) and immunoblotting to establish that the anti-HNE antibodies did not recognize protein-MDA epitopes as well as validation of specificity of anti-MDA antibodies for only protein-MDA conjugates. Subsequent ELISA analysis of anti-HNE antibodies established an absence of recognition of short-chain (C4–C6) α,β-unsaturated aldehydes or 4-hydroxy, trans-2-octenal adducted to bovine serum albumin (BSA), documenting the specificity of these antibodies for 4-HNE-specific epitopes. Likewise, ELISA analysis of the anti-MDA antibodies has verified their lack of recognition of the α,β-unsaturated aldehyde, acrolein (2-propenal), adducted to BSA, which demonstrates the specificity of these antibodies for MDA-amine haptens rather than acrolein-amine or acrolein-sulfhydral protein epitopes. Specificity also has been established by addition of excess concentrations of MDA-BSA or glutathione-HNE conjugates to ELISA or immunohistochemical incubations, which results in selective abolition of immunopositive reactions of the respective antibodies. Collectively, these results provide important evidence concerning the specificity of the antisera for MDA and HNE-specific epitopes. The use of these antibodies for immunohistochemical detection of adducted proteins in liver sections of rats treated with CCl4 is described elsewhere (Hartley et al., 1999). For the present study, paraffin-embedded liver sections, 4 μm in thickness, were placed on L-lysine-coated slides. Before immunohistochemical staining, each section was deparaffinized and rehydrated in a series of solvents (xylene, absolute ethanol, 95% ethanol, and water). Endogenous peroxidase activity was blocked by exposure to H2O2 (3% in deionized water v/v) for 15 min. 4-HNE and MDA protein adducts were detected by using antisera (1:500 in phosphate buffered saline, 7.4) to either adduct. Immunopositive reactions were detected indirectly with a secondary biotinylated goat antirabbit antibody (1:200). Visualization of primary and secondary antibody interactions was accomplished by using an avidin/biotinylated horseradish peroxidase kit (Pierce, Rockford, IL) incorporating DAB as the chromogen. Consistent with previous studies (Hartley et al., 1999), immunopositive staining was not observed in liver sections prepared from shelf-control rats. The possibility that immunopositive staining was not specific but due to nonspecific interactions of secondary antibody was eliminated by establishing the absence of positive staining using the immunostaining procedure described previously in the absence of primary antibody.
Image-Pro Plus image acquisition and analysis software (Media Cybernetics, Silver Spring, MD) integrating a Nikon Eclipse TE300 microscope equipped with a digital camera was used to capture and analyze representative images of the stained serial sections at 10× for each animal. Color detection ranges were selected for either (1) nonstaining areas of lipid on H&E-stained slides or (2) intense reddish-brown colors of the DAB chromogen, indicating positive aldehyde-adducted labeling. Images were calibrated to scale, and the extent of steatosis in H&E-stained sections was defined as the percent nonstaining area per 100× field. Similarly, the extent of aldehyde-protein epitope labeling in the corresponding serial sections was defined as the percent reddish-brown staining area per 100× field. Values from each tissue section (five fields per section) were pooled to establish mean values.
Statistical Analysis
The data were analyzed by using the Student’s t test for detection of statistically significant differences (p < 0.05) between TEN controls and ethanol-treated animals. All data are presented as the mean ± SEM.
RESULTS
Effect of Ethanol Treatment on Hepatic Histopathology and CYP2E1 Activity
At the termination of the experiments, blood ethanol concentrations in ethanol-treated rats exceeded 200 mg/dl, documenting the effectiveness of this ethanol administration procedure to maintain appreciable blood concentrations of ethanol (Table 1). At termination of 36 days of ethanol infusion, body weights of rats infused with ethanol were approximately 7.0% lower than TEN controls (p < 0.05). Consistent with the final body weights presented in Table 1 is the observation that rats infused with ethanol gained 0.5 g/day less than TEN controls. Hepatomegaly was apparent in ethanol-treated rats as evidenced by the significant 1.5-fold increase in liver/body weight ratio. Consistent with the observed hepatomegaly is the observation that chronic administration of alcohol significantly increased serum ALT (p< 0.05) values by nearly 3.5-fold, suggesting mild, ethanol-induced liver injury.
Table 1.
Effect of Chronic Ethanol Administration on Body Weight, Liver to Body Weight Ratios, Alanine Aminotransferase (ALT) Activity, and Blood Ethanol Concentrations
| Treatment | Body weight (g) | Body weight gain (g/day) | Liver wt/body wt (%) | Serum ALT (IU/liter) | Blood ethanol (mg/100 ml) |
|---|---|---|---|---|---|
| TEN control | 310 ± 9 | 1.9 ± 0.6 | 3.5 ± 0.3 | 55 ± 5 | 0 |
| Ethanol | 290 ± 7* | 1.4 ± 0.4 | 5.1 ± 0.4* | 190 ± 70* | 230 ± 70* |
All values are expressed as mean ± SEM.
