Abstract
Previous work showed that in human nuclear extracts, double-strand break substrates bearing partially complementary (-ACG) 3′-phosphoglycolate (PG)-terminated 3′ overhangs are joined by a mechanism involving annealing of the terminal CG dinucleotides, PG removal, single-base gap filling and ligation. However, in these extracts only a minority of the breaks are rejoined, and most of the 3′-PG termini remain intact even after several hours. To determine whether the presence of a persistent 3′-PG prevents patching and ligation of the opposite strand, a substrate was constructed with two -ACG overhangs, one PG-terminated and one hydroxyl-terminated. after incubation in HeLa cell nuclear extracts, two major repair products of similar yield were formed: a fully repaired duplex and a nicked duplex in which the initial 3′-PG terminus remained intact. These results indicate that patching and ligation can proceed to completion in the unmodified strand despite persistence of the 3′-PG-terminated break in the opposite strand. The break in the PG-containing strand could then presumably be rejoined by a single-strand break repair pathway.
INTRODUCTION
The nonhomologous end joining (NHEJ) pathway of DNA double-strand break (DSB) repair possesses a remarkable ability to join DNA ends of diverse geometry, sequence and chemical structure (1–3). This versatility is attributable at least in part to a high tolerance of the gap-filling DNA polymerases λ and μ (4–6), as well as the XRCC4/DNA ligase IV (X4L4) complex (7), for substrates with missing, damaged or mismatched DNA bases. This tolerance is further enhanced by the presence of the accessory factor XLF/Cernunnos (8, 9). Many free radical-mediated DSBs bear the 2-carbon sugar fragment phosphoglycolate (PO4-CH2-COOH, hereafter PG) at the 3′ end (10, 11), which must be removed prior to patching or ligation of that strand of the DSB. In cell extracts, 3′-PG processing, primarily by tyrosyl-DNA phosphodiesterase, is slow and incomplete (2, 12–14), suggesting that PG removal could be a rate-limiting step in repair. Because many free radical-mediated DSBs will likely have a PG at only one 3′ terminus, it is possible that the opposite strand could be rejoined first and the 3′-PG removed later, perhaps by single-strand break repair pathways. As described below, experiments examining repair of such substrates in HeLa cell nuclear extracts confirm that one strand of a DSB can be patched and rejoined without prior processing of a closely opposed 3′-PG-terminated strand break.
MATERIALS AND METHODS
Materials
To create the model DSB substrate (Fig. 1), one 3′-PG and one 3′-hydroxyl oligomer were ligated into 5′ overhangs of the vector pRZ56, as described previously (2, 15). DNA polymerase λ (polλ) was a gift of Kasia Bebenek and Tom Kunkel (NIEHS). Human recombinant TDP1 with a 6×histidine tag was overexpressed in E. coli and purified by nickel affinity and anion exchange (MonoQ) chromatography, as described previously (16). All other enzymes were from New England Biolabs and were used in the buffers provided.
