Abstract
Objective
This study examines the role of self-reported trait positive affect (PA) on skin barrier recovery after skin disruption, and whether the role of trait PA in wound healing is consistent with the direct effects model or the stress-buffering model of PA and health.
Design
Sixty healthy participants (mean age 22.7 ± 3.9 years) completed a self-report measure of trait positive and negative affect, underwent a “tape-stripping” procedure that disrupts normal skin barrier function, and were randomly assigned to a Stress (Trier Social Stress Test) or No Stress (reading task) condition.
Main Outcome Measures
Skin barrier recovery was assessed by measuring transepidermal water loss up to 2 hr after skin disruption.
Results
Multilevel modeling indicated that greater trait PA was related to faster skin barrier recovery (p < .05). The effects of PA on skin barrier recovery were independent of levels of trait NA.
Conclusion
These findings suggest that trait PA may influence skin barrier recovery following a brief stressor. In addition, these results provide additional evidence that trait PA can positively impact objective health outcomes.
Keywords: acute stress, trait positive affect, wound healing, skin barrier recovery
The experience of positive affect (PA) is related to a variety of health benefits such as increased longevity, decreased morbidity, and less experience of pain (reviewed in Pressman & Cohen, 2005). Several prospective studies show that PA predicts decreased morbidity, such as lower incidence of stroke in older adults (Ostir, Markides, Peek, & Goodwin, 2001), and lower incidence of developing symptoms of the common cold after inoculation with cold virus (Cohen, Doyle, Turner, Alper, & Skoner, 2003). iThese studies importantly demonstrated that the effects of PA are independent of disease processes, as participants were healthy at baseline, and that dispositional or trait PA showed effects on morbidity independent of the effects of negative affect. Moreover, prospective effects of trait PA were observed in a range of health outcomes (e.g., pregnancy outcomes, injury, stroke, and hospital readmission).
The specific mechanisms through which trait PA influences health operate through two general frameworks proposed by Pressman and Cohen (2005). One proposed framework is a direct effects model, in which trait PA impacts behaviors and biological systems relevant for health in general, irrespective of its effects on responses to stress. For example, individuals with high trait PA may be more likely to engage in health-promoting behaviors or have lower tonic levels of catecholamines or glucocorticoid hormones. By contrast, in a stress-buffering model, dispositional levels of PA may “buffer” against the harmful biological and health consequences of exposure to stressors. Individuals with high trait PA may be able to cope more effectively with stressful events and consequently, may not experience the negative health consequences of psychological stress. This stress-buffering hypothesis is consistent with Fredrickson’s “Broaden and Build” theory of positive emotions (Fredrickson, 1998), in which positive emotions result in the building of social, intellectual, and physical resources by broadening action tendencies and generating psychological resources (Salovey, Rothman, Detweiler, & Steward, 2000). Thus, social and psychological resources available to individuals high in trait PA may be drawn upon when challenged with stressful events. Regardless of whether PA influences health through direct effects or by buffering stress, there is some evidence for specific biological mechanisms that may tie PA to health, including neuroendocrine and immune function (Cohen et al., 2003; Marsland, Cohen, Rabin, & Manuck, 2006; Steptoe, Wardle, & Marmot, 2005).
An additional biological mechanism that may explain links between trait PA and health is processes involved in skin repair and wound healing, which are clinically relevant and can be measured in a brief period of time in healthy individuals. The skin is the largest organ of the body. The outermost layer of the skin, the stratum corneum, has a number of protective “barrier” functions, including defending against microbes; keeping the skin cohesive and intact despite physical damage; protecting the interior against chemical absorption, ultraviolet rays, and extreme temperature; and preventing water loss (Elias, 2005). If the skin barrier is compromised by wounding, all the previously described functions are impaired, which further impair recovery from skin damage, protection against future damage, and susceptibility to infectious illness. Much like inflammatory processes, which have multiple effects on the organism and are of considerable interest to health psychologists (Kiecolt-Glaser, McGuire, Robles, & Glaser, 2002), problems with skin repair and wound healing extend to other important biological activities that protect and preserve our health. More generally, wound healing provides a model system for understanding the impact of psychological factors on health within a short period of time.
