Abstract
A kidney perfusion machine, model MOX-100 (Waters Instruments, Ltd, Rochester, MN) was modified to allow continuous perfusion of the portal vein and pulsatile perfusion of the hepatic artery of the liver. Additional apparatus consists of a cooling system, a membrane oxygenator, a filter for foreign bodies, and bubble traps. This system not only allows hypothermic perfusion preservation of the liver graft, but furthermore enables investigation of ex vivo simulation of various circulatory circumstances in which physiological perfusion of the liver is studied. We have used this system to evaluate the viability of liver allografts preserved by cold storage. The liver was placed on the perfusion system and perfused with blood with a hematocrit of approximately 20%, and maintained at 37°C for 3 h. The flows of the hepatic artery and portal vein were adjusted to 0.33 mL and 0.67 mL/g of liver tissue, respectively. Parameters of viability consisted of hourly bile output, oxygen consumption, liver enzymes, electrolytes, vascular resistance, and liver histology. This method of liver assessment in large animals will allow the objective evaluation of organ viability for transplantation and thereby improve the outcome of organ transplantation. Furthermore, this pump enables investigation into the pathophysiology of liver ischemia and preservation.
Keywords: Liver, reperfusion, transplantation
Remarkable progress has been achieved in the field of liver transplantation during the last 25 years.1 Research in experimental animals has been a major factor responsible for these advances, including the operative technique,2,3 venovenous bypass,4 immunosuppression,5,6 and preservation.7 In spite of these major achievements, substantial knowledge of the biochemical and hemodynamic changes of the liver allograft following revascularization is lacking. This is partly attributable to difficulty in obtaining reproducible stable hemodynamics after revascularization in experimental large-animal liver-transplantation models, which is due to multiple factors involved in the actual transplant procedure.
Presently, the introduction of the University of Wisconsin solution allows safe prolonged slush preservation of the liver.1,7,8 However, further analyses on the significance of perfusion preservation of solid organs and its potential additive role to current practice remain to be clarified.9
In this report, we describe a new perfusion system that allows physiologic investigations of the liver with sanguinous perfusion and of perfusion preservation protocols in large animals.
Experimental Model
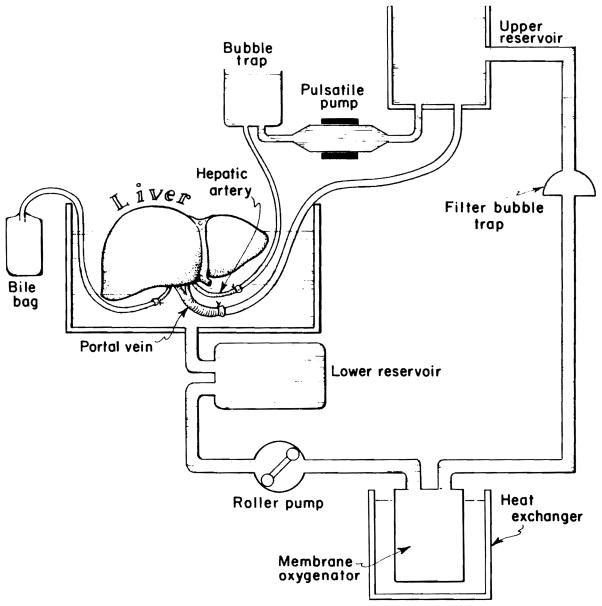
As shown in Figures 1 and 2, a kidney perfusion machine, model MOX-100 (Waters Instruments, Ltd, Rochester, MN) was modified to allow continuous perfusion of the portal vein and pulsatile perfusion of the hepatic artery of the liver. This represents a more physiologic and natural perfusion system of the large liver than was previously available. Continuous flow of the portal vein is obtained by gravity drainage of the perfusate from a reservoir, which is maintained at a preset level with the use of a roller pump combined with an automatic level control. A perfusion pump is used for pulsatile perfusion of the hepatic artery. Adjunctive pieces of equipment consist of a heat exchanger that allows the maintenance of the perfusate at a given temperature between 4 and 37°C, a membrane oxygenator, a filter that entraps foreign bodies larger than 37 nm, and bubble traps for the systemic as well as the arterial circuits. A total perfusate volume of 3000 mL is required for the circuit.