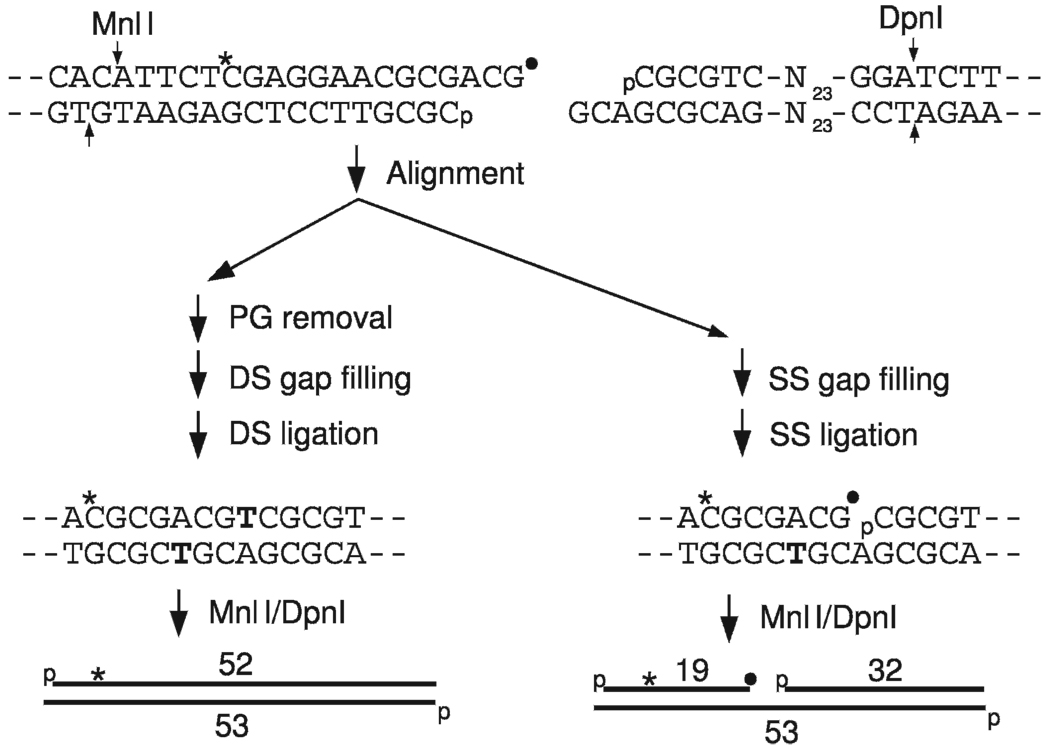
FIG. 1.
Experimental design. An internally labeled (*) plasmid substrate bearing one hydroxyl-terminated and one PG-terminated (●) 3′ overhang was prepared. Complete repair of both strands (DS) by PG removal, end alignment/annealing, patching (bolded T) and ligation results in an intact duplex that can be released as a labeled 52-mer/53-mer by cleavage with MnlI and DpnI. Repair in the hydroxyl-terminated strand only (SS) followed by MnlI/DpnI cleavage would yield a nicked duplex retaining a PG-terminated 19-mer.
End-Joining Reactions
Reactions contained HeLa cell nuclear extract (27 µg protein, Promega in vitro transcription grade), 0.1 µg of X4L4 (Trevigen), 50 mM triethanolammonium acetate, 10 mM Tris HCl, pH 7.9, 1 mM Mg(OAc)2, 40 mM KOAc, 0.5 mM dithiothreitol, 1 mM ATP, 50 µM of each dNTP or dideoxynucleoside 5′-triphosphate (ddNTP), 50 µg/ml BSA, and 20 ng DNA substrate in a total volume of 16 µl. Some reactions also contained 0.12 µg polλ, as indicated. After substrate addition, samples were incubated at 37°C, usually for 6 h. The DNA was then deproteinized by treatment with proteinase K followed by phenol and chloroform extractions (2) and digested with DpnI and MnlI.
Repair Product Analysis
After deproteinization and restriction cleavage, samples were run on a 12% nondenaturing polyacrylamide (30:1) gel for 28 h at 8 V/cm. Images were developed on a phosphorimage screen for 16 h at 8°C. For isolation of individual bands, gels were exposed to X-ray film for 24 h at 8°C and bands were excised, eluted in 2 ml 1 mM EDTA, pH 8, evaporated to 0.4 ml and ethanol-precipitated in the presence of 1 µg/ml tRNA. Samples were redissolved in 40 µl of 20 mM Tris, pH 7.6, 1 mM dithiothreitol, 0.1 mM EDTA and 50 µg/ml BSA. Half the sample was treated with 4 µg/ml human recombinant TDP1 for 1 h at 37°C, and TDP1 was inactivated by heating at 90°C for 5 min. Aliquots (10 µl) were then treated (or not) with 1 U calf intestinal phosphatase (CIP) in the buffer provided by the vendor (New England Biolabs) for 1 h at 37°C. Samples were denatured and analyzed on sequencing gels.