Exposure to acute and chronic stress is related to delayed healing of full-thickness wounds (e.g., punch biopsy) that penetrate both the epidermis and underlying dermis (reviewed in Christian, Graham, Padgett, Glaser, & Kiecolt-Glaser, 2006). Psychological stress may also disrupt the skin’s ability to recover its function as a barrier against moisture loss and pathogens after more minor disruption, such as damage to the stratum corneum. Across several studies, skin barrier recovery was reduced by 10%–15% following a brief laboratory stressor (Altemus, Rao, Dhabhar, Ding, & Granstein, 2001; Robles, 2007). Approximately 30% recovery was achieved at 3 h after skin disruption during medical school examinations, compared to 45% recovery during the end of winter and spring vacation (Garg et al., 2001). These studies suggest that skin barrier recovery may be a useful model for examining the impact of psychosocial factors such as psychological stress on health and suggest a pathway through which trait PA may impact health. However, few studies published to date have explored the role of individual difference characteristics, such as trait PA, on wound healing in response to stress.
This study tested whether individual differences in trait PA are related to skin barrier recovery, and whether the role of trait PA in wound healing is consistent with the direct effects model or the stress-buffering model. In this study, we assessed trait levels of PA and negative affect (NA) in participants, who were then randomly assigned to go through a brief psychological stressor or no stressor. The direct effects model predicts that individuals with greater trait PA will show faster skin barrier recovery, regardless of the presence of psychological stress, while the stress-buffering model predicts that greater trait PA will be related to faster skin barrier recovery in individuals undergoing a stressor. Based on previous work on the effects of PA on reducing the negative psychological and biological impact of psychological stress (Steptoe et al., 2005; Tugade & Fredrickson, 2004) and the effects of psychological stress on skin barrier recovery (Altemus et al., 2001; Garg et al., 2001), we predicted that greater trait PA would be related to faster skin barrier recovery following exposure to a laboratory stressor, consistent with the stress-buffering model.
Method
Participants
Healthy individuals ages 18 – 44 years were recruited from the community surrounding a large Midwest university using publicly posted fliers. Exclusion criteria included pregnancy; medical conditions or medications with obvious immunological, dermatological, or endocrinological consequences; allergies to tape or other adhesives; smoking; taking hormone-based contraception; and excessive caffeine or alcohol use. A total of 99 individuals participated in the study, of which 12 women did not report taking hormonal-based contraception on our initial screening form, but reported it during the experimental session, and two individuals were unable to complete the entire protocol. Data from those 14 individuals were not included in the data analyses that follow. In addition, the trait affect measure was included approximately 1/3 of the way through data collection. Therefore, we report findings from participants who completed the trait affect measure. The final sample (n = 60, mean age = 22.7, SD = 3.9) included 27 female and 33 male participants, 75% white, 13% Asian, 8% black, and 2% of other ethnicities. Most participants (70%) had some college education, and the remainder had completed college (30%).
Procedures
Participants were run individually during one 3.5-hr session at The Ohio State University General Clinical Research Center beginning at 1:30 p.m. Participants refrained from eating, vigorous exercise, and smoking for 1 h prior to the appointment. After providing informed consent, participants completed the self-report measure of trait PA and NA, followed by baseline skin measurements and skin disruption. Participants were randomly assigned to one of three groups: No Stress (n = 13), Stress (n = 20), or Stress + Support (n = 27). Each group received instructions for a task, prepared for the task for 10 min, and then performed the 10 min task. After the tasks, skin barrier recovery was measured up to 2 hr after skin disruption. Participants were debriefed by the experimenter at the end of the session. All procedures were approved by The Ohio State University Biomedical Sciences Institutional Review Board.
The No Stress group read an article silently and alone during the preparation period, followed by reading the article and an additional article out loud into a tape recorder for 10 min. They were informed that they were not being evaluated, and performed the task alone. Video recording equipment was present, turned off and pointed toward a wall. Participants in the Stress and Stress + Support groups were provided instructions for the Trier Social Stress Test (TSST), a 5-min speech and 5-min mental arithmetic task (Kirschbaum, Pirke, & Hellhammer, 1993). Participants in the Stress group prepared for their tasks alone. Participants in the Stress + Support group were greeted by a supportive confederate who provided social support for 10 minutes (for details, see Robles, 2007). After preparation, the two Stress groups began the TSST in front of an evaluative and harassing audience and were videotaped throughout. We found no differences in rate of skin barrier recovery between the Stress and Stress + Support groups (βlinear = −7.19, t = −0.50, df = 56, p = .62; βquadratic = 3.91, t = 0.64, df = 56, p = .53), similar to results from the full sample (Robles, 2007). In addition, the groups did not differ in trait PA and trait NA (all ps > .15). Therefore, the groups were combined for the remaining analyses.