Significant difference (p < 0.05) between TEN and ethanol-treated animals.
Photomicrographs of H&E-stained liver sections prepared from representative isocaloric and alcohol-treated rats are presented in Fig. 1A and 1B, respectively. Sections prepared from control rats (Fig. 1A) displayed normal histologic and morphologic characteristics, whereas macrosteatosis and microsteatosis were the most prominent histopathologic features resulting from this paradigm of alcohol administration. The lobular fat deposition was not uniform among all alcohol-treated animals, with most animals displaying panlobular or defined foci of microsteatosis in periportal, midzonal, or centrilobular regions of the hepatic lobule. Likewise, macrosteatosis was observed in periportal, midzonal, or centrilobular regions. However, periportal and midzonal macrosteatosis appeared to be a more common feature among the alcohol-treated rats. Inflammation was not a prominent histopathological consequence of this ethanol treatment. The magnified (40×) region of Fig. 1B displays representative foci of inflammatory cells dispersed among regions of micro- and macrosteatosis present in some of the ethanol-treated animals. A graphic summary of the histopathological assessment of liver sections prepared from TEN control and ethanol-treated rats is presented in Fig. 2. For ethanol-fed rats, the mean score of 4 for micro- and macrosteatosis indicates the presence of fat in greater than 75% of the hepatocytes. Steatosis was detected in some TEN control animals, resulting in a mean score of 1.5, which, given the variation in this measure, is increased only slightly above the baseline value of 1.0, a value that would be assigned to rats consuming standard laboratory chow. With respect to inflammatory cell infiltration and necrosis, a mean score of 2 was assigned to ethanol-treated animals, reflecting small but statistically significant increases in the occasional foci of inflammatory cells or the presence of 5 to 10% necrotic hepatocytes in the field of evaluation. These ethanol-related effects contribute to the significantly higher total pathology scores, which reflect the prominent contribution of micro- and macrosteatosis to the overall histopathology.
Fig. 1.
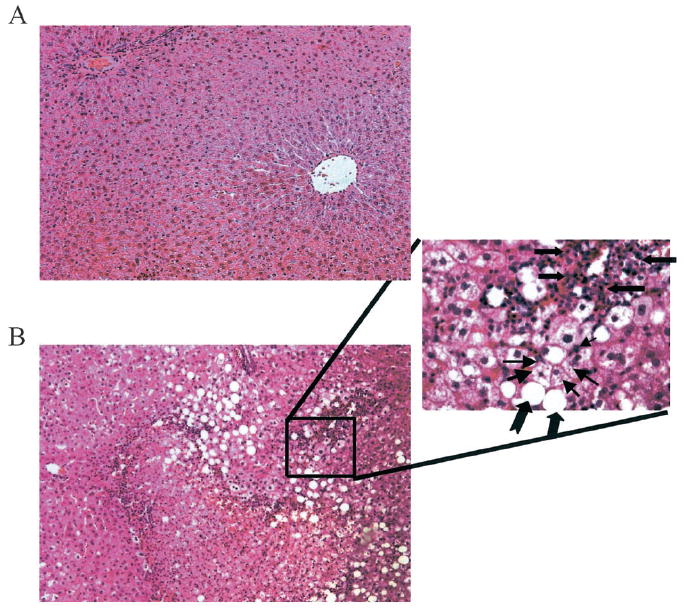
Photomicrographs of representative hematoxylin and eosin-stained liver sections prepared from an isocaloric-fed control rat (A) and a rat treated with ethanol for 36 days (B). A 40× magnification of the designated region reveals a foci of inflammatory cells by block arrows. Hepatocytes containing microsteatosis are designated with small arrows, whereas notched arrows designate macrosteatosis. Steatosis in the photomicrograph with 10× magnification (left) is concentrated in the periportal region.
Fig. 2.
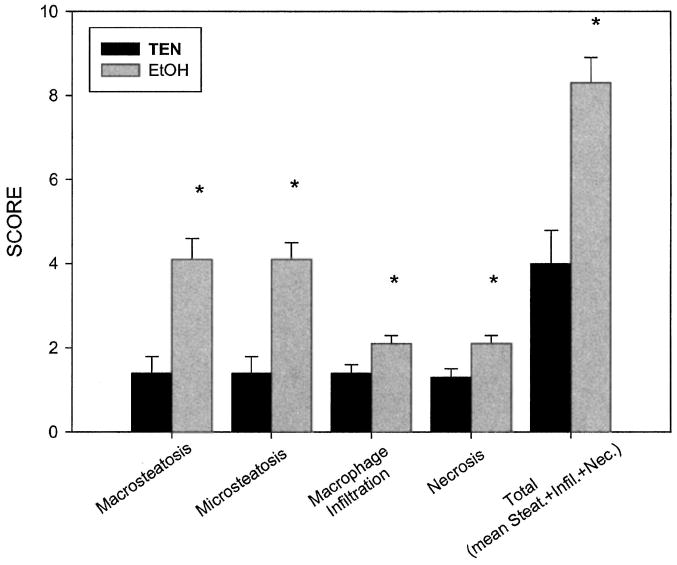
Effects of chronic enteral ethanol administration on hepatic histopathology scores. Histopathologic assessment was performed as described in the text. Data are presented as mean ± SEM. *p < 0.05 compared with TEN controls.