RESULTS
Rejoining of a DSB Bearing 3′-PG and 3′-Hydroxyl Termini Yields Two Major Repair Products
Repair of a free radical-mediated DSB in cell extracts requires several steps, including resolution of damaged termini, replacement of fragmented bases, and ligation. However, it may not be essential that removal of damaged termini and replacement of missing bases be completed in both strands before one of the strands is religated. To assess this possibility, a model DSB substrate was constructed that had a 3-base -ACG overhang at each end but with a 3′-PG terminus at one end and a 3′-hydroxyl terminus at the other (Fig. 1). To follow the progress of repair, the substrate was internally labeled 14 bases from the 3′-PG terminus.
After incubation in X4L4-supplemented HeLa cell nuclear extracts (17), the DNA was cut with DpnI and MnlI to release short fragments from each end of the substrate. From the initial unprocessed substrate, a labeled 19-mer/17-mer duplex is released. Complete, accurate repair by PG removal, alignment-based gap filling and ligation of both strands, would yield a labeled 52-mer/53-mer duplex (Fig. 1), and indeed one of the major extract-dependent products (product 1) comigrated with a synthetic duplex having the predicted length and sequence (Fig. 2A).
FIG. 2.
Multiple end joining products from a 3′-hydroxyl/3′-PG DSB. Panel A: Nondenaturing gel electrophoresis of the products of repair of the substrate shown in Fig. 1. The substrate was incubated for 6 h in X4L4-supplemented HeLa cell nuclear extracts, with or without exogenous polλ, then cut with MnlI and DpnI. The rightmost lanes contain a synthetic 5′-32P-labeled 53-mer with the same sequence as the expected repair product, annealed to either a 5′-phosphate 52-mer (“DS 52/53”) or a 5′-phosphate 3′-phosphate 19-mer and a 5′-phosphate 32-mer (“Nicked”, see Fig. 1). Panel B: Quantitative analysis of formation of products 1 and 2, at 6 h incubation time, in four replicate experiments (means ± SEM). Panel C: Dependence of repair product formation on X4L4. Reactions were performed as in panel A except that X4L4 was added or not, as indicated; no polλ was added. Panel D: Time course for formation of repair products 1 (○) and 2 (●) in the presence of added polλ; a replicate experiment (not shown) gave similar results.
A second labeled product (product 2) was also detected that migrated more slowly than the 52-mer/53-mer duplex (Fig. 2A). The formation of product 2, but not product 1, was stimulated by supplementation with exogenous polλ (Fig. 2B), which we previously showed was the primary gap filling polymerase in these extracts (18). Formation of both products was completely blocked by substitution of dTTP with ddTTP, indicating that gap filling was required in both cases (Fig. 2A, lane 4). Moreover, both products were almost completely dependent on addition of X4L4 (Fig. 2C), which is required for gap filling as well as ligation but is not present in sufficient quantity to support end joining in unsupplemented HeLa cell nuclear extracts (17). Thus product 2 could be a nicked fragment resulting from gap filling and rejoining of the DSB in one strand only (see Fig. 1). Indeed, product 2 approximately comigrated with a nicked duplex that had the same structure as the predicted singly ligated end-joining product, except that it bore a 3′-phosphate rather than 3′-PG at the 3′ end of the nick. Unexpectedly, the nicked marker consistently gave a more diffuse band than either the intact duplex or any of the putative repair products. Nevertheless, the results show that under these electrophoresis conditions, a single-strand nick reduces the mobility of a DNA duplex, consistent with the proposal that product 2 represents a nicked duplex. Whereas product 1 accumulated gradually over the course of several hours, formation of product 2 was largely complete within 30 min (Fig. 2D). However, these data do not exclude the possibility that product 2 continued to form at later times but that its formation was balanced by slow, partial conversion to product 1 (i.e., conversion of the putative nicked product to a fully repaired duplex). A third, very minor band of intermediate mobility (“m” in Fig. 2A) was also present.