Trait Positive and Negative Affect
The trait version of the Positive and Negative Affect Schedule (PANAS) (Watson, Clark, & Tellegen, 1988) was administered at the beginning of the laboratory session, about 30–40 min prior to informing participants of their group assignment. Internal consistency in this sample was excellent (PA α = .91, NA α = .86). Trait PA (M = 31.3, SD = 8.2) and NA (M = 15.7, SD = 5.4) were slightly below, but within 1 SD of the standardization sample (Watson et al., 1988). Similar to other studies (Crawford & Henry, 2004; Tellegen, Watson, & Clark, 1999), trait PA and trait NA were not correlated (r = .01, p = .95). There were no gender differences in trait PA, t(58) = −1.41, p = .16, or relationships between trait PA and age (r = .04) or education, F(3, 56) = 1.17, p = .33. Trait PA was not related to alcohol and caffeine intake, or body mass index (data not shown), but individuals reporting worse sleep quality on the Pittsburgh Sleep Quality Index (Buysse, Reynolds III, Monk, Berman, & Kupfer, 1989) reported lower trait PA (r = −.40, p = .002).
Skin Measurement and Skin Barrier Disruption
Baseline skin barrier function on the palm side of the dominant forearm was measured by obtaining transepidermal water loss (TEWL) readings using two evaporimeter probes (cyberDERM, Cortex DermaLab; Media, PA; Grove, Grove, Zerweck, & Pierce, 1999). TEWL indicates the skin’s ability to prevent water loss from interior layers. Greater TEWL reflects decreased barrier function, and lower TEWL following disruption indicates increasing barrier recovery. Following baseline measurements, a 2.54 × 5.08 cm rectangular area was disrupted using a procedure known as “tape-stripping” (Nickoloff & Naidu, 1994), and we measured TEWL from three adjacent sites (each 1 cm in diameter) within that area. A 2.54 × 2.54 cm area was left undisturbed for basal TEWL measurements. Cellophane tape (Tesa, Inc. 4101; Charlotte, NC), was applied repeatedly (6–51 times) to remove superficial layers of skin cells from the disrupted site. Stripping stopped when TEWL was elevated from the basal levels (M = 7.46g/m2h, SD = 2.48) to at least 20g/m2h at the disrupted site, or a maximum of 51 strips (M = 38.2 strips, SD = 13.8). Skin measurements were repeated at 1, 1.5, and 2 hr after tape-stripping (or 35, 60, and 90 min after the tasks). The three measurement times were chosen because we wanted to determine if the effects of stress on skin barrier recovery could be observed in less than 3 hr (other studies measured skin barrier recovery starting 3 hr after tape-stripping) to minimize the time that participants were in the laboratory. Moreover, biological processes that initiate skin barrier recovery begin within the first hours after skin disruption (Elias, 2005; Nickoloff & Naidu, 1994). The two (out of three) disrupted sites that showed TEWL values closest to one another were averaged together, and recovery was computed from a widely used formula (Altemus et al., 2001; Garg et al., 2001) where t represents hr since tape-stripping: Recoveryt = (TEWLafter tape-stripping – TEWL t )/ (TEWLafter tape-stripping – TEWLat baseline) × 100%.
Data Analysis
For our primary analyses, we used multilevel modeling to model skin barrier recovery using HLM 6 (Version 6.06, Scientific Software International; Raudenbush, Bryk, Cheong, & Congdon, 2004). Measurement occasions (level-1) were nested within individuals (level-2), and time was the level-1 predictor of change over measurement occasions. The data and fit indices with different models of change showed that a model with linear and quadratic (U-shaped) slopes provided the best fit to the data. The intercept value was centered at % recovery immediately after disruption (equal to 0 across all participants). Time was scaled such that 1 unit of time equaled 1 hr of real time. Across all the models, level-1 error variances were specified as homogeneous. Effect sizes are reported as r’s by taking the square root of the quantity t2/(t2 + df), (Rosenthal & DiMatteo, 2001). The level-2 variables in the final model testing our hypothesis that trait PA would predict faster skin barrier recovery were as follows: Group (No Stress vs. Stress), trait PA, trait NA, the Group × PA interaction, and the Group × NA interaction. We included trait NA and the Group × NA interaction in these analyses because of previous recommendations that PA and NA should be simultaneously included in statistical analyses to account for potential interdependence between PA and NA (Pressman & Cohen, 2005).