Figure 1.

Photographic view of the liver perfusion and preservation system developed by modifying a kidney perfusion machine, model MOX-100 (Waters Instruments, Ltd, Rochester, MN).
Figure 2.
Schematic view of liver perfusion and preservation system.
Technique of Allograft Hepatectomy
The donor pig, weighing 15–30 kg, is anesthetized with 25 mg/kg of thiamylal iv, and 100% oxygen is administered through an endotracheal tube. The abdomen is prepped and draped in a sterile fashion. The peritoneal cavity is then entered through a midline incision and the terminal aorta is encircled. Hilar dissection of the liver is performed by dividing the common bile duct, the gastroduodenal and right gastric artery, and areolar structures of the hepatoduodenal ligament just above the pancreas between silk ligatures. The pancreatic branch of the portal vein is divided between silk ties, and the portal vein is dissected bluntly down to the confluence of the splenic and the superior mesenteric veins. The common hepatic artery is dissected proximally and the left gastric artery is transected between silk ligatures. Then the spleen and the stomach are retracted medially and the suprarenal abdominal aorta is dissected from the left lateral aspect to expose the celiac axis and the superior mesenteric artery. The splenic artery is divided between silk ties, and the celiac axis as well as the common hepatic artery are completely dissected. The origin of the superior mesenteric artery is encircled. The gallbladder is entered in the fundus and irrigated to avoid autolysis of the bile duct epithelium during cold preservation. Following intravenous administration of 3 mg/kg of heparin, cannulae are inserted into the portal vein through the distal splenic vein and into the distal aorta through the terminal aorta. Following ligation of the superior mesenteric artery and vein, a total of 1000 mL of blood is drawn through the aortic cannula, while 500 mL of lactated Ringer’s solution (4°C) is administered through the portal cannula. When 1000 mL of blood is obtained, the liver is flushed with lactated Ringer’s solution (4°C) with an additional 1500 mL of lactated Ringer’s solution (4°C) through the portal cannula with 100 cm H2O. At the same time, the inferior vena cava is transected in the pericardial cavity and the thoracic aorta is cross-clamped.
After ascertaining the flushing and cooling of the liver, the left hemidiaphragm is incised and the aorta is transected at the level of midthoracic aorta proximally and just above the origin of the superior mesenteric artery distally. The lesser omentum is divided cephalad, while an assistance retracts the stomach and the esophagus laterally and away from the liver. The aorta is then delivered anterior to the stomach through the lesser omentum. The right hemidiaphragm is incised and the liver is allowed to rotate posterosuperiorly, which facilitates better exposure of the liver hilum. The splenic and the superior mesenteric veins are divided and the infrahepatic vena cava is transected, while the right kidney is retracted caudad. The liver is then sharply detached from the retroperitoneum from the inferior aspect while the liver is held anteriorly in the surgeon’s left hand from the superior aspect through the pleural and pericardial cavities. The liver is then placed in a sterile bag, where a preservation solution (studied) is administered through the portal cannula. The arterial system is flushed passively in a retrograde fashion by reflux of the preservation solution. The distal common bile duct is cannulated with a 16F catheter with side holes and the cystic duct is ligated with a silk tie. The diaphragm is excised and cannulae are placed in the proximal aorta and the portal vein (½ and ⅜ in., respectively). The liver is weighed and stored in an ice chest.
Sanguinous Perfusion
A total of 3000 mL of pig blood is obtained from two blood donor animals as well as from a liver donor. The perfusion system is primed with 1000 mL of lactated Ringer’s solution, followed by the blood collected. The hematocrit of the perfusate is adjusted to approximately 20%. One liter per minute of oxygen is administered through the oxygenator, while the circuit is warmed by a heat exchanger to maintain the temperature at 37°C. The pH of the perfusate is adjusted to 7.4 with sodium bicarbonate and carbon dioxide gas. The liver is reperfused by connecting the portal and aortic cannulae of the liver with the circuits. The biliary cannula is connected to an extension tube for external drainage, to measure bile output. The total liver flow of 1 mL/g of liver tissue is maintained for 3 h. The flow is divided into 0.67 mL/g of liver tissue through the portal vein and 0.33 mL/g of liver tissue through the hepatic artery. This flow rate and distribution were selected based on previous studies in animals and humans.10 The blood flow is measured using a Transonic full-flow illumination transit-time ultrasonic flow-meter, Model T201 (Transonic Systems Inc, Ithaca, NY). The portal venous flow is maintained by adjusting the height of the upper reservoir, and the hepatic arterial flow is controlled by manipulating the stroke volume of the pulsatile pump. The pulse rate of the pulsatile pump is constantly maintained at 70/min. Parameters measured following revascularization of the liver are listed in Table 1. Blood samples and bile output are obtained every 30 min, and liver biopsy is acquired hourly for the histological evaluation of the liver tissue.