The effect of ethanol administration on hepatic CYP2E1 apoprotein expression and activity is presented in Fig. 3. Immunoblot analysis (Fig. 3A) revealed a 2.8-fold increase in CYP2E1 protein content in livers of ethanol-treated rats compared with control animals, a difference also reflected in an approximate 4-fold increase in CYP2E1 activity shown in Fig. 3B. Collectively, the data presented in Table 1 as well as Figs. 1 and 2 validate the effectiveness of this ethanol administration paradigm to sustain moderate increases in body weight while initiating mild changes in hepatic morphology reflected in primarily steatosis and, to a lesser degree, inflammation and necrosis. The data presented in Fig. 3 confirm the ability of the TEN procedure of ethanol administration to increase CYP2E1 activity proposed to play an important role in ethanol-induced liver injury.
Fig. 3.
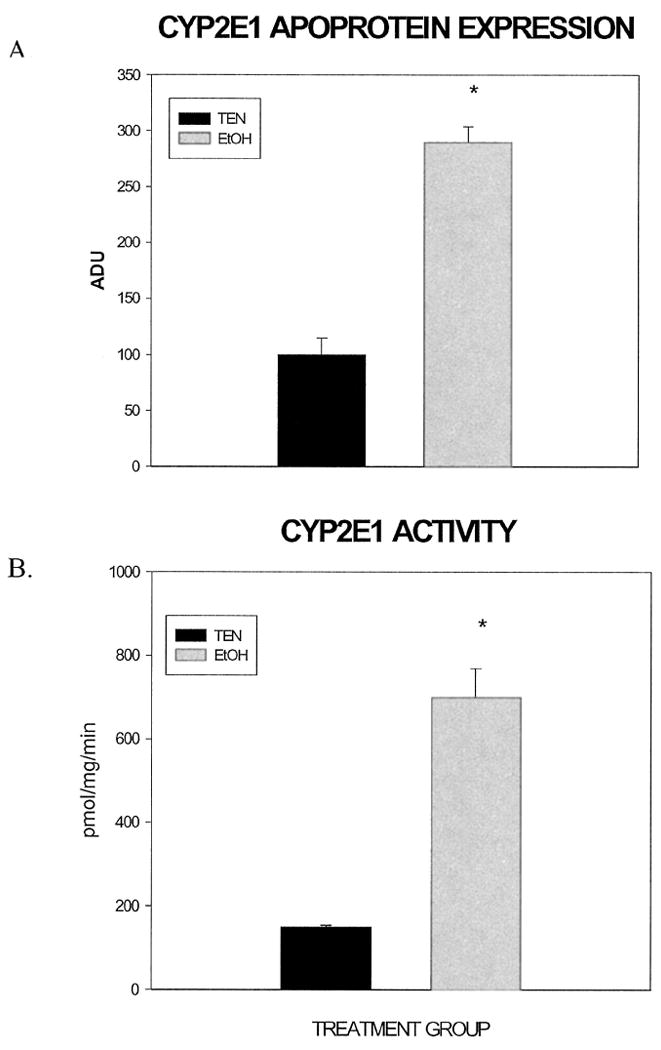
Effect of chronic ethanol administration on CYP2E1 apoprotein and activity. Hepatic microsomes were isolated from TEN and ethanol-treated rats as described for determination of CYP2E1 apoprotein (A) expressed in arbitrary densitometric units (ADU) and activity (B). *p < 0.05 compared with TEN controls.
Effect of Ethanol Administration on MDA- and 4-HNE Immunohistochemical Staining
Polyclonal antibodies specific for 4-HNE or MDA-protein epitopes (Hartley et al., 1997, 1999) were used for immunohistochemical detection of the respective aldehyde-modified proteins in liver sections prepared from TEN- and alcohol-treated rats. Figure 4 shows patterns of MDA- and 4-HNE-modified proteins in representative liver sections prepared from rats receiving the TEN diet or ethanol-containing diets. Figure 4A and 4B reveals only very light immunopositive staining of MDA- or 4-HNE-modified proteins in sections prepared from TEN control rats. The very light immunopositive staining for MDA-modified proteins was diffuse with some concentrated areas of staining residing near the portal triads, whereas 4-HNE immunopositive staining was randomly dispersed throughout the lobule. There was, however, some 4-HNE-positive staining in pericentral and perivenous endothelial cells. Figure 4 C and 4D demonstrates marked immunopositive staining for MDA- and 4-HNE-modified proteins in liver sections prepared from ethanol-treated rats. Immunopositive staining for MDA-modified proteins (Fig. 4C) was moderate throughout the hepatic lobule with heavy staining in mid-zonal or periportal areas that contained islands of cells characterized by micro- or macrovesicular steatosis. Immunopositive staining of 4-HNE-modified proteins (Fig. 4D) was also moderate throughout the hepatic lobule and, like the MDA positive staining, was more concentrated in periportal or pericentral regions containing hepatocytes with deposits of micro- or macrovesicular fat. As is evident from the magnified inserts presented in Fig. 4, important features of the MDA- or 4-HNE positive immunostaining include a prominent cytosolic localization in hepatocytes with an overall preserved morphology and a general absence of adducts in cells contained within or adjacent to areas of inflammation.