Results
We determined that the most appropriate model of skin barrier recovery should specify the intercept as fixed across participants because values at the 0-hr time point were zero for all participants, and linear and quadratic slopes as varying across participants (random). The mean parameter estimates in the unspecified model were very similar to the final model and did not change the interpretation of the results (slopes specified as random, data not shown). A U-shaped pattern of skin barrier recovery indicated a large initial increase in skin barrier recovery 1 h after tape-stripping, which eventually decreased but still represented an increase in recovery from baseline. Recovery increased by an average of 63% per hour, and decreased by 24% per hour squared. Linear increases in skin barrier recovery were almost perfectly negatively correlated with quadratic decreases in recovery over time, which is expected for a U-shaped pattern of change. There was significant variance in linear and quadratic slopes; therefore, including our level 2 variables (Main effects: Group, PA, NA; Interactions: Group × PA, Group × NA) as predictors of linear and quadratic slopes was appropriate.
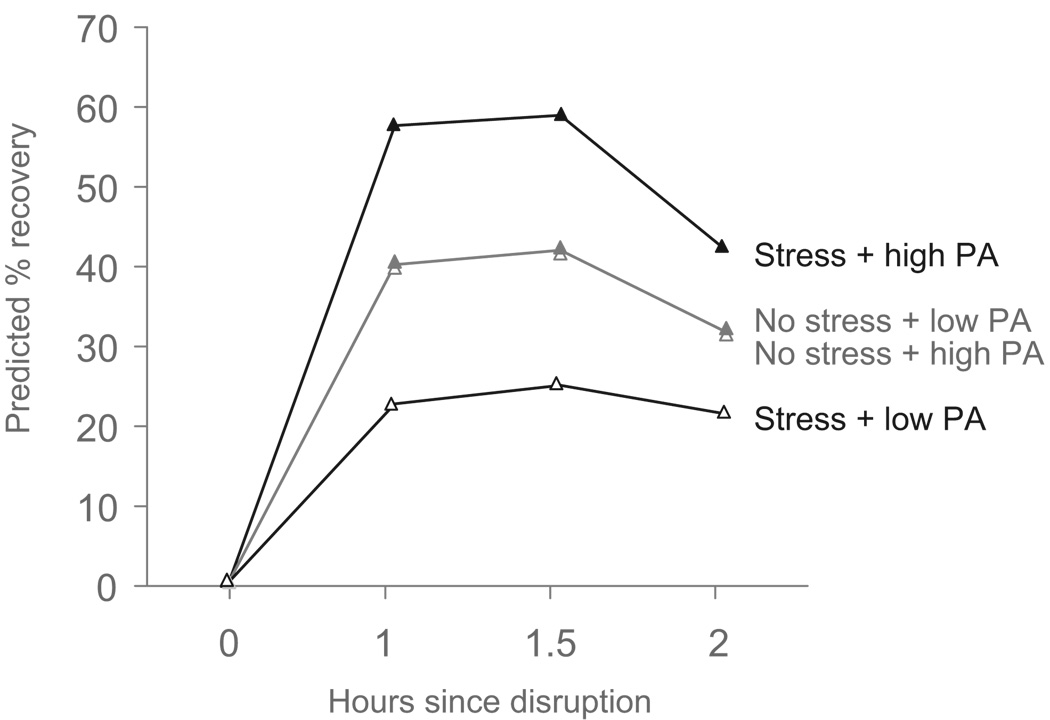
Our primary hypothesis was that trait PA would buffer the negative effects of psychological stress on skin barrier, indicated by a significant Group × PA interaction predicting skin barrier recovery slopes, with high PA predicting faster increases in recovery compared to low PA, but only in the Stress group. A main effect of PA on skin barrier recovery slopes would support a direct effects model. As shown in the Table 1, indicated that there were no significant main effects of group, PA, or NA on linear or quadratic slopes. Significant Group × PA interactions for linear and quadratic slopes, depicted in the Figure 1, indicated that greater trait PA was related to faster skin barrier recovery for participants in the Stress group, while trait PA was not related to skin barrier recovery in the No Stress group. In the Stress group, participants who scored + 1 SD on trait PA showed almost double the skin barrier recovery compared to participants scoring −1 SD.