In an attempt to assess the dependence of the two repair products on PG removal by TDP1, a similar experiment was performed with extracts from TDP1-mutant SCAN1 cells. While a trace of product 1 was detected in SCAN1 extracts supplemented with X4L4, TDP1 and polλ, no product 2 was detected under any condition, and there appeared to be extensive degradation of the substrate, as indicated by loss of most of the radiolabel (Supplementary Fig. 1).
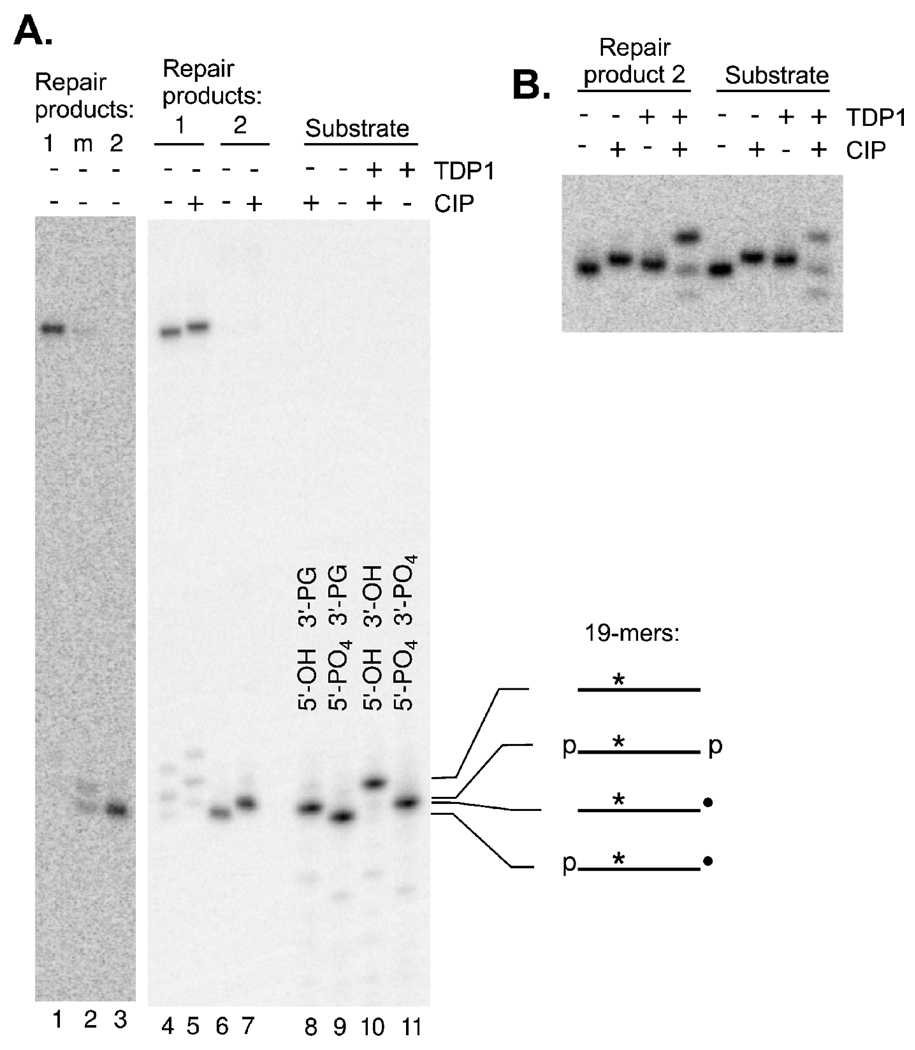
A Nicked Repair Product Retains a 3′-PG
To examine the repair products in more detail, each product was excised from the nondenaturing gel, eluted, dephosphorylated (or not), and then rerun on a denaturing gel. The putative fully repaired product 1 migrated as a single band at a position consistent with its predicted length (Fig. 3A). Label from the putative nicked repair product 2 migrated at precisely the same position as a 5′-phosphate 3′-PG 19-mer generated by MnlI cleavage of the untreated starting substrate. Furthermore, removal of the 5′-phosphate by CIP treatment produced the same small decrease in mobility for both the repair product and the marker. Finally, conversion of the 3′-PG to a 3′-phosphate by treatment with TDP1 likewise produced identical mobility shifts in the two species, while treatment with both TDP1 and CIP produced a larger decrease in mobility, yielding a product with the mobility of a 3′-hydroxyl, 5′-hydroxyl 19-mer (Fig. 3B). These results strongly suggest that an unprocessed 3′-PG 19-mer is still present in repair product 2. Thus the presence of a persistent, closely opposed 1-base gap with a 3′-PG terminus in one strand did not prevent gap filling and ligation of the other strand.