Table 1.
Parameters Used for the Assessment of Sanguinous Perfusion of the Liver
| Blood (Every 30 min) | Bile (Every 30 min) | Biopsy (Every Hour) |
|---|---|---|
| Blood gas | Bile output | Photomicroscopy |
| Oxygen consumption | Electon microscopy | |
| SGOTa | ||
| SGPTb | ||
| Alkaline phosphatase | ||
| Lactate dehydrogenase | ||
| Total bilirubin | ||
| Sodium | ||
| Potassium |
SGOT, serum glutamic–oxaloacetic transaminase.
SGPT, serum glutamic–pyruvic transaminase.
Oxygen consumption was calculated according to the following formula:
where AO2 Sat is the oxygen saturation of the affluent (%); EO2 Sat is the oxygen saturation of the effluent (%); Hgb is the hemoglobin of the perfusate (g/dL); FHA is the hepatic arterial flow (mL/min); and FPV is the portal venous flow (mL/min).
Results and Use of This Model
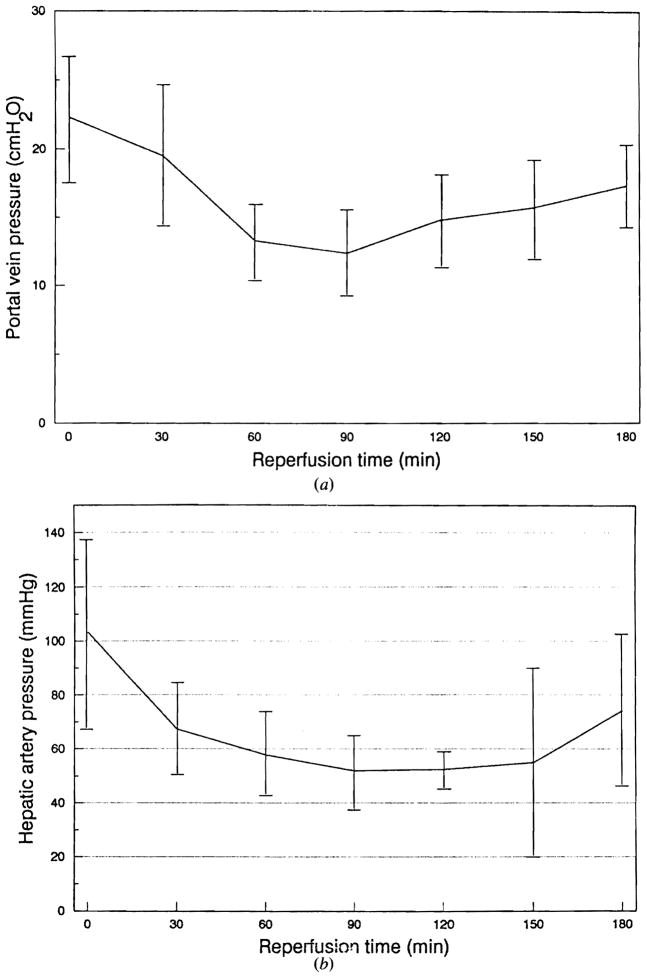
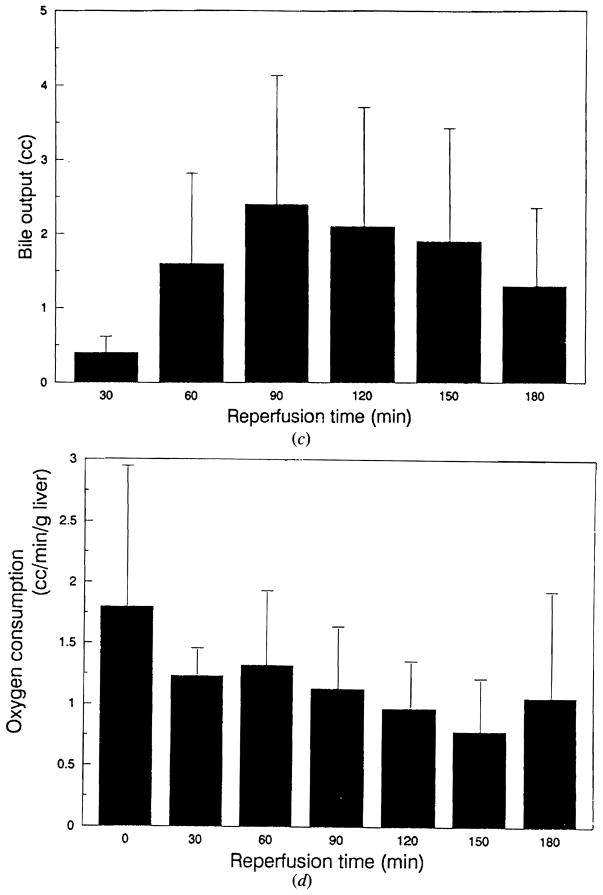
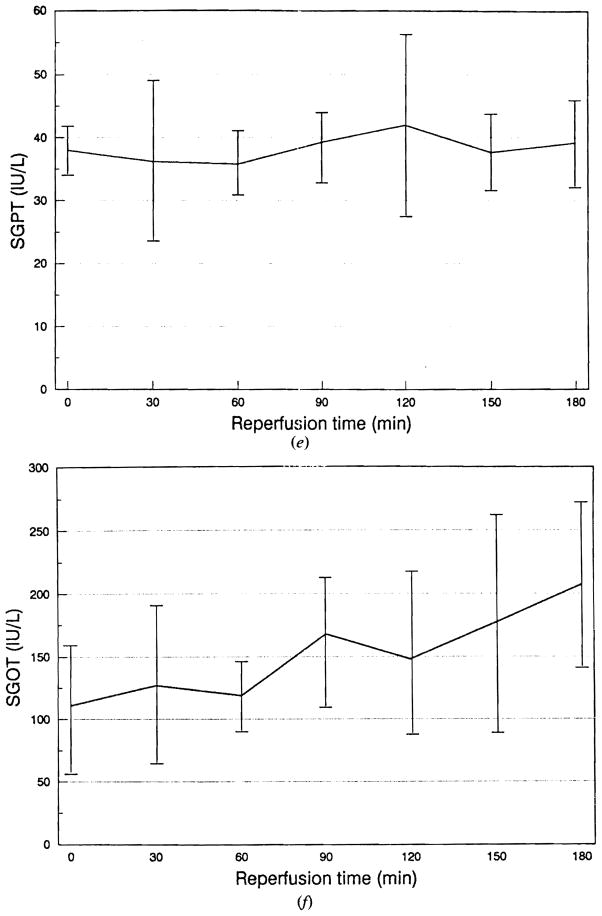
Figure 3 shows the results of sanguinous perfusion of the porcine liver preserved for 50–150 min (mean 83.3 min) in lactated Ringer’s solution. The portal vein and the hepatic artery pressures showed rapid reduction during the initial 60 min following reperfusion (Fig 3a, b). Both circuits showed an increase in pressure at the end of the perfusion period. Bile output reached a maximum at 90 min after reperfusion and started to decline afterwards (Fig 3c). The oxygen consumption demonstrated a tendency to decline after reperfusion (Fig 3d). Serum glutamic–pyruvic transaminase (SGPT) remained unchanged, whereas serum glutamic–oxaloacetic transaminase (SGOT) showed an almost continuous rise during the sanguinous perfusion (Fig 3e, f). Lactate dehydrogenase showed changes identical to SGOT, while total bilirubin and alkaline phosphatase showed no change during perfusion. Figure 4 shows typical histological changes in the liver before and after sanguinous reperfusion. The biopsy specimen obtained 3 h after initiation of reperfusion demonstrates mild periportal sinusoidal congestion, scattered necrotic hepatocytes and microvesicular steatosis, which are consistent with ischemic changes.