Table 1.
Parameter Estimates For % Skin Barrier Recovery
| Parameter | Parameter estimate | Effect size r |
|---|---|---|
| Fixed effects | ||
| Intercept | 0.52 (0.25) | .14 |
| Linear slope | 63.79 (14.79)*** | .51 |
| Group | −19.20 (16.32) | .16 |
| PA | −1.98 (0.99) | .26 |
| NA | 2.03 (4.21) | .07 |
| Group × PA | 3.74 (1.89)** | .38 |
| Group × NA | −1.14 (4.31) | .04 |
| Quadratic slope | −24.08 (7.39)*** | .41 |
| Group | 10.81 (7.96) | .18 |
| PA | 0.87 (0.46) | .25 |
| NA | −1.99 (2.08) | .13 |
| Group × PA | −1.55 (0.57)** | .35 |
| Group × NA | 1.76 (2.15) | .11 |
| Variance components | ||
| Linear slope | 1126.33*** | — |
| Quadratic slope | 150.68*** | — |
| Within-subject | 215.51 | — |
Note. Intercept values reflect % skin barrier recovery (g/m2h) at baseline. Linear slopes reflect change in % recovery per 1 h of time, and quadratic slopes reflect change in % recovery per (1 hr of time)2. Linear and quadratic slopes correlated −.99. Values in parentheses are standard errors. Intercept df = 207, slope df = 53, level-2 predictors of slopes df = 53, variances df = 49.
p < .01.
p < .001.
Figure 1.
Predicted skin barrier recovery as a function of group assignment and trait PA based on parameter estimates in Table 1. High trait PA represents 1 SD above the mean, and low trait PA represents 1 SD below the mean on trait PA. The lines representing low and high trait PA in the No Stress group are superimposed upon one another.
As shown in the Table, trait NA did not predict skin barrier recovery. Removing trait NA and the Group × NA interaction did not change the results for the Group × PA interaction on linear and quadratic change in skin barrier recovery, which remained statistically significant (βlinear = 3.33, SE = 1.13, t = 2.95, df = 55, p = .005, effect size r = .37; βquadratic = −1.20, SE = 0.50, t = −2.40, df = 55, p = .02, effect size r = .31). The number of strips to criterion was not significantly related to trait PA (r = −.24, p = .07) or NA (r = −.02, p = .88) and neither trait PA nor trait NA predicted basal TEWL before tape-stripping (r’s from −.08 to −.02, ns) or immediately after tape stripping (r’s from .01 −.03, ns). Basal TEWL at the control site did not significantly change during the session, and there were no significant main effects of group, trait PA, or trait NA, or interactions for control site TEWL (data not shown).
Discussion
This study extends previous work on stress and wound healing by demonstrating that trait PA buffered the effects of stress on skin barrier recovery. Specifically, individuals with greater trait PA showed faster skin barrier recovery within 1 hr after skin disruption, and that faster recovery was maintained up to 2 hr after disruption. Trait PA was not significantly related to skin barrier recovery in individuals who did not undergo the psychological stressor. Thus, these data support the stress-buffering model of the effects of trait PA on health (Pressman & Cohen, 2005). The effects of trait PA on skin barrier recovery were independent of the effects of trait NA, which is worth noting because considerable evidence suggests that NA is related to poor health outcomes (Kiecolt-Glaser et al., 2002). We included NA in our analyses to ensure that the effects of PA on skin barrier recovery were not driven by the effects of NA. The magnitude of the effect of PA on skin barrier recovery was similar to the effect size of psychological stress on skin barrier recovery reported in previous studies (r = .36, derived from Altemus et al., 2001, and Robles, 2007). Notably, we found effects for trait PA on biological responses to stress, whereas most studies of PA and biological responses to stress have focused on inducing positive mood states.