FIG. 3.
Denaturing gel electrophoresis of the repaired products. Panel A: Each repair product from experiments similar to those in Fig. 2 was eluted from the gel, treated (or not) with CIP and then run on a 20% denaturing gel (lanes 2–7). Lanes 1–3 are from a separate experiment in which the intermediate band m (see Fig. 2A) was also isolated. Markers with known termini (lanes 8–11) were generated by treating the initial MnlI-cut substrate (see Fig. 1) with TDP1 and/or CIP. Panel B: Repair product 2, or the MnlI-cut substrate, was treated with TDP1 and/or CIP as indicated. Higher-mobility minor bands in samples treated with both enzymes presumably reflect a trace of exonuclease in the CIP incubation, resulting in partial conversion of the 3′-hydroxyl 5′-hydroxyl 19-mer to shorter products.
Denaturing electrophoresis of the intermediate-mobility product (band “m”) indicated that it was contaminated with small amounts of products 1 and 2 (due to incomplete resolution on the nondenaturing gel). However, it also contained a unique product whose mobility was consistent with a 5′-phosphate 3′-hydroxyl 19-mer. This result suggests that, throughout the incubation period, small amounts of a repair intermediate were present in which PG was removed from one strand and the opposite strand was patched and ligated, although the order of these events could not be distinguished. When the end-joining products were deproteinized and incubated in fresh extract, there was an X4L4-independent increase in band m, at the expense of band 2, indicating that PG removal can indeed occur after gap filling and ligation of the opposite strand (Supplementary Fig. 2).
DISCUSSION
No enzyme specific for the resolution of 3′-PG termini has yet been identified, but several enzymes with diverse canonical substrates can act on 3′-PGs. At single-strand breaks, the abasic endonuclease APE1 appears to be the primary PG-processing enzyme (19), directly yielding the 3′-hydroxyl terminus required for gap filling and ligation. Although APE1 is capable of removing 3′-PGs from blunt and 3′-recessed DSBs, the efficiency of this reaction is very low, about 20 times lower than for a single-strand break and 1000 times lower than the efficiency of abasic site cleavage (20). Moreover, APE1 has no activity toward PGs on 3′ overhangs (20), and there is no evidence that it interacts with or is recruited or stimulated by the core NHEJ proteins.
TDP1 can also act on 3′-PG termini of DSBs, whether overhanging, blunt or recessed, converting them to 3′-phosphates that can then be removed by polynucleotide kinase/phosphatase (13, 14). Again, however, TDP1 is ~100-fold less efficient in processing PG ends than its canonical 3′-pTyr substrate, and there is no evidence of its being recruited by other NHEJ proteins (14). Alternatively, 3′-PG DSB ends can be trimmed by Artemis in a DNA-PK-dependent manner, with the PG being released as a mono-, di- or oligonucleotide, depending on the geometry of the end (21). In vitro, this reaction is very efficient for long 3′-overhangs (22), but for short overhangs and blunt ends, it is very slow, with about half of the ends remaining unprocessed even after several hours (21). Thus, while at least three enzymes are available for resolving 3′-PG DSB ends, in vitro data suggest that none of them work very efficiently on these lesions.