Figure 3.
Results of sanguinous perfusion of fresh porcine liver. (a) Changes in portal venous pressure. (b) Changes in hepatic arterial pressure. Data are expressed as mean ± SD.
(c) Changes in bile output. (d) Changes in oxygen consumption. Data are expressed as mean ± SD.
(e) Changes in serum glutamic-pyruvic transaminase (SGPT). (f) Changes in serum glutamic–oxaloacetic transaminase (SGOT). Data are expressed as mean ± SD.
Figure 4.
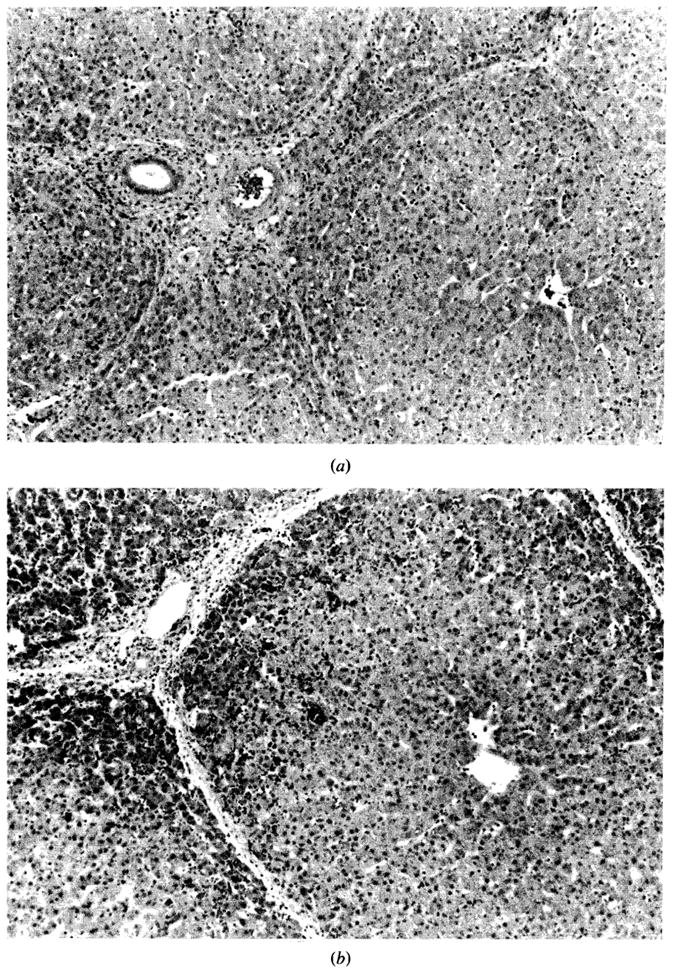
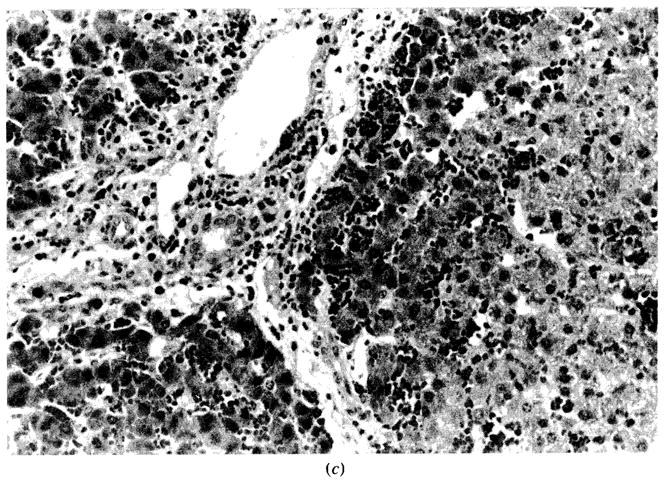
Histological changes before and after sanguinous perfusion of fresh porcine liver. (a) Liver specimen just prior to sanguinous reperfusion; lobular architecture is well maintained. No steatosis, cholestasis, or hepatocellular necrosis is present. (b) Liver specimen after sanguinous reperfusion (180 min); lobular architecture is still maintained. However, mild sinusoidal congestion is seen in periportal area with scattered necrosis of the hepatocytes. No pathologic changes are noted in midzonal or centrilobular area. (H–E stain; original magnification × 100.)