To impact skin barrier recovery, psychological factors would have to affect key biological processes involved in skin barrier recovery (lipid synthesis and inflammation; Elias, 2005) through mechanisms that communicate psychosocial “messages” to the skin. Several candidate messenger systems that could explain links between trait PA and skin barrier recovery are systemic glucocorticoids, neuropeptide release from afferent nerves in the peripheral nervous system, and activation of immune and inflammatory processes in the skin (Garg et al., 2001). Although studies in rodent models clearly show that elevated glucocorticoids can delay skin barrier recovery (Choi et al., 2005), we and others previously showed that salivary cortisol levels during stress were not related to skin barrier recovery (Altemus et al., 2001; Robles, 2007). Thus, beyond glucocorticoid levels, psychosocial factors may influence neuropeptide and neurotransmitter release in the skin (e.g., substance P, norepinephrine, acetylcholine; Arck, Slominski, Theoharides, Peters, & Paus, 2006), but no empirical studies to date have studied psychological factors in humans and skin levels of neuropeptides and neurotransmitters. Finally, psychological factors such as trait PA may influence immune processes in the skin. While no studies have explicitly studied trait PA and immune mechanisms involved in wound healing, several studies suggest that state PA can influence skin response to allergens. A reduction in allergic response was found when pleasantness and relaxation were induced by hypnosis (Laidlaw, Booth, & Large, 1996) and when humor was induced by a movie (Kimata, 2001). Moreover, greater self-reported vigor was related to smaller allergy responses (Laidlaw, Booth, & Large, 1994). Thus, the precise biological mechanisms through which trait PA acts on the epidermis are not known, but several candidates are possible for future exploration.
One notable finding in this study is that skin barrier recovery was highest level at 1 – 1.5 hr after disruption, and decreased slightly at 2 hr after disruption. Few studies have reported the hour-to-hour kinetics of skin barrier recovery. Thus, the extent to which our U-shaped results are similar to other work is unknown. Potential explanations for the increase, followed by a decrease in recovery must be consistent with several observations in our data. The explanations and mechanisms must covary with performing a mental task (No Stress and Stress groups showed a U-shaped curve), and be specific to processes related to skin barrier recovery and not basal TEWL, as basal TEWL did not significantly change across the session, and the kinetics of basal TEWL did not differ across groups. One possibility is sweating, which can increase TEWL and thereby decrease skin barrier recovery (Rogiers & EEMCO Group, 2001). Individuals low in trait PA may have been sweating more during the TSST, which may explain why their TEWL was higher in the 1 – 1.5 hr after tape-stripping. However, increased sweating would not be consistent with our basal TEWL findings or with lower skin barrier recovery 2 hr after tape-stripping (up to 1.5 hr after the TSST). A more plausible explanation is increased skin surface temperature, potentially caused by increased blood flow to the skin. Experimentally raising skin surface temperature for 1 hr after tape-stripping results in faster recovery, which persists up to 6 h after skin disruption (Denda, Sokabe, Fukumi-Tominaga, & Tominaga, 2007). Moreover, increased skin blood flow after removing occlusion corresponds with decreased TEWL and faster recovery after disruption (Rodrigues, Pinto, Magro, Fernandes, & Alves, 2004). However, increased skin surface temperature should be related to elevated basal TEWL, which is not consistent with our results. Future work should consider measuring these potential mechanisms, including local skin blood flow and skin conductance.
The limitations of this study provide areas of improvement for future work. We used a single self-report measure of trait affect, which may be less reliable compared to multiple measures of daily affect over several weeks (Cohen et al., 2003). In addition, our trait affect measure was not administered to the entire sample (n = 85, compared to n = 60 in this study). Moreover, our findings may reflect a limited range of PA and NA, and future work should obtain a wider range of individual differences in trait affect through larger and more representative samples. For example, this study does not generalize to older, less educated, or less healthy individuals. Future work should also incorporate additional facets of positive emotions, such as those developed in the expanded version of the PANAS (PANAS-X; Watson & Clark, 1999). Although we experimentally manipulated stress, we did not manipulate levels of PA; thus, the conclusions regarding the relationship between PA and skin barrier recovery should be considered correlational and not causal. Finally, future work should measure the skin barrier recovery process as it unfolds over several days, rather than hours.