For DSBs bearing a 3′-PG at only one end of the break, an additional possibility is that the 3′-PG could remain unprocessed even as rejoining is completed in the opposite strand. After dissociation of end-joining proteins, the 3′-PG strand could then be repaired as a single-strand break, with the PG being removed by APE1 (19). The results presented here indicate that the end-joining machinery can complete the repair of one strand, including gap filling, despite an unprocessed PG-terminated break and 1-base gap only 3 bases away in the opposite strand. The formation of resulting the 3′-PG nicked product was stimulated by addition of exogenous polλ (Fig. 2B), suggesting that the PG-containing annealed structure (see Fig. 1) may be a less favorable substrate than the same substrate with two 3′-hydroxyl termini. Attempts to directly demonstrate progression of the nicked product 2 to the fully repaired product 1 by examining the kinetics of repair were inconclusive. Although product 1 accumulated more slowly than product 2, the absolute amount of product 2 did not decrease with time. Furthermore, the finding that addition of polλ increased the formation of product 2 without increasing the yield of product 1 (Fig. 2A and 2B) suggests that there was relatively little conversion of product 2 to product 1 over the course of the reaction. Previous work showed that a fraction of 3′-PG DSB termini are removed very rapidly, and it appears more likely that product 1 arises primarily from this fraction of the breaks. As in most other end-joining studies (17, 23), the assays in Fig. 2 were performed in the presence of low Mg++ (1 mM), which is adequate for DNA ligase IV but not other ligases (24). When the reaction products were deproteinized and incubated in fresh extract with 10 mM Mg++, there was still little if any conversion of product 2 to product 1, but there was a significant (two- to threefold) increase in the intermediate band m, indicating further PG removal from the singly ligated product in fresh extract (Supplmentary Fig. 2).
While there is no doubt that free radicals produce PG ends, the exact fraction of DSBs that bear PG ends is difficult to determine and may be different for isolated DNA than for DNA in intact cells. In the specific case of ionizing radiation, early biochemical studies suggested that approximately half of all strand breaks had 3′-PG termini (11), while a more recent measurement by mass spectrometry suggested approximately 10% (25). Even if this lower estimate is correct, it predicts that while only ~1% of DSBs will have a PG terminus at both ends, ~18% will have a PG at one end. Thus failure to resolve such ends would result in large numbers of unrepaired DSBs and a significant decrease in radiation survival. However, if the breaks with only one PG terminus can be repaired by the mechanism described above (Fig. 1), then only the ~1% of breaks with PG at both ends would strictly require PG removal prior to rejoining. Thus this mechanism provides a potential explanation for the lack of a significant DSB repair defect in irradiated TDP1-mutant SCAN cells (26) or Tdp1−/− mouse fibroblasts (27), even though experiments in cell extracts suggest that lack of TDP1 confers a severe deficiency in 3′-PG processing at DSBs ends (12, 16).
Supplementary Material
ACKNOWLEDGMENTS
We thank T. A. Knukel and K. Bebenek for providing polλ. This work was supported by Grants CA40615 and AG023783 from the National Institutes of Health, USDDHS.