(c) High-power view of Figure 4b. The hepatic artery, the portal vein, and the bile ducts are intact. Microvesicular steatosis is observed around the congested areas (H–E stain; original magnification × 250.)
Comments
Liver transplantation in large animals is imperative for the evaluation of immunosuppressants as well as preservation fluids, and is an excellent model for the development of technical expertise. However, experimental liver transplantation in large animals requires a large supportive team as well as considerable financial support. Furthermore, multiple factors are involved in the actual liver transplantation procedures. In the donor, the factors include the condition of the donor before and during organ harvesting, as well as the quality of liver procurement. In the recipient, the factors include blood loss, fluid and electrolyte balance, efficacy of the venovenous bypass, manipulation of the liver, and the quality of and the time required for the vascular anastomoses. These numerous variables make it difficult to obtain a standardized and reproducible environment for the evaluation of postperfusion changes.
Our perfusion system allows completely reproducible reperfusion of the liver in large animals, which offers unique opportunity to investigate various biochemical and hemodynamic changes associated with revascularization of the liver. This is an excellent model for the analysis of the pathophysiology of liver ischemia/reperfusion injury and preservation. Furthermore, this system requires only one person to do the study and the overall cost of this perfusion experiment is much lower than the actual surgical transplant model in large animals.
Although the University of Wisconsin solution enables slush preservation of the liver for up to 24 h,1 investigation into perfusion preservation of solid organs must continue.9 The perfusion system described herein allows maintenance of flows and temperature of the perfusate, thereby facilitating the evaluation of perfusion preservation of the liver.
This perfusion system will require minimal, if any, significant modification, should it be applied clinically.
Acknowledgments
This project was supported by research grants from the Veterans Administration and Project Grant AM 29961 from the National Institutes of Health, Bethesda, MD.
References
- 1.Starzl TE, Todo S, Tzakis AG, et al. Liver transplantation: An unfinished product. Transplant Proc. 1989;21:2197. [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Kaupp HA, Brock DR, et al. Reconstructive problems in canine liver homotransplantation with special reference to postoperative role of hepatic venous flow. Surg Gynecol Obstet. 1960;111:733. [PMC free article] [PubMed] [Google Scholar]
- 3.Moore FD, Wheeler HB, Demissianos HV, et al. Experimental whole-organ transplantation of the liver and of the spleen. Ann Surg. 1960;152:374. [PMC free article] [PubMed] [Google Scholar]
- 4.Denmark SW, Shaw BW, Jr, Griffith BP, Starzl TE. Venovenous bypass without systemic anticoagulation in canine and human liver transplantation. Surg Forum. 1983;34:380. [PMC free article] [PubMed] [Google Scholar]
- 5.Starzl TE, Marchioro TL, Porter KA, et al. The use of heterologous antilymphoid agents in canine renal and liver homotransplantation, and in human renal homotransplantation. Surg Gynecol Obstet. 1967;124:301. [PMC free article] [PubMed] [Google Scholar]
- 6.Williams JW, Peters TG, Haggitt R, et al. Cyclosporin A in orthotopic canine hepatic transplants. J Surg Res. 1982;32:576. doi: 10.1016/0022-4804(82)90142-1. [DOI] [PubMed] [Google Scholar]
- 7.Jamieson NV, Sundberg R, Lindell S, et al. Preservation of the canine liver for 24–28 hours using simple cold storage with UW solution. Transplantation. 1988;46:517. doi: 10.1097/00007890-198810000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation. 1988;45:673. doi: 10.1097/00007890-198804000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann RM, Stratta RJ, D’Alessandro AM, et al. Combined cold storage-perfusion preservation with a new synthetic perfusate. Transplantation. 1989;47:32. doi: 10.1097/00007890-198901000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Greenway CV, Start RD. Hepatic vascular bed. Physiol Rev. 1971;51:23. doi: 10.1152/physrev.1971.51.1.23. [DOI] [PubMed] [Google Scholar]