Fig. 4.
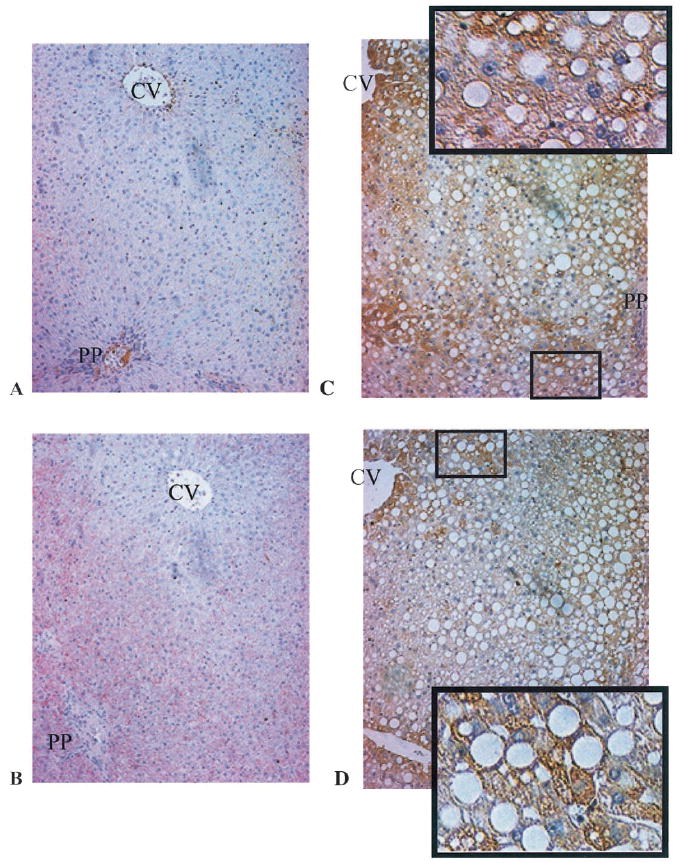
Immunohistochemical detection of MDA- and 4-HNE-modified proteins in liver sections prepared from TEN and ethanol-treated rats (magnification 10×). CV designates the central vein, whereas the periportal vein is identified by PP. Panels A and B are representative of MDA- or 4-HNE-stained sections from TEN control rats, respectively. Panels C (MDA) and D (4-HNE) are representative sections prepared from ethanol-treated rats. The enlarged areas of panels C and D show localization of immune-positive staining in hepatocytes characterized by normal morphology and microsteatosis. Quantitation of the immunostaining is presented in Table 2.
Because hepatic steatosis was the prominent histopathological outcome, quantitation of the lipid-containing, non-stained H&E areas also was performed. Consistent with histopathologic assessment of steatosis (Figs. 1 and 2), quantitation of the lipid-containing, nonstained H&E regions of liver sections from alcohol-treated rats comprised approximately 27 ± 1.3% of the average field compared with only 5 ± 0.2% of the field for control rats (data not presented). The quantitative data presented in Table 2 corroborate the immunohistochemical results in Fig. 4 in that, when compared with the respective controls, alcohol treatment resulted in a 5-fold increase in the percentage of immunopositive stained area occupied by MDA-modified proteins and a nearly 6-fold increase in the percentage of the total field area occupied by 4-HNE-modified proteins.
Table 2.
Effect of Ethanol Administration on Immunohistochemical Staining of Hepatic MDA- and 4-HNE-Modified Proteins
| Treatment | % of field staining positive |
|
|---|---|---|
| MDA | 4-HNE | |
| TEN control | 0.43 ± 0.13 | 0.31 ± 0.12 |
| Ethanol | 2.48 ± 0.37* | 1.71 ± 0.31* |
All values are expressed as mean ± SEM.
Significant difference (p < 0.05) between TEN and ethanol-treated animals.
DISCUSSION
Ethanol-mediated liver injury is a multifactorial process characterized by steatosis that may progress to steatohepatitis, necrosis, fibrosis, and potentially cirrhosis. The early events of ethanol-induced liver injury are thought to result from sensitizing factors such as lipid accumulation, which predisposes hepatocytes to subsequent priming events potentially involving CYP2E1 induction, reactive oxygen species production, and lipid peroxidation, all of which promote dysregulation of hepatocellular homeostasis potentially leading to necrosis (DeCarli and Lieber, 1967; Diehl 2001; Tsukamoto and Shelly, 2001). Using an intragastric ethanol administration paradigm to ensure adequate nutrition and sustain blood ethanol concentrations, we have demonstrated that during the early stages of ethanol-mediated liver injury, the accumulation of hepatocellular lipids and lipid peroxidation occurs as evidenced by immunodetection of hepatic proteins adducted with MDA or 4-HNE.