REFERENCES
- 1.Lieber MR, Lu H, Gu J, Schwarz K. Flexibility in the order of action and in the enzymology of the nuclease, polymerases, and ligase of vertebrate non-homologous DNA end joining: Relevance to cancer, aging, and the immune system. Cell Res. 2008;18:125–133. doi: 10.1038/cr.2007.108. [DOI] [PubMed] [Google Scholar]
- 2.Chen S, Inamdar KV, Pfeiffer P, Feldmann E, Hannah MF, Yu Y, Lee JW, Zhou T, Lees-Miller SP, Povirk LF. Accurate in vitro end-joining of a DNA double-strand break with partially cohesive 3′-overhangs and 3′-phosphoglycolate termini: Effect of Ku on repair fidelity. J. Biol. Chem. 2001;276:24323–24330. doi: 10.1074/jbc.M010544200. [DOI] [PubMed] [Google Scholar]
- 3.Pfeiffer P, Thode S, Hancke J, Keohavong P, Thilly WG. Resolution and conservation of mismatches in DNA end joining. Mutagenesis. 1994;9:527–535. doi: 10.1093/mutage/9.6.527. [DOI] [PubMed] [Google Scholar]
- 4.Picher AJ, Blanco L. Human DNA polymerase lambda is a proficient extender of primer ends paired to 7,8-dihydro-8-oxoguanine. DNA Repair (Amst.) 2007;6:1749–1756. doi: 10.1016/j.dnarep.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Zhou RZ, Blanco L, Garcia-Diaz M, Bebenek K, Kunkel TA, Povirk LF. Tolerance for 8-oxoguanine but not thymine glycol in alignment-based gap filling of partially complementary double-strand break ends by DNA polymerase λ in human nuclear extracts. Nucleic Acids Res. 2008;36:2895–2905. doi: 10.1093/nar/gkn126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruiz JF, Dominguez O, Lain de Lera T, Garcia-Diaz M, Bernad A, Blanco L. DNA polymerase mu, a candidate hypermutase? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001;356:99–109. doi: 10.1098/rstb.2000.0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma Y, Lu H, Tippin B, Goodman MF, Shimazaki N, Koiwai O, Hsieh CL, Schwarz K, Lieber MR. A biochemically defined system for mammalian nonhomologous DNA end joining. Mol. Cell. 2004;16:701–713. doi: 10.1016/j.molcel.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Gu J, Lu H, Tsai AG, Schwarz K, Lieber MR. Single-stranded DNA ligation and XLF-stimulated incompatible DNA end ligation by the XRCC4-DNA ligase IV complex: Influence of terminal DNA sequence. Nucleic Acids Res. 2007;35:5755–5762. doi: 10.1093/nar/gkm579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai CJ, Kim SA, Chu G. Cernunnos/XLF promotes the ligation of mismatched and noncohesive DNA ends. Proc. Natl. Acad. Sci. USA. 2007;104:7851–7856. doi: 10.1073/pnas.0702620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertoncini CR, Meneghini R. DNA strand breaks produced by oxidative stress in mammalian cells exhibit 3′-phosphoglycolate termini. Nucleic Acids Res. 1995;23:2995–3002. doi: 10.1093/nar/23.15.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henner WD, Rodriguez LO, Hecht SM, Haseltine WA. Gamma-ray induced deoxyribonucleic acid strand breaks. 3′ glycolate termini. J. Biol. Chem. 1983;258:711–713. [PubMed] [Google Scholar]
- 12.Zhou T, Lee JW, Tatavarthi H, Lupski JR, Valerie K, Povirk LF. Deficiency in 3′-phosphoglycolate processing in human cells with a hereditary mutation in tyrosyl-DNA phosphodiesterase (TDP1) Nucleic Acids Res. 2005;33:289–297. doi: 10.1093/nar/gki170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inamdar KV, Pouliot JJ, Zhou T, Lees-Miller SP, Rasouli-Nia A, Povirk LF. Conversion of phosphoglycolate to phosphate termini on 3′ overhangs of DNA double-strand breaks by the human tyrosyl-DNA phosphodiesterase hTdp1. J. Biol. Chem. 2002;276:24323–24330. doi: 10.1074/jbc.M204688200. [DOI] [PubMed] [Google Scholar]