What are the implications of these findings for the larger literature on PA and health? Moreover, how might the effects of trait PA on the healing of a relatively minor wound translate to long-term effects of trait PA on health? The skin serves as the first line of defense against foreign pathogens (Elias, 2005). Thus, delays in skin barrier recovery in a particular area of the skin represent a “leak” in the body’s defenses, increasing the probability of foreign pathogens penetrating the skin and spreading to inner tissues. Therefore, a faster healing skin barrier during stressful periods, perhaps observed in individuals with greater trait PA, may reduce the probability of certain pathogens migrating from the skin surface to the interior. Beyond risk of infection, which would be relevant to most individuals (though more pronounced in older adults), trait PA may play a role in disease progression in individuals who have certain skin diseases that may be exacerbated by stress, such as psoriasis or atopic dermatitis, though this is an empirical question that should be addressed with future research. More broadly, relationships between trait PA and skin barrier recovery may reflect a larger link between PA and biological mechanisms that influence health and disease in other organ systems, such as the cardiovascular system. Establishing that trait PA can literally “get under the skin” is an important first step in determining the many ways in which trait PA benefits health.
Acknowledgments
This project was supported by a the following awards to T. F. R., Graduate Research Award from the American Psychological Association (APA), Division 38; an Alumni Grant for Graduate Research and Scholarship from the Graduate School, The Ohio State University; an APA Dissertation Research Award; the Department of Psychology, The Ohio State University; and National Institutes of Health Grant MO1-RR-0034 (Ohio State University General Clinical Research Center); also supported in part by a National Science Foundation Integrative Graduate Education and Research Traineeship to K. P. B.; and to the Pittsburgh Mind-Body Center at the University of Pittsburgh and Carnegie Mellon University (NIH HL65111, HL 65112, HL76858, and HL76852) to S.D.P. This study is based on a doctoral dissertation by T. F. R. We thank Janice Kiecolt-Glaser and the OSU Stress and Health Study for their support of this project, and the undergraduate research assistants for their work.
Footnotes
Portions of this study were presented at the 2008 annual meeting of the American Psychosomatic Society in Baltimore, MD.
Contributor Information
Theodore F. Robles, Department of Psychology, University of California, Los Angeles
Kathryn P. Brooks, Department of Psychology, University of California, Los Angeles
Sarah D. Pressman, Department of Psychology, University of Kansas
References
- Altemus M, Rao B, Dhabhar FS, Ding W, Granstein RD. Stress-induced changes in skin barrier function in healthy women. Journal of Investigative Dermatology. 2001;117:309–317. doi: 10.1046/j.1523-1747.2001.01373.x. [DOI] [PubMed] [Google Scholar]
- Arck PC, Slominski A, Theoharides TC, Peters EM, Paus R. Neuroimmunology of stress: Skin takes center stage. Journal of Investigative Dermatology. 2006;126:1697–1704. doi: 10.1038/sj.jid.5700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Choi EH, Brown BE, Crumrine D, Chang S, Man MQ, Elias PM, et al. Mechanisms by which psychologic stress alters cutaneous permeability barrier homeostasis and stratum corneum integrity. Journal of Investigative Dermatology. 2005;124:587–595. doi: 10.1111/j.0022-202X.2005.23589.x. [DOI] [PubMed] [Google Scholar]
- Christian LM, Graham JE, Padgett DA, Glaser R, Kiecolt-Glaser JK. Stress and wound healing. Neuroimmunomodulation. 2006;13:337–346. doi: 10.1159/000104862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Turner RB, Alper CM, Skoner DP. Emotional style and susceptibility to the common cold. Psychosomatic Medicine. 2003;65:652–657. doi: 10.1097/01.psy.0000077508.57784.da. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Henry JD. The Positive and Negative Affect Schedule (PANAS): Construct validity, measurement properties and normative data in a large non-clinical sample. British Journal of Clinical Psychology. 2004;43:245–265. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- Denda M, Sokabe T, Fukumi-Tominaga T, Tominaga M. Effects of skin surface temperature on epidermal permeability barrier homeostasis. Journal of Investigative Dermatology. 2007;127:654–659. doi: 10.1038/sj.jid.5700590. [DOI] [PubMed] [Google Scholar]