In the present study, rats receiving ethanol by intragastric infusion for 36 consecutive days were well nourished as evidenced by the 20 to 30% increase in body weight for ethanol-treated and TEN control rats, respectively. Hepatic histopathologies consisting primarily of micro- and macrosteatosis with low levels of inflammation and necrosis were observed, all of which are histologic features characteristic of early events of ethanol-induced hepatic morphology. The current study was specifically designed to examine early events in the pathogenesis of alcohol-induced liver injury before development of extensive inflammation and necrosis. The ethanol-related changes reported in the present communication can be considered mild compared with our prior studies, which involved administration of a ethanol-containing diet to rats for up 42 days (Korourian et al., 1999) by using an ethanol infusion schedule that was increased from 10 g/kg/day to 12 to 13 g/kg/day over the first 14 days and maintained at that level for the remaining 28 days. Thus, the high-ethanol/low-carbohydrate diet was maintained for a much longer time (28 days) compared with the 7 day duration used in the current study. Collectively, the data presented here suggest that this procedure of ethanol administration was effective in initiating the early stages of ethanol-induced liver injury in that significant steatosis, the induction of CYP2E1, and lipid peroxidation were evident in the absence of marked inflammation and hepatocellular necrosis.
A reproducible result of oral or intragastric alcohol administration to rats is hepatic steatosis (Kono et al., 2001; Korourian et al., 1999; Rowlands et al., 2000; Tsukamoto and Shelly, 2001). Consistent with those reports are the present results documenting the potential of ethanol administration to produce an observable progression of hepatic steatosis (Figs. 1 and 2). Specifically, the alcohol administration procedure used for the present study produced a spectrum of steatosis ranging from micro to macro in nature, thus making it possible to evaluate the temporal relationship of hepatocellular lipid accumulation with other pathomechanisms of ethanol-induced liver injury. In this context, and of particular interest to the present study, are the results showing colocalization of MDA- or 4-HNEmodified proteins in hepatocytes containing primarily microsteatosis (Fig. 4). In addition, the pattern of MDA- or 4-HNE adducted proteins was panlobular and localized to the greatest extent in hepatocytes with conserved morphology as opposed to a near absence in hepatocytes with macrosteatosis or in association with cells adjacent to foci of inflammation or necrosis. Whereas the occurrence of 4-HNE-modified proteins has been described by using an animal model of ethanol-induced liver injury resulting in marked hepatic damage (Kono et al., 2001; Tsukamoto et al., 1995), detailed descriptions of the cellular site of adduct deposition were not presented. Consistent with the data presented here is a recent report describing the colocalization of acetaldehyde and MDA adducts with areas of hepatic fatty infiltration in livers of rats treated with alcohol (Niemela et al., 2002). Unlike the present study, however, this latter study used a longer period of intragastric alcohol administration resulting in significant hepatocellular inflammation and necrosis. Likewise, the cellular and extracellular sites of aldehyde-protein adducts in liver biopsy specimens obtained from humans with liver disease are variable, which is likely attributable to the advanced stages of alcohol-induced liver fibrosis/cirrhosis (Paradis et al., 1997a) or marked hepatocellular injury resulting from hepatitis C (Paradis et al., 1997b).
It is likely that the formation and lobular accumulation of hepatic aldehyde-adducted proteins, as a consequence of chronic alcohol administration, is dependent on the animal model and mode of alcohol administration. For instance, centrilobular localization of MDA- and 4-HNE-adducted proteins has been observed in livers of micropigs subjected to chronic alcohol administration (Niemela et al., 1999). In another study, liver biopsy specimens obtained from human alcoholics revealed the centrilobular distribution of MDA- and 4-HNE adducts colocalized with fatty deposits (Niemela et al., 2000). The centrilobular localization of aldehyde-modified proteins is predictable given the centrilobular distribution of cytochrome P-450 enzymes, which have the ability to generate a microenvironment with high pro-oxidant potential. However, relatively low and inconsistent correlations also have been reported between the immunostaining of protein adducts and cytochrome enzyme apoproteins (Niemela et al., 1999), which implicates the multifactorial nature of alcohol-induced liver injury. In the present study we did not observe a consistent centrilobular localization of steatosis or protein adduct staining, an observation suggesting that these histologic features of alcohol-induced liver injury are influenced by the duration and mode of ethanol administration. Our results imply that during the early stages of alcohol-induced liver injury produced by this mode of alcohol administration, pro-oxidant mechanisms independent of CYP2E1 or other cytochrome enzymes are functionally sufficient to support the autocatalytic process of lipid peroxidation. For example, nicotinamide adenine dinucleotide phosphate oxidase-derived free radicals produced by Kupffer cells have been implicated as key oxidants in liver injury produced by chronic alcohol administration (Kono et al., 2000). The data presented here point to lipid peroxidation and subsequent formation of aldehyde-modified proteins as early events associated with accumulation of an oxidizable lipid substrate in hepatocytes with conserved morphology and, presumably, metabolic capacities capable of oxidizing ethanol and generating or responding to pro-oxidative intermediates produced by nonparenchymal cells. Given the multifactorial nature of alcohol-induced liver injury, time course studies that use well-characterized procedures of ethanol administration will be necessary to establish causal relationships between alcohol-induced steatosis and ensuing hepatocellular damage.