- 14.Zhou T, Akopiants K, Mohapatra S, Lin PS, Valerie K, Ramsden DA, Lees-Miller SP, Povirk LF. Tyrosyl-DNA phosphodiesterase and the repair of 3′-phosphoglycolate-terminated DNA double-strand breaks. DNA Repair (Amst.) 2009;8:901–911. doi: 10.1016/j.dnarep.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett RAO, Gu XY, Povirk LF. Construction of a vector containing a site-specific DNA double-strand break with 3′-phosphoglycolate termini and analysis of the products of end-joining in CV-1 cells. Int. J. Radiat. Biol. 1996;70:623–636. doi: 10.1080/095530096144509. [DOI] [PubMed] [Google Scholar]
- 16.Hawkins AJ, Subler MA, Akopiants K, Wiley JL, Taylor SM, Rice AC, Windle JJ, Valerie K, Povirk LF. In vitro complementation of Tdp1 deficiency indicates a stabilized enzyme-DNA adduct from tyrosyl but not glycolate lesions as a consequence of the SCAN1 mutation. DNA Repair (Amst.) 2009;8:654–663. doi: 10.1016/j.dnarep.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang J, Dynan WS. Reconstitution of the mammalian DNA double-strand break end-joining reaction reveals a requirement for an Mre11/Rad50/NBS1-containing fraction. Nucleic Acids Res. 2002;30:667–674. doi: 10.1093/nar/30.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JW, Blanco L, Zhou T, Bebenek K, Garcia-Diaz M, Kunkel TA, Wang Z, Povirk LF. Implication of DNA polymerase lambda in alignment-based gap filling for nonhomologous DNA end joining in human nuclear extracts. J. Biol. Chem. 2004;279:805–811. doi: 10.1074/jbc.M307913200. [DOI] [PubMed] [Google Scholar]
- 19.Parsons JL, Dianova II, Dianov GL. APE1 is the major 3′-phosphoglycolate activity in human cell extracts. Nucleic Acids Res. 2004;32:3531–3536. doi: 10.1093/nar/gkh676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suh D, Wilson DM, III, Povirk LF. 3′-phosphodiesterase activity of human apurinic/apyrimidinic endonuclease at DNA double-strand break ends. Nucleic Acids Res. 1997;25:2495–2500. doi: 10.1093/nar/25.12.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yannone SM, Khan IS, Zhou RZ, Zhou T, Valerie K, Povirk LF. Coordinate 5′ and 3′ endonucleolytic trimming of terminally blocked blunt DNA double-strand break ends by artemis nuclease and DNA-dependent protein kinase. Nucleic Acids Res. 2008;36:3354–3365. doi: 10.1093/nar/gkn205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Povirk LF, Zhou T, Zhou R, Cowan MJ, Yannone SM. Processing of 3′-phosphoglycolate-terminated DNA double strand breaks by artemis nuclease. J. Biol. Chem. 2007;282:3547–3558. doi: 10.1074/jbc.M607745200. [DOI] [PubMed] [Google Scholar]
- 23.Baumann P, West SC. DNA end-joining catalyzed by human cell-free extracts. Proc. Natl. Acad. Sci. USA. 1998;95:14066–14070. doi: 10.1073/pnas.95.24.14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Zeng ZC, Perrault AR, Cheng X, Qin W, Iliakis G. Genetic evidence for the involvement of DNA ligase IV in the DNA-PK-dependent pathway of non-homologous end joining in mammalian cells. Nucleic Acids Res. 2001;29:1653–1660. doi: 10.1093/nar/29.8.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen B, Zhou X, Taghizadeh K, Chen J, Stubbe J, Dedon PC. GC/MSmethods to quantify the 2-deoxypentos-4-ulose and 3′-phosphoglycolate pathways of 4′ oxidation of 2-deoxyribose in DNA: Application to DNA damage produced by γ-radiation and bleomycin. Chem. Res. Toxicol. 2007;20:1701–1708. doi: 10.1021/tx700164y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Khamisy SF, Hartsuiker E, Caldecott KW. TDP1 facilitates repair of ionizing radiation-induced DNA single-strand breaks. DNA Repair (Amst.) 2007;6:1485–1495. doi: 10.1016/j.dnarep.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 27.Katyal S, El-Khamisy SF, Russell HR, Li Y, Ju L, Caldecott KW, McKinnon PJ. TDP1 facilitates chromosomal single-strand break repair in neurons and is neuroprotective in vivo. EMBO J. 2007;26:4720–4731. doi: 10.1038/sj.emboj.7601869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.