- Elias PM. Stratum corneum defensive functions: An integrated view. Journal of Investigative Dermatology. 2005;125:183–200. doi: 10.1111/j.0022-202X.2005.23668.x. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL. What good are positive emotions? Review of General Psychology. 1998;2:271–299. doi: 10.1037/1089-2680.2.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg A, Chren M, Sands LP, Matsui MS, Marenus KD, Feingold KR, et al. Psychological stress perturbs epidermal permeability barrier homeostasis. Archives of Dermatology. 2001;137:53–59. doi: 10.1001/archderm.137.1.53. [DOI] [PubMed] [Google Scholar]
- Grove GL, Grove MJ, Zerweck C, Pierce E. Computerized evaporimetry using the DermaLab TEWL probe. Skin Research and Technology. 1999;5:9–13. [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Emotions, morbidity, and mortality: New perspectives from psychoneuroimmunology. Annual Review of Psychology. 2002;53:83–107. doi: 10.1146/annurev.psych.53.100901.135217. [DOI] [PubMed] [Google Scholar]
- Kimata H. Effect of humor on allergen-induced wheal reactions. Journal of the American Medical Association. 2001;285:738. doi: 10.1001/jama.285.6.738. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke K, Hellhammer DH. The ‘Trier Social Stress Test’—A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Laidlaw TM, Booth RJ, Large RG. The variability of Type I hypersensitivity reactions: The importance of mood. Journal of Psychosomatic Research. 1994;38:51–61. doi: 10.1016/0022-3999(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Laidlaw TM, Booth RJ, Large RG. Reduction in skin reactions to histamine after a hypnotic procedure. Psychosomatic Medicine. 1996;58:242–248. doi: 10.1097/00006842-199605000-00008. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Cohen S, Rabin BS, Manuck SB. Trait positive affect and antibody response to hepatitis B vaccination. Brain, Behavior, and Immunity. 2006;20:261–269. doi: 10.1016/j.bbi.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Nickoloff BJ, Naidu Y. Perturbation of epidermal barrier function correlates with initiation of cytokine cascade in human skin. Journal of the American Academy of Dermatology. 1994;30:535–546. doi: 10.1016/s0190-9622(94)70059-1. [DOI] [PubMed] [Google Scholar]
- Ostir GV, Markides KS, Peek MK, Goodwin JS. The association between emotional well-being and the incidence of stroke in older adults. Psychosomatic Medicine. 2001;63:210–215. doi: 10.1097/00006842-200103000-00003. [DOI] [PubMed] [Google Scholar]
- Pressman SD, Cohen S. Does positive affect influence health? Psychological Bulletin. 2005;131:925–971. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS, Cheong YF, Congdon R. HLM 6: Hierarchical linear and nonlinear modeling. Lincolnwood, IL: Scientific Software International; 2004. [Google Scholar]
- Robles TF. Stress, social support, and delayed skin barrier recovery. Psychosomatic Medicine. 2007;69:807–815. doi: 10.1097/PSY.0b013e318157b12e. [DOI] [PubMed] [Google Scholar]
- Rodrigues LM, Pinto PC, Magro JM, Fernandes M, Alves J. Exploring the influence of skin perfusion on transepidermal water loss. Skin Research and Technology. 2004;10:257–262. doi: 10.1111/j.1600-0846.2004.00080.x. [DOI] [PubMed] [Google Scholar]
- Rogiers V EEMCO Group. EEMCO guidance for the assessment of transepidermal water loss in cosmetic sciences. Skin Pharmacology and Applied Skin Physiology. 2001;14:117–128. doi: 10.1159/000056341. [DOI] [PubMed] [Google Scholar]
- Rosenthal R, DiMatteo MR. Meta-analysis: Recent developments in quantitative methods for literature reviews. Annual Review of Psychology. 2001;52:59–82. doi: 10.1146/annurev.psych.52.1.59. [DOI] [PubMed] [Google Scholar]
- Salovey P, Rothman AJ, Detweiler JB, Steward WT. Emotional states and physical health. American Psychologist. 2000;55:110–121. doi: 10.1037//0003-066x.55.1.110. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Wardle J, Marmot M. Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processes. Proceedings of the National Academy of Sciences, USA of the United States of America. 2005;102:6508–6512. doi: 10.1073/pnas.0409174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellegen A, Watson D, Clark LA. On the dimensional and hierarchical structure of affect. Psychological Science. 1999;10:297–303. [Google Scholar]
- Tugade MM, Fredrickson BL. Resilient individuals use positive emotions to bounce back from negative emotional experiences. Journal of Personality and Social Psychology. 2004;86:320–333. doi: 10.1037/0022-3514.86.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA. The PANAS-X: Manual for the Positive and Negative Affect Schedule—Expanded Form. The University of Iowa; 1999. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]