The role of steatosis as an early event in the progression of alcoholic liver disease is widely accepted (Diehl, 2001) and has been attributed to the multiple hyperlipidemic actions of tumor necrosis factor-α and prostaglandin E2 released from endotoxin-activated Kupffer cells (Diehl, 2001; Enomoto et al., 2000; Martinez et al., 1992; Yin et al., 1999). Data obtained in the present study document colocalization of the established biomarkers of lipid peroxidation, 4-HNE- and MDA-modified proteins, primarily with microvesicular lipid deposits in hepatocytes. This observation suggests that concurrent with the development of steatosis, aldehydic products of lipid peroxidation are produced that, through a variety of biological activities, could initiate a number of effects on hepatic parenchymal and nonparenchymal cells. For instance, the reported biological actions of 4-HNE include chemotaxis (Curzio et al., 1985, 1990), which could initiate infiltration of polymorphonucleocytes and monocytes. Likewise, the ability of 4-HNE to activate activator protein-1 has potential consequences with respect to modulation of the proinflammatory, profibrogenic cytokine tumor growth factor-β1 (Feingold and Grunfeld, 1987) and increased fibrogenesis through regulation of collagen type 1 gene activity (Armendariz- Borunda et al., 1994; Parola et al., 1992). As well, 4-HNE is also reported to induce expression of monocyte chemotactic protein-1 involved in recruitment of monocytes in response to liver injury (Marra et al., 1999).
CONCLUSION
The results presented in this communication suggest that alcohol-induced hepatic steatosis may be a sensitizing event that sets the stage for priming mechanisms such as reactive oxygen species production and lipid peroxidation resulting in production of bioactive molecules such as 4-HNE. The presence of 4-HNE- and MDA-adducted proteins indicates that these major products of lipid peroxidation accumulate and have the potential to alter a variety of cellular signaling processes, some of which are involved in the hepatopathogenesis of alcohol.
Acknowledgments
Supported by NIH Grants AA-09300 (DRP), AA-0845 (TMB), and AA-12931 (TMB).
Contributor Information
Brante P. Sampey, Department of Pharmaceutical Sciences, University of Colorado Health Sciences Center Denver, Colorado
Soheila Korourian, Department of Pathology, University of Arkansas for Medical Sciences, Little Rock, Arkansas.
Martin J. Ronis, Department of Pediatrics, University of Arkansas for Medical Sciences, Little Rock, Arkansas
Thomas M. Badger, Department of Physiology, University of Arkansas for Medical Sciences, Little Rock, Arkansas
Dennis R. Petersen, Department of Pharmaceutical Sciences, University of Colorado Health Sciences Center Denver, Colorado
References
- Armendariz-Borunda J, Simkevich CP, Roy N, Raghow R, Kang AH, Seyer JM. Activation of Ito cells involves regulation of AP-1 binding proteins and induction of type I collagen gene expression. Biochem J. 1994;304:817–824. doi: 10.1042/bj3040817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger TM, Crouch J, Irby D, Hakkak R, Shahare M. Episodic excretion of ethanol during chronic intragastric ethanol infusion in the male rat: continuous vs. cyclic ethanol and nutrient infusions. J Pharmacol Exp Ther. 1993;264:938–943. [PubMed] [Google Scholar]
- Camandola S, Scavazza A, Leonarduzzi G, Biasi F, Chiarpotto E, Azzi A, Poli G. Biogenic 4-hydroxy-2-nonenal activates transcription factor AP-1 but not NF-kB in cells of the macrophage lineage. Biofactors. 1997;6:173–179. doi: 10.1002/biof.5520060211. [DOI] [PubMed] [Google Scholar]
- Cambiaggi C, Dominici S, Comporti M, Pompella A. Modulation of human T lymphocyte proliferation by 4-hydroxynonenal, the bioactive product of neutrophil-dependent lipid peroxidation. Life Sci. 1997;61:777–785. doi: 10.1016/s0024-3205(97)00559-6. [DOI] [PubMed] [Google Scholar]
- Curzio M, Esterbauer H, Dianzani MU. Chemotactic activity of hydroxyalkenals on rat neutrophils. Int J Tissue React. 1985;7:137–142. [PubMed] [Google Scholar]
- Curzio M, Esterbauer H, Mauro C, Dianzani MU. Influence of the lipid peroxidation product 4-hydroxynonenal on human neutrophil migration. Int J Tissue React. 1990;6:13–18. [Google Scholar]
- DeCarli LM, Lieber CS. Fatty liver in the rat after prolonged intake of ethanol with nutritionally adequate new liquid diet. J Nutr. 1967;91:131–138. doi: 10.1093/jn/91.3_Suppl.331. [DOI] [PubMed] [Google Scholar]
- Diehl AM. Nonalcoholic fatty liver disease: implications for alcoholic liver disease. Alcohol Clin Exp Res. 2001;25:8S–14S. doi: 10.1097/00000374-200105051-00004. [DOI] [PubMed] [Google Scholar]
- Enomoto N, Ikejima K, Yamashina S, Enomoto A, Nishiura T, Nishimura T, Brenner DA, Schemmer P, Bradford BU, Rivera CA, Zhong Z, Thurman RG. Kupffer cell-derived prostaglandin E2 is involved in alcohol-induced fat accumulation in rat liver. Am J Physiol Gastrointest Liver Physiol. 2000;279:G100–G106. doi: 10.1152/ajpgi.2000.279.1.G100. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Zollner H, Schaur RJ. Aldehydes formed by lipid peroxidation: Mechanisms of formation, occurrence and determination. In: Vigo-Pelfrey C, editor. Membrane Lipid Peroxidation. Vol. 1. CRC Press; Boca Raton, FL: 1990. pp. 240–268. [Google Scholar]
- Esterbauer H, Zollner H, Schaur RJ. Chemistry and biochemistry of 4-hydroxynonenal, malondialdehyde, and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Fang C, Lindros KO, Badger TM, Ronis MJ, Ingelman-Sundberg M. Zonated expression of cytokines in rat liver: effect of chronic ethanol and cytochrome P450 2E1 inhibitor, chlormethiazole. Hepatology. 1998;27:1304–1310. doi: 10.1002/hep.510270516. [DOI] [PubMed] [Google Scholar]
- Feingold KR, Grunfeld C. Tumor necrosis factor-alpha simulates hepatic lipogenesis in the rat in vivo. J Clin Invest. 1987;80:184–190. doi: 10.1172/JCI113046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley DP, Kolaja KL, Reichard J, Petersen DR. 4-Hydroxynonenal and malondialdehyde hepatic protein adducts in rats treated with carbon tetrachloride: immunochemical detection and lobular localization. Toxicol Appl Pharmacol. 1999;161:23–33. doi: 10.1006/taap.1999.8788. [DOI] [PubMed] [Google Scholar]
- Hartley DP, Kroll DJ, Petersen DR. Prooxidant-initiated lipid peroxidation in isolated rat hepatocytes: detection of 4-hydroxynonenaland malondialdehyde-protein adducts. Chem Res Toxicol. 1997;10:895–905. doi: 10.1021/tx960181b. [DOI] [PubMed] [Google Scholar]
- Kono H, Arteel GE, Rusyn I, Sies H, Thurman RG. Ebselen prevents early alcohol-induced liver injury in rats. Free Radic Biol Med. 2001;30:403–411. doi: 10.1016/s0891-5849(00)00490-1. [DOI] [PubMed] [Google Scholar]
- Kono H, Rusyn I, Yin M, Gabele E, Yamashina S, Dikalova A, Kadiiska MB, Connor HD, Mason RP, Segal BH, Bradford BU, Holland SM, Thurman RG. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest. 2000;106:867–872. doi: 10.1172/JCI9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korourian S, Hakkak R, Ronis MJ, Shelnutt SR, Waldron J, Ingelman-Sunberg M, Badger TM. Diet and risk of ethanol-induced hepatotoxicity: carbohydrate-fat relationships in rats. Toxicol Sci. 1999;47:110–117. doi: 10.1093/toxsci/47.1.110. [DOI] [PubMed] [Google Scholar]
- Marra F, DeFranco R, Grappone C, Parola M, Milan S, Leonarduzzi G, Pastacaldi S, Wenzel UO, Pinzani M, Dianzani MU, Laffi G, Gentilini P. Expression of monocyte chemotactic protein 1 precedes monocyte recruitment in a rat model of acute liver injury: modulation by vitamin E pretreatment. J Invest Med. 1999;47:66–75. [PubMed] [Google Scholar]
- Martinez F, Abril ER, Earnest DL, Watson RR. Ethanol and cytokine secretion. Alcohol. 1992;9:455–458. doi: 10.1016/0741-8329(92)90080-t. [DOI] [PubMed] [Google Scholar]
- Niemela O, Parkkial S, Bradford B, Imuro Y, Pasanen M, Thurman RG. Effect of Kupffer cell inactivation on ethanol-induced protein adducts in liver. Free Radic Biol Med. 2002;33:350–355. doi: 10.1016/s0891-5849(02)00894-8. [DOI] [PubMed] [Google Scholar]
- Niemela O, Parkkial S, Juvonen RO, Viitala K, Gelboin HV, Pasanen M. Cytochromes P450 2A6, 2E1 and 3A and production of proteinaldehyde adducts in the liver of patients with alcoholic and nonalcoholic liver diseases. J Hepatol. 2000;33:893–901. doi: 10.1016/s0168-8278(00)80120-8. [DOI] [PubMed] [Google Scholar]
- Niemela O, Parkkial S, Pasanen M, Viitala K, Vilianueva JA, Halsted CH. Induction of cytochrome P450 enzymes and generation of protein-aldehyde adducts are associated with sex-dependent sensitivity to alcohol-induced liver disease in micropigs. Hepatology. 1999;30:1011–1017. doi: 10.1002/hep.510300413. [DOI] [PubMed] [Google Scholar]
- Okada K, Wangpoengtrakul C, Osawa T, Toyokuni S, Tanaka K, Uchida K. 4-Hydroxy-2-nonenal-mediated impairment of intracellular proteolysis during oxidative stress: identification of proteasomes as target molecules. J Biol Chem. 1999;274:23787–23793. doi: 10.1074/jbc.274.34.23787. [DOI] [PubMed] [Google Scholar]
- Page S, Fisher C, Baumgartner B, Haas M, Kreusel U, Loidl G, Hayn M, Zieglerheitbrock HWL, Neumeier D, Brand K. 4-Hydroxynonenal prevents NF-kappa B activation and tumor necrosis factor expression by inhibiting I-kappa B phosphorylation and subsequent proteolysis. J Biol Chem. 1999;274:11611–11618. doi: 10.1074/jbc.274.17.11611. [DOI] [PubMed] [Google Scholar]
- Paradis V, Kollinger M, Fabre M, Holstege A, Poynard T, Bedossa P. In situ detection of lipid peroxidation by-products in chronic liver diseases. Hepatology. 1997a;26:135–142. doi: 10.1053/jhep.1997.v26.pm0009214462. [DOI] [PubMed] [Google Scholar]
- Paradis V, Mathurin P, Kollinger M, Imbert-Bismut F, Piton A, Opolon P, Holstege A, Poynard T, Bedossa P. In situ detection of lipid peroxidation in chronic hepatitis C: correlation with pathological features. J Clin Pathol. 1997b;50:401–406. doi: 10.1136/jcp.50.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola M, Muraca R, Dianzani I, Barrera G, Leonarduzzi G, Bendinelli P, Piccoletti R, Poli G. Vitamin E dietary supplementation inhibits transforming growth factor β1 gene expression in rat liver. FEBS Lett. 1992;308:267–270. doi: 10.1016/0014-5793(92)81290-3. [DOI] [PubMed] [Google Scholar]
- Parola M, Pinzani M, Casini A, Leonarduzzi G, Marra F, Caligiuri A, Cenri E, Biondi P, Poli G, Dianzani MU. Induction of procollagen type I gene expression and synthesis in human hepatic stellate cells by 4-hydroxy-2, 3-nonenal and other 4-hydroxy-2, 3-alkenals is related to their molecular structure. Biochem Biophys Res Commun. 1996;222:261–264. doi: 10.1006/bbrc.1996.0732. [DOI] [PubMed] [Google Scholar]
- Poli G, Schaur RJ. 4-Hydroxynonenal in the pathomechanisms of oxidative stress. IUBMB Life. 2000;50:315–321. doi: 10.1080/713803726. [DOI] [PubMed] [Google Scholar]
- Rowlands JC, Wang H, Hakkak R, Ronis MJ, Strobel HW, Badger TM. Chronic intragastric infusion of ethanol-containing diets induces CYP3A9 while decreasing CYP3A2 in male rats. J Pharmacol Exp Ther. 2000;295:747–752. [PubMed] [Google Scholar]
- Schaur RJ, Zollner H, Esterbauer H. Biological effects of aldehydes with particular attention to 4-hydroxynonenal and malondialdehyde. In: Vigo-Pelfrey C, editor. Membrane Lipid Peroxidation. Vol. 3. CRC Press; Boca Raton, FL: 1990. pp. 141–163. [Google Scholar]
- Tsukamoto H, Horne W, Kamimura S, Niemela O, Parkkila S, Yla-Herttuala S, Brittenham GM. Experimental liver cirrhosis induced by alcohol and iron. J Clin Invest. 1995;96:620–630. doi: 10.1172/JCI118077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto H, Shelly CL. Current concepts in the pathogenesis of alcoholic liver injury. FASEB J. 2001;15:1335–1349. doi: 10.1096/fj.00-0650rev. [DOI] [PubMed] [Google Scholar]
- Uchida K, Stadtman KJ. Covalent attachment of 4-hydroxynonenalprotein adducts to glyceraldehydes-3-phosphate dehydrogenase: potential of intra- and intermolecular reactions. J Biol Chem. 1993;268:6388–6393. [PubMed] [Google Scholar]
- Yin M, Wheeler MD, Kono H, Bradford B, Gallucci RM, Luster MI, Thurman RG. Essential role of tumor necrosis factor α in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–952. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]


